kinetics of an aminated PEGMA catalyst
Continuous flow investigation of the aldol reaction
Academic year 2019-2020
Master of Science in Chemical Engineering
Master's dissertation submitted in order to obtain the academic degree of Counsellor: Bert Biesemans
Supervisors: Prof. dr. ir. Joris Thybaut, Dr. ir. Jeroen Lauwaert Student number: 01501502
kinetics of an aminated PEGMA catalyst
Continuous flow investigation of the aldol reaction
Academic year 2019-2020
Master of Science in Chemical Engineering
Master's dissertation submitted in order to obtain the academic degree of Counsellor: Bert Biesemans
Supervisors: Prof. dr. ir. Joris Thybaut, Dr. ir. Jeroen Lauwaert Student number: 01501502
LABORATORY FOR CHEMICAL TECHNOLOGY Technologiepark 125, 9052 Gent, Belgium
Declaration concerning the accessibility of the master thesis
Undersigned, Mariska Creten
Graduated from Ghent University, academic year 2019-2020 and is author of the master thesis with title:
Continuous flow investigation of the aldol reaction kinetics of an aminated PEGMA catalyst The author(s) gives (give) permission to make this master dissertation available for consultation and to copy parts of this master dissertation for personal use. In the case of any other use, the copyright terms have to be respected, in particular with regard to the obligation to state expressly the source when quoting results from this master dissertation.
31/05/2020 Mariska Creten
Acknowledgement
Now that the end of my master thesis and in general the end of my studies is nearing, I realize that I would not have been able to do all of this without the help given to me. Therefore I would like to thank a few people.
First of all, I would like to thank my supervisors prof. dr. ir. Joris W. Thybaut and dr. ir. Jeroen Lauwaert, for offering me the chance to work on this interesting project. Many thanks for giving me the opportunity to be a part of the CaRe group for a year, and for the follow-up of my work.
A special thanks to my coach and support throughout the entire year, Bert Biesemans. Whenever I had a question he provided the answer or the direction to look in. Thank you for guiding me throughout this project and for the many hours spent on the reading and revising of my writing. I also appreciate the many efforts you have made to help me with the troubles that, inevitably, arose during lab experiments.
I would like to thank my fellow classmates, for making all the hours spent in class rooms and the thesis room a lot of fun. Ordering pizza to eat together during breaks, bursting into the thesis room to tell a joke or a tale, helping each other with projects; it was a wonderful two years.
And finally, I would like to thank my family and friends for their support and always being there for me. Without you I would never have made it.
Mariska Creten Ghent, May 2020
“And so, does the destination matter? Or is it the path we take? I declare that no accomplishment has substance nearly as great as the road used to achieve it. We are not creatures of destinations. It is the journey that shapes us. Our callused feet, our backs strong from carrying the weight of our travels, our eyes open with the fresh delight of experiences lived.”
Preamble to the Master’s Dissertation
In context of the worldwide outbreak of the coronavirus disease COVID-19, measures were taken by Ghent University. Because of these measures, research requiring lab work could no longer be carried out. Since this master’s dissertation was initially an experimental thesis, the project was partially redirected so that its completion was still possible.
In specific, as of 16 March 2020 all experiments in context of this thesis were ended. In Chapter 4 (Results and Discussion), a distinction is made between experiments that were carried out and provided results, and experiments that were planned but not carried out. For the latter, hypotheses are provided on expected results and on possible conclusions that would have been made, to clarify the purpose of these planned experiments.
As an alternative to unperformed experiments, a review paper (Chapter 5) was written on existing 3D printing techniques for the fabrication of structured polymer catalysts. This review serves as a perspective on future work that will be carried out with the catalytic material investigated in this thesis, in the transition of academic research on fundamental catalytic properties to applied research on the optimization of the reactor performance.
Continuous Flow Investigation of the Aldol Reaction
Kinetics of an Aminated PEGMA Catalyst
Mariska Creten
Supervisors: Prof. dr. ir. Joris Thybaut, Dr. ir. Jeroen Lauwaert Counsellor: Bert Biesemans
Master's dissertation submitted in order to obtain the academic degree of Master of Science in Chemical Engineering
Faculty of Engineering and Architecture Academic year 2019-2020
___________________________________________________________________________
Abstract: The catalytic performance of a newly developed stable aminated polyethylene glycol
(PEG) resin catalyst was assessed in the aldol reaction of acetone with 4-nitrobenzaldehyde, using water as a solvent. Poly(ethylene glycol) methacrylate (PEGMA) organic hydrogels were synthesized via free radical suspension polymerization, and functionalized by nucleophilic substitution of ethylenediamine (EDA). The catalytic activity of the primary amine PEGMA-EDA catalysts was first evaluated in a batch reactor. A turnover frequency (TOF) was obtained comparable to the TOF of state-of-the-art primary amine functionalized silica catalysts evaluated for the same reaction in hexane. Subsequently, the catalyst was filtered and recycled for a second batch experiment to assess the reusability, maintaining 92% of the initial TOF. Finally, a thorough evaluation of the catalyst stability was made by monitoring the conversion as a function of time on stream in a continuous reactor. The catalyst performance in the aqueous aldol reaction remained stable during at least 12 hours on stream, also under varying reaction conditions, demonstrating the material’s potential as a stable and reusable catalyst.
As the developed polymeric resin is ideally suited for shaping into a structured catalyst, the existing 3D printing techniques for the fabrication of such structured polymer catalysts were reviewed, providing a perspective on future process intensification.
Continuous Flow Investigation of the Aldol
Reaction Kinetics of an Aminated PEGMA Catalyst
Mariska CretenCounsellor: Bert Biesemans
Supervisor(s): Prof. dr. ir. Joris Thybaut, Dr. ir. Jeroen Lauwaert
Abstract: The catalytic performance of a newly developed
stable aminated polyethylene glycol (PEG) resin catalyst was assessed in the aldol reaction of acetone with 4-nitrobenzaldehyde, using water as a solvent. Poly(ethylene glycol) methacrylate (PEGMA) organic hydrogels were synthesized via free radical suspension polymerization, and functionalized by nucleophilic substitution of ethylenediamine (EDA). The catalytic activity of the primary amine PEGMA-EDA catalysts was first evaluated in a batch reactor. A turnover frequency (TOF) was obtained comparable to the TOF of state-of-the-art primary amine functionalized silica catalysts evaluated for the same reaction in hexane. Subsequently, the catalyst was filtered and recycled for a second batch experiment to assess the reusability, maintaining 92% of the initial TOF. Finally, a thorough evaluation of the catalyst stability was made by monitoring the conversion as a function of time on stream in a continuous reactor. The catalyst performance in the aqueous aldol reaction remained stable during at least 12 hours on stream, also under varying reaction conditions, demonstrating the material’s potential as a stable and reusable catalyst.
As the developed polymeric resin is ideally suited for shaping into a structured catalyst, the existing 3D printing techniques for the fabrication of such structured polymer catalysts were reviewed, providing a perspective on future process intensification.
Keywords: aldol reaction, batch reactor, continuous reactor,
polymer resin, catalyst
I. INTRODUCTION
The aldol reaction is considered one of the most powerful synthetic tools for carbon-carbon bond-forming reactions, with applications in the pharmacological industry [1], fine chemicals synthesis [2], and in the production of biomass-derived hydrocarbon fuels [3]. By yielding new carbon-carbon bonds, larger and more complex molecules are constructed.
Currently, aqueous sodium hydroxide and other strong bases are typically employed as the standard catalyst for the industrial aldol reaction [4]. As a replacement for these materials, heterogeneous catalysts are promising alternatives. Related advantages are a reduced energy cost for separating the catalyst from the product stream, a longer catalyst lifetime, and reduced equipment corrosion [5]. Zeolites, layered double hydroxides, functionalized silicas and organosilicas have already been investigated, but generally lack the required stability. Recently, the feasibility of amine functionalized resins as stable and selective aldol reaction catalysts has been demonstrated by De Vylder et al [6].
In this thesis, the newly developed stable aminated resin catalyst was synthesized and the catalyst performance was assessed in the aldol reaction of acetone with 4-nitrobenzaldehyde, using water as a solvent.
II. PROCEDURES
A. Catalyst Synthesis
The synthesis procedure of the PEGMA-EDA catalyst consisted of three main steps, and was adapted from De Vylder [6]. Figure 1 shows the catalyst structure.
Figure 1. Chemical structure of the PEGMA catalyst functionalized
with a primary ethylenediamine (EDA). The secondary amine that is formed in PEGMA-EDA is not catalytically active. Adapted from De Vylder [6].
In a first step, cross-linked poly(ethylene glycol) methacrylate hydrogel beads were synthesized via suspension polymerization. 0.40 g polyvinylpyrrolidone (Sigma-Aldrich, K30, MW 40000) was dissolved in 80 mL deionized water. 5.5 mL cyclohexanol (Sigma-Aldrich, ReagentPlus, 99%), 2.0 mL octanol (Acros, 99%), 4.1 mL poly(ethylene glycol) methacrylate (Sigma-Aldrich, average Mn 360), 0.6 mL poly(ethylene glycol) dimethacrylate (Sigma-Aldrich, average Mn 750) and 0.12 g benzoyl peroxide (Luperox® A98, reagent grade, ≥98%) were mixed separately and added to the aqueous solution. The mixture was then stirred at 700 rpm for 4 hours at 85 °C and for 1 hour at 95 °C. The obtained hydrogel beads were filtered off, washed twice with acetone (VWR, 99.5%) for 30 min at 500 rpm and dried for 17 hours on a high vacuum line at 50 °C.
Next, the hydroxyl groups were partially substituted with chlorine groups. The hydrogel beads were covered with 40 mL dry toluene (Sigma-Aldrich, anhydrous, 99.8%), and 0.33 mL pyridine (JT Baker, ACS Reagent, 100%) and 0.3 mL thionyl chloride (Sigma-Aldrich, ReagentPlus, >99%) were added. The mixture was stirred at 450 rpm for 4 hours at 75 °C, filtered off, and washed twice with ethanol (Koptec, 200 proof) for 30 min at 450 rpm.
In the last step, the chlorine groups were replaced by amine groups. The hydrogel beads were covered with 50 mL dry
toluene, 5 mL ethylenediamine (Sigma-Aldrich, ReagentPlus, 99%), and 13 mL di-isopropylethylamine (Sigma-Aldrich, ReagentPlus, >99%). The mixture was stirred at 450 rpm and refluxed for 24 hours at 110 °C. Finally, the catalyst was filtered off, washed twice with ethanol for 30 min at 450 rpm and dried for 17 hours on a high vacuum line at 50 °C.
B. Catalyst Characterization
SEM images of the resin beads were collected with a Hitachi SU 8010 scanning electron microscope, after applying a thin layer of gold using sputter coating. The concentration of amine groups was measured via CHN-analysis, using a Thermo Flash 2000 elemental analyser with V2O5 as catalyst,
in order to ensure total oxidation of the hydrogel sample. The active site loading was determined as half of the concentration of amine groups.
C. Performance Evaluation of the Catalyst
The catalyst performance was evaluated for the aldol reaction of acetone with 4-nitrobenzaldehyde, yielding 4-hydroxy-4-(4-nitrophenyl)butan-2-one as aldol product and 4-(4-nitrophenyl)-3-buten-2-one as enone product (Figure 2). Water was used as solvent.
Figure 2. Aldol reaction of acetone with 4-nitrobenzaldehyde.
1) Batch Reactor
First, the catalyst performance was assessed in a batch-type reactor (Parr® 4560 mini, 300 mL). The reactor was loaded with 55 g water (double deionized, Milli-Q) as solvent, 0.14 g catalyst, and 0.25 g methyl 4-nitrobenzoate (Sigma-Aldrich, >99%) as internal standard. This mixture was heated to 55 °C, under constant stirring at 400 rpm. 45 g acetone (Acros, 99.6%) was preheated separately to the same temperature and used to dissolve 0.45 g 4-nitrobenzaldehyde (Acros, 99%), before being injected in the reactor using a syringe. The reaction was monitored for 4 hours by taking a sample (0.3 mL) of the reaction mixture every 30 minutes.
To assess the reusability, the catalyst was reused in a second batch experiment. After filtering off (MN 640 d, Filter Paper), the catalyst was washed twice with ethanol (Koptec, 200 proof) for 30 min at 450 rpm and dried for 17 hours on a high vacuum line at 50 °C.
2) Continuous Reactor
Next, the catalyst was also evaluated in a packed-bed plug-flow reactor (Liquid-Solid Lab-Scale (LS)²). The reactor was loaded with catalyst material, applied between inert material, which consists of round alumina beads of different sizes (0.5 – 3.5 mm). Attention should be paid that the catalyst layer is inserted at the height of the inner thermocouple in the reactor, with layers of the inert beads below and above. The feed bottle (containing 389 g water, 318 g acetone, 3.18 g 4-nitrobenzaldehyde, and 1.76 g methyl 4-nitrobenzoate) was connected to a plunger pump. The pump was then started and the temperature was set for the pre-heater and reactor heating jacket. The time at which the thermocouple inside the catalyst bed measured the desired reaction temperature was taken as the start of the reaction (t = 0 min), after which the reaction
was monitored by taking a sample (0.3 mL) of the reaction mixture every 60 minutes.
3) Sample Analysis
The samples taken during the experiments were analysed using a reversed-phase high-performance liquid chromato-graph (RP-HPLC) from Agilent (1100 series). The composition of the reaction mixture was determined by relating the peak surface areas of the components to the amount and peak area of internal standard added to the reactor. The catalyst activity was quantified by the turnover frequency (TOF).
III. RESULTS AND DISCUSSION
Three batches of catalyst were synthesized, characterized and subsequently tested in the aqueous aldol reaction of acetone with 4-nitrobenzaldehyde. A fourth batch was synthesized and used in a continuous flow experiment, but not characterized.
A. Catalyst Characterization
SEM images of the PEGMA hydrogel beads indicated that, initially, spherical particles in the order of 100 – 500 μm were formed during the suspension polymerization, as reported by De Vylder [6]. Analysis of the images showed furthermore that during subsequent washing and refluxing, the polymer particles were subject to fragmentation. However, performance evaluation of the catalysts in the batch reactor indicated that the degree of fragmentation has no effect on the catalytic activity.
Table 1 reports the active site loading of the synthesized catalysts. These values are in the same order of magnitude as reported by De Vylder [6].
Table 1. Active site loading for the different batches of
PEGMA-EDA catalyst.
Sample Active site loadinga (mmol g-1)
PEGMA-EDA1 0.54 PEGMA-EDA2 0.50 PEGMA-EDA3 0.40
aThe active site loading corresponds to the total nitrogen content obtained from CHN-analysis divided by 2.
B. Batch Reactor
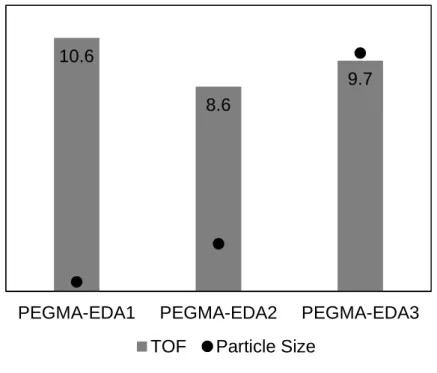
The TOF of the PEGMA-EDA catalysts is displayed in Figure 3, and amounts to 9.6 ± 1.0 10-4 s-1 on average. The
aldol and enone product selectivity was found to be 96% and 4% respectively, and remained constant throughout the entire experiment. The values for the TOF are in the same order of magnitude as previously reported by De Vylder [6] for the same catalyst and reaction. Moreover, the obtained TOF is 4 times higher than the state-of-the-art primary amine functionalized mesoporous silica catalyst evaluated in water [7], and is comparable to the TOF of this primary amine functionalized silica catalyst evaluated in hexane [8].
Figure 3. Turnover frequency for the PEGMA-EDA catalysts,
determined in the Parr® batch reactor, for the aldol reaction of acetone with 4-nitrobenzaldehyde. (T = 55 °C, 45 g acetone, 0.45 g 4-nitrobenzaldehyde, 55 g water, 0.14 g catalyst)
When reusing the spent catalyst in a second batch experiment, 92% of the initial TOF was maintained for PEGMA-EDA1. Characterization of the spent catalyst was performed by means of nitrogen elemental analysis. The total nitrogen content of the spent catalyst was found to have increased to 2.1 wt%, as compared to 1.5 wt% for the unused catalyst. Moreover, color of the originally white catalyst changed to dark orange and remained like that even after additional stirring in ethanol and drying. This color change, together with the higher nitrogen content, indicates that certain surface species, imines derived from 4-nitrobenzaldehyde, remain on the catalyst and possibly cause the changes in catalytic behavior, although the effect on the activity is small. Under ‘dry’ reaction conditions, e.g. using DMSO as a solvent, these species would block the active sites, resulting in significantly poorer performance in consecutive batch experiments [9]. It is, however, assumed that hydrolysis of the imine is fast in aqueous conditions, thereby shifting the equilibrium away from the imine [10], which could explain why the effect is only slightly noticeable when using water as a solvent.
C. Continuous Reactor
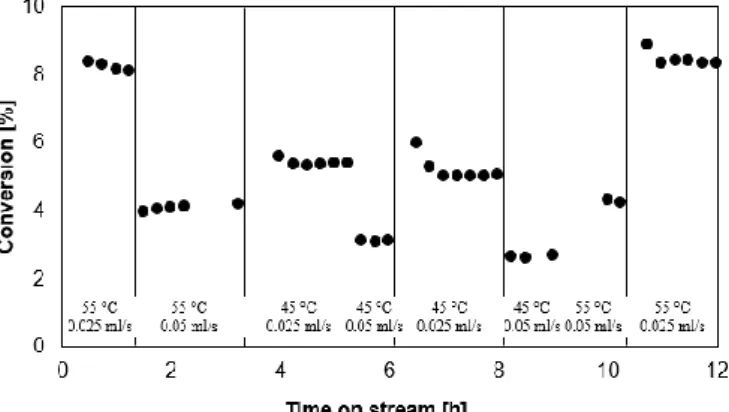
The conversion for the aldol reaction of acetone and 4-nitrobenzaldehyde in the continuous flow reactor is given as a function of time-on-stream in Figure 4. From the reactor hydrodynamics’ point of view, no steady-state operation was obtained in the first hour of reaction. From 1 hour on-stream up to 4 hours on-stream, the conversion decreased slightly (from 8.4% to 7.4%). This can be attributed to the formation of stable imines derived from 4-nitrobenzaldehyde, in line with the observations made in the batch reactor. From 4 hours on-stream onwards, the inhibitory phenomena stabilized and a stable conversion was obtained. These results indicate that a stable operating regime can be reached in the continuous flow aldol reaction with water as solvent, in contrast to the aminated mesoporous silica catalysts that were found to continuously deactivate due to silica hydrolysis and leaching caused by water [10,12].
For this continuous reactor experiment with PEGMA-EDA1, a TOF of 2.2 10-4 s-1 was calculated. This value is 5
times smaller than the TOF that was obtained for the same catalyst in the batch reactor experiment. A hypothesis was formulated that this was due to a non-optimal loading of catalyst in the reactor. In the experiment, the catalyst was deposited in the reactor tube in a dry state, then water was
added to wet the catalyst, and inert alumina beads were deposited on top afterwards. The low TOF, and the high reactor pressure (3 – 5 bar) that was measured during the experiment, indicated that the polymer resin was only able to swell to a limited degree, leading to an inefficient use of the active sites. In order to not inhibit the swelling of the polymer catalyst beads, the catalyst should be loaded in the reactor in the swollen state. A short repeat experiment was carried out with PEGMA-EDA3 in the continuous reactor. Before loading in the reactor, the resin catalyst was allowed to swell in excess deionized water for 60 minutes. During the experiment, the pressure remained below 2 bar, and a TOF of 8.3 10-4 s-1 was
obtained, which is in the same order of magnitude as the TOF that was obtained for the same catalyst in the batch reactor experiment.
Figure 4. Conversion as a function of time on stream for
PEGMA-EDA1 in the aldol reaction of acetone with 4-nitrobenzaldehyde. (T = 55 °C, P = 180 kPa, site time = 354 molsite.s.mol-1,
0.45 wt% 4-nitrobenzaldehyde, 44.6 wt% acetone, 54.5 wt% water)
A preliminary test with different reaction temperatures was carried out, of which the results are shown in Figure 5. A lower temperature results in a lower conversion, in accordance with the Arrhenius law. At the end of the experiment, the initial conditions were repeated, and the same conversion was obtained as for these conditions at the start of the experiment. These results indicate that the catalyst is stable for at least 12 hours on stream, also under varying reaction conditions.
Figure 5. Conversion as a function of time on stream for
PEGMA-EDA4 in the aldol reaction of acetone with 4-nitrobenzaldehyde. (T = varied, P = 180 kPa, site time = varied, 0.45 wt% 4-nitrobenzaldehyde, 44.6 wt% acetone, 54.5 wt% water, 0.81 g catalyst)
D. Structured Catalysts
A fixed bed tubular reactor packed with catalyst pellets, which was used as the set-up in this thesis, is one of the simplest continuous flow reactor types. Typically, the reactor tubes are relatively long with a small diameter and thin walls, enabling an efficient supply or removal of heat. However, the pressure drop occurring over such a packed bed may become unpractically high, and non-idealities in the hydrodynamic flow pattern may develop as the reactor tube becomes too small or too large. These issues can be overcome by structuring the catalyst bed, using catalyst materials of a well-defined spatial structure. The developed polymeric resin is ideally suited for shaping into a structured catalyst by means of a technique called three-dimensional (3D) printing. A review on existing 3D printing techniques for the fabrication of such structured polymer catalysts serves as a perspective on future process intensification.
IV. CONCLUSIONS
This work has shown that amine functionalized polymer resins are stable heterogeneous catalysts for the aqueous aldol reaction. Previous results were successfully reproduced, and a reliable method for the synthesis and performance evaluation of the catalyst was determined.
Poly(ethylene glycol) methacrylate (PEGMA) organic hydrogels were synthesized via free radical suspension polymerization, and functionalized by nucleophilic substitution of ethylenediamine (EDA). Scanning electron microscopy (SEM) images confirmed the formation of spherical beads during the polymerization, and elemental analysis allowed for the determination of the active site loading of the primary amine resin catalysts, which was in the range of 0.40 – 0.54 mmol/g. The catalytic activity of the PEGMA-EDA catalysts was first evaluated in a batch reactor for the aldol reaction of acetone with 4-nitrobenzaldehyde, using 50 vol% water as solvent and 50 vol% acetone as excess reagent. A turnover frequency (TOF) of 9.6 ± 1.0 10-4 s-1 was
obtained, which is comparable to the TOF of the state-of-the-art primary amine functionalized silica catalyst evaluated in hexane. Subsequently, the catalyst was filtered and recycled for a second batch experiment to assess the reusability, maintaining 92% of the initial TOF. Finally, a thorough evaluation of the catalyst stability was made by monitoring the conversion as a function of time on stream in a continuous reactor. The results indicated a stable catalyst operation for the aqueous aldol reaction during at least 12 hours on stream, under varying reaction conditions, demonstrating the material’s potential as a stable and reusable catalyst.
The developed polymeric resin allows shaping into a structured catalyst, which offers several advantages as compared to packed-bed reactors, by means of a technique called three-dimensional (3D) printing. A review on existing 3D printing techniques for the fabrication of such structured polymer catalysts serves as a perspective on future process intensification.
V. FUTURE PERSPECTIVE
Further improvements and tuning of the amine function, catalyst support and experimental conditions could lead to an optimized catalytic activity. For example, other amines can be attached to the PEGMA support material to serve as active sites, or the catalytic support can be tuned by altering the composition or degree of cross-linking of the copolymer for
an optimized absorption of reagents in the catalyst pores. Investigating the effect of experimental conditions on the catalytic activity would allow for the determination of the set of reaction conditions that optimizes the catalyst performance. The gathering of a kinetic data set could also serve as a basis for a reaction model describing the kinetics.
REFERENCES
[1] H. Suwito, A. Novi Kristanti, and N. Nyoman Tri Puspaningsih, “Chalcones: Synthesis, structure diversity and pharmacological aspects,” J. Chem. Pharm. Res., no. 6, 2014.
[2] S. Cheng, X. Wang, and S. Y. Chen, “Applications of amine-functionalized mesoporous silica in fine chemical synthesis,” in Topics in Catalysis, 2009, vol. 52, no. 6–7, pp. 681–687.
[3] J. N. Chheda and J. A. Dumesic, “An overview of dehydration, aldol-condensation and hydrogenation processes for production of liquid alkanes from biomass-derived carbohydrates,” Catal. Today, vol. 123, no. 1–4, pp. 59–70, May 2007.
[4] H. Bahrmann, H.-D. Hahn, D. Mayer, and G. D. Frey, “2-Ethylhexanol,” in Ullmann’s Encyclopedia of Industrial
Chemistry, American Cancer Society, 2013.
[5] C. Lucarelli and A. Vaccari, “Examples of heterogeneous catalytic processes for fine chemistry,” Green Chemistry, vol. 13, no. 8. Royal Society of Chemistry, pp. 1941–1949, 08-Aug-2011.
[6] A. De Vylder, J. Lauwaert, J. De Clercq, P. Van Der Voort, C. W. Jones, and J. W. Thybaut, “Aminated poly(ethylene glycol) methacrylate resins as stable heterogeneous catalysts for the aldol reaction in water,” J. Catal., vol. 381, pp. 540– 546, Jan. 2020.
[7] D. Singappuli-Arachchige et al., “Interfacial Control of Catalytic Activity in the Aldol Condensation: Combining the Effects of Hydrophobic Environments and Water,” ACS
Catal., vol. 9, no. 6, pp. 5574–5582, Jun. 2019.
[8] J. Lauwaert, E. De Canck, D. Esquivel, P. Van Der Voort, J. W. Thybaut, and G. B. Marin, “Effects of amine structure and base strength on acid–base cooperative aldol condensation,” Catal. Today, vol. 246, pp. 35–45, 2015. [9] A. De Vylder et al., “The role of water in the reusability of
aminated silica catalysts for aldol reactions,” J. Catal., vol. 361, pp. 51–61, May 2018.
[10] K. Kandel et al., “Substrate inhibition in the heterogeneous catalyzed aldol condensation: A mechanistic study of supported organocatalysts,” J. Catal., vol. 291, pp. 63–68, Jul. 2012.
[11] M. Etienne and A. Walcarius, “Analytical investigation of the chemical reactivity and stability of aminopropyl-grafted silica in aqueous medium,” Talanta, vol. 59, no. 6, pp. 1173–1188, May 2003.
Table of Contents i
Table of Contents
TABLE OF CONTENTS I
LIST OF FIGURES IV
LIST OF SCHEMES VI
LIST OF TABLES VII
LIST OF SYMBOLS VIII
CHAPTER 1 INTRODUCTION 1
1.1 GREEN CHEMISTRY 1
1.1.1 RENEWABLE FEEDSTOCKS 1 1.1.2 SAFER SOLVENTS 2 1.1.3 CATALYSIS 2 1.2 CONTINUOUS FLOW OPERATION 3 1.3 STRUCTURE OF THE THESIS 3
1.4 REFERENCES 4
CHAPTER 2 LITERATURE STUDY 6
2.1 ALDOL REACTIONS 6
2.1.1 BRØNSTED ACID CATALYZED REACTION MECHANISM 8
2.1.2 BRØNSTED BASE CATALYZED REACTION MECHANISM 9
2.1.3 LEWIS BASE CATALYZED REACTION MECHANISM 9
2.2 HETEROGENEOUS CATALYSTS FOR THE ALDOL REACTION 11
2.2.1 ZEOLITES 11
2.2.2 LAYERED DOUBLE HYDROXIDES 12
2.2.3 FUNCTIONALIZED SILICAS 12
2.2.4 FUNCTIONALIZED ORGANOSILICAS 14
2.2.5 FUNCTIONALIZED POLYMER RESINS 16
2.3 CATALYST CHARACTERZIATION 21
2.3.1 SCANNING ELECTRON MICROSCOPY 21
2.3.2 ELEMENTAL ANALYSIS 22
Table of Contents ii
2.4 EFFECTS OF REACTION CONDITIONS ON THE AMINE CATALYZED ALDOL
REACTION 24
2.4.1 TEMPERATURE AND FEED COMPOSITION 24
2.4.2 SOLVENT 25 2.5 AIM OF THE THESIS 26 2.6 REFERENCES 26 CHAPTER 3 PROCEDURES 33 3.1 CATALYST SYNTHESIS 33 3.2 CATALYST CHARACTERIZATION 35
3.2.1 SCANNING ELECTRON MICROSCOPY 35
3.2.2 ELEMENTAL ANALYSIS 35
3.2.3 SWELLING CAPACITY 35
3.3 PERFORMANCE EVALUATION OF THE CATALYST 36
3.3.1 BATCH REACTOR CONFIGURATION 36
3.3.2 CONTINUOUS FLOW REACTOR CONFIGURATION 38
3.3.3 SAMPLE ANALYSIS AND DEFINING THE CATALYST ACTIVITY 39
3.3.4 REACTION CONDITIONS 40
3.4 REFERENCES 40
CHAPTER 4 RESULTS AND DISCUSSION 42
4.1 CATALYST CHARACTERIZATION 42
4.1.1 SCANNING ELECTRON MICROSCOPY 42
4.1.2 ELEMENTAL ANALYSIS 43
4.1.3 SWELLING CAPACITY 43
4.2 PERFORMANCE EVALUATION OF THE CATALYST 44
4.2.1 BATCH REACTOR 44
4.2.2 CONTINUOUS FLOW REACTOR 46
4.3 REFERENCES 48
CHAPTER 5 A REVIEW ON 3D PRINTING TECHNOLOGY FOR STRUCTURED POLYMER CATALYSTS 50
5.1 INTRODUCTION 50
5.2 STRUCTURED CATALYSTS 51
Table of Contents iii
5.3 3D PRINTING TECHNOLOGIES 53
5.3.1 EXTRUSION-BASED PROCESSES 55
5.3.2 VAT PHOTOPOLYMERIZATION PROCESSES 58
5.3.3 POWDER BED FUSION PROCESSES 65
5.3.4 MATERIAL AND BINDER JETTING PROCESSES 67
5.3.5 SHEET LAMINATION PROCESSES 70
5.4 DISCUSSION 71
5.5 CONCLUSION 72
5.6 REFERENCES 73
CHAPTER 6 CONCLUSIONS AND PERSPECTIVES 80
List of Figures iv
List of Figures
Figure 2-1. Schematic representation of the structure of hydrotalcite materials. Bivalent and
trivalent cations (e.g. Mg2+ and Al3+) are sixfold coordinated to form octahedra that share
edges to constitute infinite layers. Small spheres drawn in the interlayer region represent the compensating anions (e.g., CO32−). Adopted from Debecker et al54.
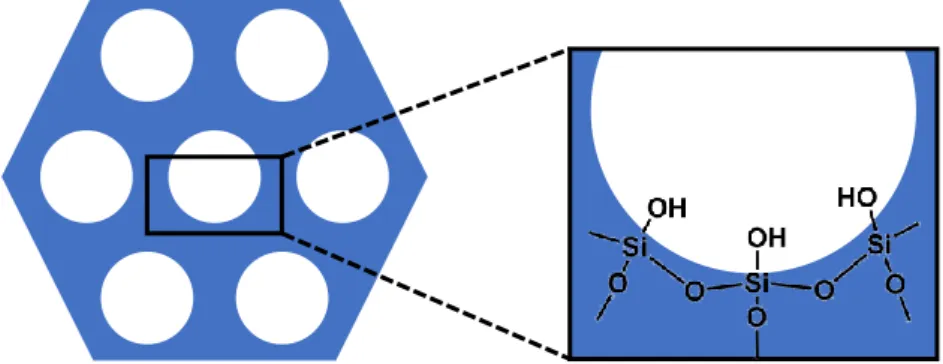
Figure 2-2. General structure of mesoporous silica material.
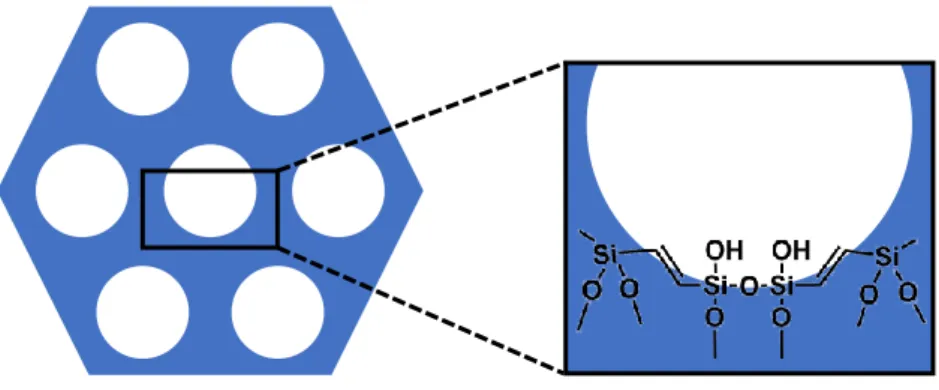
Figure 2-3. General structure of periodic mesoporous organosilica material with an ethylene
bridge.
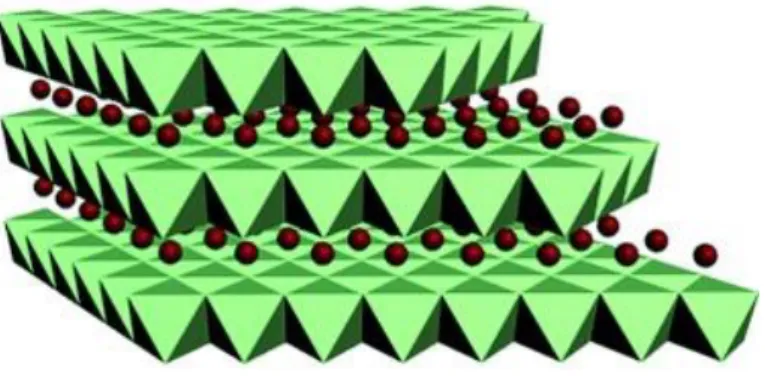
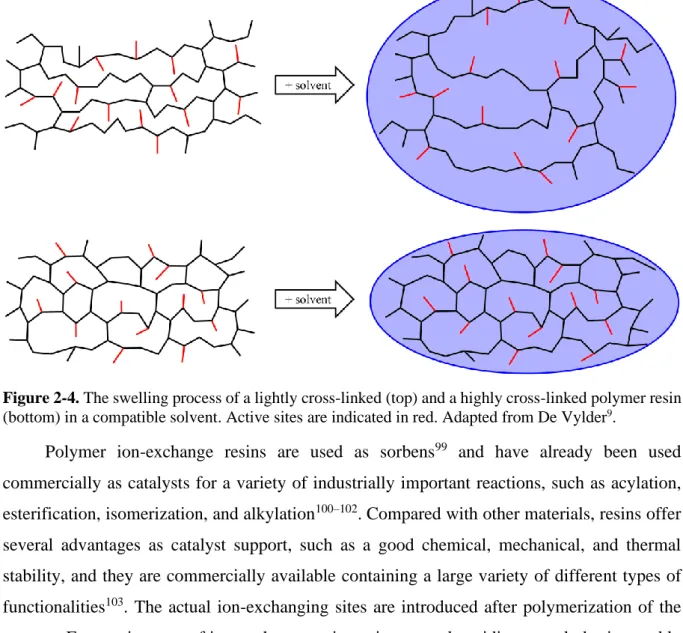
Figure 2-4. The swelling process of a lightly cross-linked (top) and a highly cross-linked
polymer resin (bottom) in a compatible solvent. Active sites are indicated in red. Adapted from De Vylder9.
Figure 2-5. Amine precursors used for functionalization of PEGMA resin, (a) methylamine
(MA), (b) ethylenediamine (EDA), (c) N,N′-dimethylethylenediamine (DED). Adopted from De Vylder et al115.
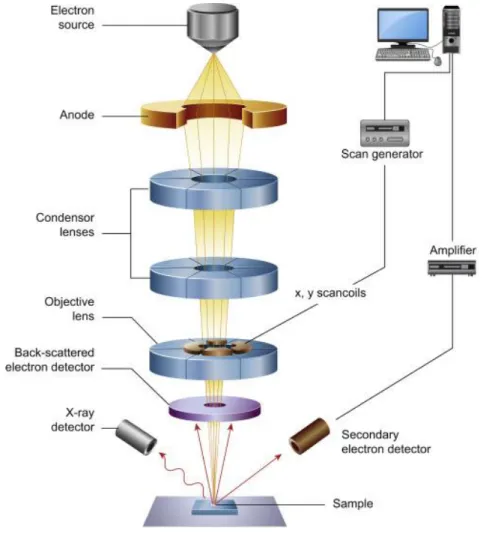
Figure 2-6. Schematic representation of a scanning electron microscope. Adopted from
Inkson122.
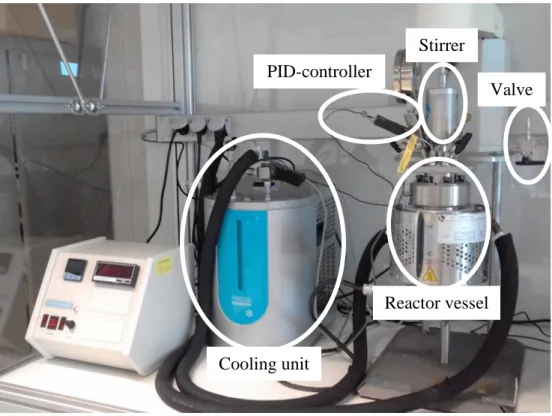
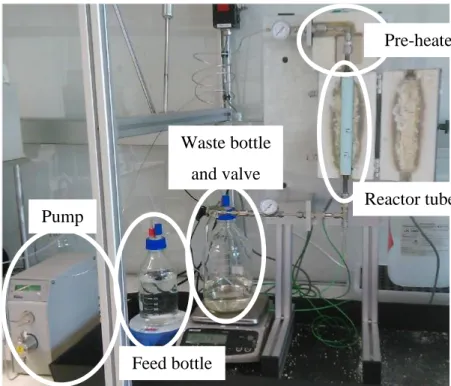
Figure 3-1. Batch-type reactor (Parr® 4560 mini, 300 mL). Figure 3-2. Plug-flow reactor (Liquid-Solid Lab-Scale (LS)²).
Figure 4-1. Turnover frequency for the PEGMA-EDA catalysts, determined in the Parr®
batch-type reactor, for the aldol condensation of acetone with 4-nitrobenzaldehyde (left axis). (T = 55 °C, 45 g acetone, 0.45 g 4-nitrobenzaldehyde, 55 g water, 0.14 g catalyst). Average particle size, determined from the SEM images (right axis).
Figure 4-2. Turnover frequency for first and second run of the PEGMA-EDA1 catalyst,
determined in the Parr® batch-type reactor, for the aldol condensation of acetone with
4-nitrobenzaldehyde. (T = 55 °C, 45 g acetone, 0.45 g 4-nitrobenzaldehyde, 55 g water, 0.14 g catalyst)
Figure 4-3. Conversion as a function of time on stream for PEGMA-EDA1 in the aldol
reaction of acetone with 4-nitrobenzaldehyde. (T = 55 °C, P = 180 kPa, site time = 354 molsite.s.mol-1, 0.45 wt% 4-nitrobenzaldehyde, 44.6 wt% acetone, 54.5 wt% water)
List of Figures v Figure 4-4. Conversion as a function of time on stream for PEGMA-EDA4 in the aldol
condensation of acetone with 4-nitrobenzaldehyde. The reaction temperature and feed flow rate are indicated for every section. (T = varied, P = 180 kPa, site time = varied, 0.45 wt% 4-nitrobenzaldehyde, 44.6 wt% acetone, 54.5 wt% water, 0.81 g catalyst)
Figure 5-1. Different reactor concepts for heterogeneous catalysis in continuous-flow
systems. (a) Random reactor. (b-d) Structured reactors. Adapted from Schwieger et al13.
Figure 5-2. Additive manufacturing process. (A) A three-dimensional model is designed
using computer-assisted design software, (B) the model is converted to a 3D printable file format, (C) the model is digitally sliced, (D) the object is printed by layering building material. Adopted from Parra-Cabrera et al31.
Figure 5-3. Schematic representation of the fused deposition modeling process. Figure 5-4. Schematic representation of the direct ink writing process.
Figure 5-5. Schematic representation of the stereolithography process. Figure 5-6. Schematic representation of the direct light processing system.
Figure 5-7. Schematic representation of the continuous liquid interface production process. Figure 5-8. Two-photon polymerization. (a) The principle of the fabrication of 3D
microstructures by two-photon polymerization; (b) Longitudinal profile of a laser beam and a photopolymerizable region (voxel) formed by two-photon absorption (TPA). Adopted from Shpichka et al103.
Figure 5-9. Schematic representation of the selective laser sintering process. Figure 5-10. Schematic representation of the material jetting process.
Figure 5-11. Schematic representation of the binder jetting process.
Figure 5-12. Schematic representation of the laminated object manufacturing process. Figure A-1. SEM images of PEGMA hydrogel beads (above) and PEGMA-EDA catalyst (below), synthesis batch 1.
Figure A-2. SEM images of PEGMA hydrogel beads (above) and PEGMA-EDA catalyst (below), synthesis batch 2.
Figure A-3. SEM images of PEGMA hydrogel beads (above) and PEGMA-EDA catalyst (below), synthesis batch 3.
List of Schemes vi
List of Schemes
Scheme 2-1. Generic example of an aldol condensation between two carbonyl compounds
with the formation of an α,β-unsaturated aldehyde or ketone.
Scheme 2-2. Industrial production of 2-ethylhexanol from the self-aldol condensation of
butanal and subsequent hydrogenation.
Scheme 2-3. Model aldol condensation of acetone with 4-nitrobenzaldehyde to the aldol
product 4-hydroxy-4-(4-nitrophenyl)butan-2-one and subsequent dehydration to the enone product 4-(4-nitrophenyl)-3-buten-2-one.
Scheme 2-4. Generic reaction mechanism for the Brønsted acid catalyzed aldol condensation.
Adapted from De Vylder9.
Scheme 2-5. Generic reaction mechanism for the Brønsted base catalyzed aldol condensation.
Adapted from De Vylder9.
Scheme 2-6. Generic reaction mechanism for the Lewis base catalyzed aldol condensation.
All reactions are reversible. Adapted from De Vylder9.
Scheme 2-7. Base-induced disproportionation of two molecules of the non-enolizable
benzaldehyde to a primary alcohol and a carboxylic acid (Cannizzaro reaction).
Scheme 2-8. Reaction mechanism for the model aldol reaction of acetone with
4-nitrobenzaldehyde, catalyzed by a primary amine. Adapted from Lauwaert et al29.
Scheme 2-9. Formation of imine (a) or iminium ion (b) on respectively a primary and a
secondary amine.
Scheme 3-1. Synthesis procedure of the PEGMA catalyst functionalized with a primary
ethylenediamine (EDA). The secondary amine that is formed in PEGMA-EDA is not catalytically active. Adapted from De Vylder et al1.
Scheme 3-2. Model aldol reaction of 4-nitrobenzaldehyde with acetone, yielding
4-hydroxy-4-(4-nitrophenyl)butan-2-one as the aldol product and 4-(4-nitrophenyl)-3-buten-2-one as the enone product.
List of Tables vii
List of Tables
Table 3-1. Range of experimental conditions in the continuous reactor experiments. Table 4-1. Active site loading for the different batches of PEGMA-EDA catalyst.
List of Symbols viii
List of Symbols
Roman symbols
TOF turnover frequency [s-1]
𝐶𝑠𝑖𝑡𝑒 active site concentration [molsite kgcat-1]
𝐹4−𝑁𝐵0 molar inlet feed of 4-nitrobenzaldehyde [mol4-NB s-1]
𝑆𝑑 Volume of dry hydrogel sample [L]
𝑆𝑠 volume of swollen hydrogel sample [L]
𝑊𝑐𝑎𝑡 catalyst mas [kgcat]
𝑊𝑑 weight of dry hydrogel sample [g]
𝑊𝑠 weight of swollen hydrogel sample [g]
𝑆 volumetric swelling ratio [L L-1]
𝑊 weight swelling ratio [g g-1]
Acronyms
ABS acrylonitrile butadiene styrene MAP methylaminopropyl
CAD computer-assisted design PEG polyethylene glycol
CLIP continuous liquid interface production
PEGDMA polyethylene glycol dimethacrylate PEGMA polyethylene glycol methacrylate
DED N,N’-dimethylethylenediamine PMO periodic mesoporous organosilica
DIPEA di-isopropylethylamine RP-HPLC reversed-phase high performance
liquid chromatograph DIW direct ink writing
DLP direct light processing SEM scanning electron microscopy
DMSO dimethylsulfoxide SLA stereolithography
EDA ethylenediamine SLS selective laser sintering
FDM fused deposition modeling TEOS tetraethyl orthosilicate
LDH layered double hydroxide TPP two-photon polymerization
LOM laminated object manufacturing TS transition state
Introduction 1
Chapter 1
Introduction
During the 1950s and 1960s, chemistry was seen as the solution to many of society’s needs, by both scientists and the general public. Some of the greatest perceived benefits came from the pharmaceutical industry with development of painkillers, antibiotics and heart drugs, but chemistry also assisted improvements in transportation, agriculture, food industry and many other industrial sectors. Unfortunately, these numerous successes were accompanied by some adverse outcomes that chemists had not foreseen. Chlorofluorocarbons, developed as safer alternatives to sulfur dioxide and ammonia as refrigerants, played an important role in the destruction of the ozon layer2. Gas leaking from a pesticide plant in Bhopal, one of the world’s worst industrial disasters, caused major environmental damage and loss of life3. And apart from these specific disasters, general side effects came to the public’s attention through eutrophication, air pollution, resource scarcity, and damage to the ecosystem1. Nowadays, the
chemical industry is often viewed as causing more harm than good. The challenge in the twenty-first century is to continue to provide benefits we have come to rely on, in an economically viable manner, but without the adverse side effects.
1.1 Green chemistry
The concept of green chemistry was introduced in the 1980s, as a response to the escalating environmental and health concerns related to the chemical industry4. Green
chemistry, defined as ‘the design of chemical products and processes to reduce or eliminate the use and generation of hazardous substances’, is described by 12 principles5, of which at least
three are related to this thesis and are described in the following paragraphs.
1.1.1 Renewable feedstocks
Nowadays, the feedstocks of the chemical industry are predominantly fossil – coal, oil and natural gas – and only a small percentage are renewables6. These fossil resources originate from carbon deposition of eons ago, and by burning them, a net increase in atmospheric CO2
Introduction 2 depletion of crude oil resources are becoming important driving forces encouraging the search for new feedstocks, as alternative to crude oil. Biomass is one of the few resources exhibiting high potential to meet the challenges of sustainable and green energy8. Biomass-derived feedstocks have captured CO2 during a limited number of years and never deplete, which results
in a closed CO2 loop.
Because of its abundant availability, lignocellulosic biomass is considered the most appropriate long-term alternative to fossil carbon9. Several different pathways to convert lignocellulose in biofuels are currently being investigated, such as gasification10, fast pyrolysis11, liquefaction12, and hydrolysis13,14. In the hydrolysis route, rigid lignocellulose structure is first broken down into lignin and (hemi)cellulose. Hydrolysis of the cellulose fraction yields smaller aqueous sugars, and further processing via dehydration results in chemical platform molecules15. These platform molecules typically contain only 3 to 6 carbon
atoms, which is too low for applications such as hydrocarbon fuels, for which a carbon content in the range of 9 to 22 atoms per molecules is required16. Carbon-carbon coupling reactions,
such as the aldol reaction, can be employed to increase the carbon number significantly. Finally, the resulting molecules are dehydrated and hydrogenated to remove unsaturated bonds, yielding the desired liquid hydrocarbon fuels17.
1.1.2 Safer solvents
With solvents being of a very broad applicability and often used in extremely high volume, they are a determining factor in the toxicity and environmental impact of chemical processes18. If possible, efforts should be made to avoid using them, and if needed it should be tried to use harmless substances. Alternative solvents suitable for green chemistry are those that have low toxicity, are easy to recycle, are inert, and do not contaminate the products19.
Water appears as a very attractive alternative solvent from both an environmental and an economical point of view, since it is abundantly available, inherently safe, toxic, and non-flammable20–22. During the last decades, it has become clear that the unique structure and
physicochemical properties of water lead to complex new interactions induced by polarity, hydrogen bonding or hydrophobic effects that can positively influence the reaction rate and product yield in an organic reaction23–25.
1.1.3 Catalysis
Currently, many organic reactions in the fine chemical and the pharmaceutical industry make use of homogeneous catalysts, such as the bases NaOH and KOH and the mineral acid
Introduction 3 H2SO426. Despite their advantages, such as high activities and selectivities, these catalysts
exhibit, unfortunately, several important disadvantages, such as a short lifetime, environmental risks, equipment corrosion, and an energy intensive separation from the reaction products27.
Heterogeneous catalysis addresses the goals of green chemistry by providing the ease of separation of product and catalyst, thereby eliminating the need for separation through distillation or extraction28. Hence, the pursuit of a reusable, heterogeneous catalyst could lead to a more sustainable alternative production. In addition, it would be of interest to design these catalysts to exhibit a performance as selective as possible to reduce the amount of by-products formed and maximize the desired product yield.
1.2 Continuous flow operation
Continuous flow technologies have captured attention in modern chemistry as they offer a massive number of advantages over conventional batch procedures, such as faster heat and mass transfer, the efficient mixing of substrates, and shorter reaction times29,30. Furthermore, the well-regulated continuous flow reactor concept enables reactions to be performed with an unprecedented level of control. The most important reaction parameters (such as flow rate, pressure and temperature) can be adjusted and monitored quickly and precisely31,32.
Currently, batch operation is the main industrial mode of operation for aldol reactions33. However, this reaction could benefit from the advantages offered by continuous operation. By an improved control over the reaction parameters, continuous flow operation can result in safer, more sustainable and more performant processes, especially in combination with safer solvents and heterogeneous catalysis. Moreover, continuous operation also has advantages at laboratory scale, e.g. catalyst deactivation can be readily investigated by making use of a continuous flow set-up.
1.3 Structure of the thesis
In this thesis, the discussed principles of green chemistry will be applied in the development and optimization of a heterogeneous catalyst for the aldol reaction. Catalytic activity and stability will be tested in a continuous reactor set-up, and water will be used as the main solvent.
Chapter 2 describes the state-of-the-art of the aldol reaction, including its applications
Introduction 4 investigated in the past for this reaction are discussed, together with their performance. Especially functionalized polymer resins are emphasized. The effects of certain reaction conditions on the amine catalyzed aldol reaction are mentioned briefly. Finally, a more detailed description of the aim of the thesis is given.
Chapter 3 outlines the methodology employed in the synthesis and characterization of
the catalyst as well as a thorough explanation on how catalyst performance evaluation was carried out in batch and continuous operation.
Chapter 4 reports the experimental results from the characterization, as well as from the
performance evaluation of the catalyst in batch and continuous operation.
Chapter 5 provides a review on the state-of-the-art of 3D printing techniques for the
fabrication of structured polymer catalysts.
Chapter 6 gives a final and summarizing conclusion and discusses future perspectives.
1.4 References
1. Lancaster, M. Green Chemistry: An Introductory Text. (Royal Society of Chemistry, 2007). 2. Haas, P. M. Banning chlorofluorocarbons: Epistemic community efforts to protect stratospheric
ozone. Int. Organ. 46, 187–224 (1992).
3. Broughton, E. The Bhopal disaster and its aftermath: A review. Environmental Health: A Global
Access Science Source 4, 6 (2005).
4. Linthorst, J. A. An overview: Origins and development of green chemistry. Found. Chem. 12, 55–68 (2010).
5. Anastas, P. T. & Warner, J. C. Green Chemistry: Theory and Practice. (Oxford University Press, 2000).
6. Metzger, J. O. Fats and oils as renewable feedstock for chemistry. Eur. J. Lipid Sci. Technol.
111, 865–876 (2009).
7. Zou, C., Zhao, Q., Zhang, G. & Xiong, B. Energy revolution: From a fossil energy era to a new energy era. Nat. Gas Ind. B 3, 1–11 (2016).
8. Melero, J. A., Iglesias, J. & Garcia, A. Biomass as renewable feedstock in standard refinery units. Feasibility, opportunities and challenges. Energy and Environmental Science 5, 7393–7420 (2012).
9. Chheda, J. N., Huber, G. W. & Dumesic, J. A. Liquid-Phase Catalytic Processing of Biomass-Derived Oxygenated Hydrocarbons to Fuels and Chemicals. Angew. Chemie Int. Ed. 46, 7164– 7183 (2007).
10. Alauddin, Z. A. B. Z., Lahijani, P., Mohammadi, M. & Mohamed, A. R. Gasification of lignocellulosic biomass in fluidized beds for renewable energy development: A review.
Renewable and Sustainable Energy Reviews 14, 2852–2862 (2010).
11. Liu, C., Wang, H., Karim, A. M., Sun, J. & Wang, Y. Catalytic fast pyrolysis of lignocellulosic biomass. Chemical Society Reviews 43, 7594–7623 (2014).
12. Jørgensen, H., Vibe-Pedersen, J., Larsen, J. & Felby, C. Liquefaction of lignocellulose at high-solids concentrations. Biotechnol. Bioeng. 96, 862–870 (2007).
Introduction 5 13. Sun, Y. & Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review.
Bioresour. Technol. 83, 1–11 (2002).
14. Kunkes, E. L. et al. Catalytic conversion of biomass to monofunctional hydrocarbons and targeted liquid-fuel classes. Science (80-. ). 322, 417–421 (2008).
15. Bond, J. Q. et al. Production of renewable jet fuel range alkanes and commodity chemicals from integrated catalytic processing of biomass. in Energy and Environmental Science 7, 1500–1523 (Royal Society of Chemistry, 2014).
16. Simonetti, D. A. & Dumesic, J. Catalytic production of liquid fuels from biomass-derived oxygenated hydrocarbons: Catalytic coupling at multiple length scales. Catalysis Reviews -
Science and Engineering 51, 441–484 (2009).
17. Chheda, J. N. & Dumesic, J. A. An overview of dehydration, aldol-condensation and hydrogenation processes for production of liquid alkanes from biomass-derived carbohydrates.
Catal. Today 123, 59–70 (2007).
18. Constable, D. J. C., Jimenez-Gonzalez, C. & Henderson, R. K. Perspective on solvent use in the pharmaceutical industry. Organic Process Research and Development 11, 133–137 (2007). 19. Kerton, F. M. & Marriott, R. Alternative Solvents for Green Chemistry. (Royal Society of
Chemistry, 2013).
20. Li, C. J. & Chen, L. Organic chemistry in water. Chemical Society Reviews 35, 68–82 (2006). 21. Lindström, U. M. Stereoselective organic reactions in water. Chem. Rev. 102, 2751–2772 (2002). 22. Simon, M. O. & Li, C. J. Green chemistry oriented organic synthesis in water. Chemical Society
Reviews 41, 1415–1427 (2012).
23. Klijn, J. E. & Engberts, J. B. F. N. Organic chemistry: Fast reactions ‘on water’. Nature 435, 746–747 (2005).
24. Narayan, S. et al. “On Water”: Unique Reactivity of Organic Compounds in Aqueous Suspension. Angew. Chemie Int. Ed. 44, 3275–3279 (2005).
25. Hayashi, Y. In Water or in the Presence of Water? Angew. Chemie Int. Ed. 45, 8103–8104 (2006). 26. Sheldon, R. A., Arends, I. & Hanefeld, U. Green Chemistry and Catalysis. (Wiley, 2007). 27. Sheldon, R. A. E factors, green chemistry and catalysis: An odyssey. Chemical Communications
0, 3352–3365 (2008).
28. Anastas, P. T., Kirchhoff, M. M. & Williamson, T. C. Catalysis as a foundational pillar of green chemistry. Appl. Catal. A Gen. 221, 3–13 (2001).
29. Razzaq, T. & Kappe, C. O. Continuous flow organic synthesis under high-temperature/pressure :Conditions. Chemistry - An Asian Journal 5, 1274–1289 (2010).
30. Charlotte Wiles & Paul Watts. Continuous flow reactors: a perspective. Green Chem. 14, 38–54 (2012).
31. DeMello, A. J. Control and detection of chemical reactions in microfluidic systems. Nature 442, 394–402 (2006).
32. Yoshida, J., Nagaki, A. & Yamada, T. Flash Chemistry: Fast Chemical Synthesis by Using Microreactors. Chem. - A Eur. J. 14, 7450–7459 (2008).
33. Ötvös, S. B., Mándity, I. M. & Fülöp, F. Asymmetric aldol reaction in a continuous flow reactor catalyzed by a highly reusable heterogeneous peptide. J. Catal. 295, 179–185 (2012).
Literature Study 6
Chapter 2
Literature Study
This chapter provides a background on the subject of this thesis. First, aldol reactions are introduced, and the various reaction mechanisms are elaborated on. The different heterogeneous catalysts that can be used to catalyze the aldol reaction are described, and characterization techniques are discussed. The effects of certain reaction conditions on the amine catalyzed aldol reaction are mentioned briefly. Finally, the aim of this thesis is summarized.
2.1 Aldol reactions
The aldol reaction is considered one of the most powerful synthetic tools for carbon-carbon bond-forming reactions and plays an important role in catalytic organic synthesis1–3. By yielding new carbon-carbon bonds, larger and more complex molecules are constructed. Applications can be found in the pharmacological industry4, fine chemicals synthesis5, and in the production of biomass-derived hydrocarbon fuels6,7.
The aldol reaction takes place between two carbonyl compounds, i.e. ketones or aldehydes, and generates a new β-hydroxy carbonyl compound, i.e. either a β-hydroxyaldehyde (aldol) or a β-hydroxyketone (ketol). This product will be referred to as the ‘aldol product’. Dehydration of the aldol product results in either an α,β-unsaturated aldehyde or ketone, and this product will be referred to as the ‘enone product’8. The combination of an aldol reaction and subsequent dehydration is defined an ‘aldol condensation’. If the two reacting species are identical, the overall reaction is denoted a ‘self-condensation’, while the reaction of two different species is called a ‘cross-condensation’9. For the reaction to take place, it is essential
that one of the carbonyl reactants contains at least one α-hydrogen, as will be shown later. In presence of a catalyst, this carbonyl group, which is essentially electrophilic, can be converted into a stabilized carbon nucleophile and attack the other carbonyl reactant. The nucleophilic group can be either an enol, enolate or enamine, depending on the catalyst employed9. A generic example of an aldol condensation is given in Scheme 2-1.
Literature Study 7
Scheme 2-1. Generic example of an aldol condensation between two carbonyl compounds with the
formation of an α,β-unsaturated aldehyde or ketone.
The aldol reaction is employed for the construction of highly complex and multi-functional molecules. It is of industrial relevance in the large-scale production of commodity chemicals, such as the building block pentaerythritol10, and in the fine chemical and pharmacological industry, e.g. in the synthesis of the heart disease drug Lipitor11. An important
industrial example is the production of 2-ethylhexanol, which is used as a starting material in the production of plasticizers, coatings and adhesives, lube and oil additives, solvents, and fragrances. It is manufactured industrially by the self-aldol condensation of butanal, followed by hydrogenation of the resulting unsaturated aldehyde, as shown in Scheme 2-2. This process, used by Shell and Exxon, is called the ‘Aldox process’ and is catalyzed by a strong homogeneous base catalyst12. Approximately 3 300 000 tonnes of 2-ethylhexanol are produced
annually worldwide13.
Scheme 2-2. Industrial production of 2-ethylhexanol from the self-aldol condensation of butanal and
subsequent hydrogenation.
In academic research, typically a model aldol condensation is chosen to evaluate the performance of synthesized catalysts14–17. The cross-condensation of 4-nitrobenzaldehyde with acetone, depicted in Scheme 2-3, is frequently used for this purpose for several reasons. First, the self-condensation of 4-nitrobenzaldehyde is avoided due to the absence of an α-hydrogen on this reactant. For this reason, and because acetone is a symmetrical ketone, the product spectrum will remain limited. Quantitative product analysis and mass balance verification are therefore straightforward. Second, noticeable conversions can be reached at mild reaction conditions because of the electron-withdrawing nitro group on 4-nitrobenzaldehyde. This makes it possible to carry out the reaction at low temperatures, below the boiling point of acetone, avoiding the use of pressurized equipment9.
Literature Study 8
Scheme 2-3. Model aldol condensation of acetone with 4-nitrobenzaldehyde to the aldol product
4-hydroxy-4-(4-nitrophenyl)butan-2-one and subsequent dehydration to the enone product 4-(4-nitrophenyl)-3-buten-2-one.
As mentioned before, a catalyst is required to convert the electrophilic carbonyl group into a nucleophile before the aldol reaction can proceed. The catalyst can be an acid or a base, both either of the Brønsted18 or Lewis type19. In what follows, the Brønsted acid, Brønsted base and Lewis base reaction mechanisms are discussed.
2.1.1 Brønsted acid catalyzed reaction mechanism
The reaction mechanism for an aldol condensation catalyzed by a Brønsted acid is presented in Scheme 2-4. First, the acid catalyzed reversible shift of a proton from an α-carbon to the oxygen atom on the carbonyl group takes place (Scheme 2-4, 1), which leads to the formation of an electron rich enol. This chemical equilibrium reaction is known as the keto-enol tautomerism. The second step in the mechanism is the protonation of a second carbonyl compound by the acid (Scheme 2-4, 2), making the carbonyl reactant more susceptible for a nucleophilic attack by the enol. This nucleophilic attack results in the formation of a new carbon-carbon bond (Scheme 2-4, 3), subsequent deprotonation leads to the aldol product (Scheme 2-4, 4). Next, the acid protonates the hydroxyl group of the aldol (Scheme 2-4, 5), converting it into a better leaving group. Deprotonation then yields the enone product (Scheme 2-4, 6)20.
Scheme 2-4. Generic reaction mechanism for the Brønsted acid catalyzed aldol condensation. Adapted
Literature Study 9
2.1.2 Brønsted base catalyzed reaction mechanism
The reaction mechanism for an aldol condensation catalyzed by a Brønsted base is presented in Scheme 2-5. The first step in this mechanism is the abstraction of an α-hydrogen from the aldehyde or ketone (Scheme 2-5, 1), to form a resonance-stabilized enolate-anion. This nucleophilic ion will attack the carbonyl group of a second aldehyde or ketone (Scheme 2-5, 2), which results in the formation of a new carbon-carbon bond. Next, the base catalyst is regenerated by protonation of the alkoxide ion, which leads to the formation of the aldol product (Scheme 2-5, 3). The base catalyst then abstracts a proton from the aldol product (Scheme 2-5, 4) to form an enolate ion. Finally, the hydroxide ion is eliminated from this anion (Scheme 2-5, 5), which restores the catalytically active species and yields the enone product20.
Scheme 2-5. Generic reaction mechanism for the Brønsted base catalyzed aldol condensation. Adapted
from De Vylder9.
2.1.3 Lewis base catalyzed reaction mechanism
Amine-catalyzed aldol reactions were discovered in the 1970s21–24, but it was only recently that such reactions were generalized by List et al25. They demonstrated that the amino acid L-proline can act as a catalyst for the asymmetric aldol reaction of acetone with a wide variety of aldehydes. The proposed reaction mechanism for the L-proline catalyzed aldol condensation between acetone and 4-nitrobenzalehyde is presented in Scheme 2-6.
The first step in this mechanism is the nucleophilic addition of acetone to the secondary amine in L-proline (Scheme 2-6, 1), with formation of a carbinolamine intermediate. This is possible due to the formation of a hydrogen bond between acetone and the carboxylic acid group. Next, by dehydration of the carbinolamine, an enamine is formed (Scheme 2-6, 2), an
Literature Study 10 electron-rich intermediate that plays the same role as the enol or enolate in the Brønsted acid or Brønsted base catalyzed aldol reaction, respectively. An equilibrium exists between this enamine and its corresponding iminium ion, which is electrophilic in nature and not able to react with a carbonyl group. Subsequently, the enamine intermediate reacts with the carbonyl group of 4-nitrobenzaldehyde (Scheme 2-6, 3), which results in the formation of an iminium ion. In this step, enantioselectivity is obtained by the carboxylic acid group that promotes the re-facial attack. A carbinolamine is formed when hydration of the iminium ion occurs (Scheme 2-6, 4), and subsequently the aldol product detaches, thereby liberating the amine site (Scheme 2-6, 5). In a next step, the catalyst supported enolization of the aldol takes place (Scheme 2-6, 6). The resulting enol is less stable than the aldol product and can dehydrate spontaneously to the enone product (Scheme 2-6, 7)25.
Scheme 2-6. Generic reaction mechanism for the Lewis base catalyzed aldol condensation. All reactions
are reversible. Adapted from De Vylder9.
The carboxylic acid group in L-proline is key to establishing a high enantioselectivity, but although it increases the reaction rate by assisting in proton transfers, it is not required for
Literature Study 11 the reaction to take place26. Therefore, the carboxylic acid group can be seen as a promoting group that acts cooperatively with the amine.
It has been demonstrated that both primary and secondary amines catalyze the aldol reaction through an enamine intermediate27. On a primary amine, an equilibrium exists between the reactive enamine intermediate and an imine. Similar to the iminium ion on a secondary amine, an imine is electrophilic in nature and is not able to directly react with a carbonyl group, thus inhibiting the aldol reaction when its formation is stabilized.
2.2 Heterogeneous catalysts for the aldol reaction
Currently, aqueous sodium hydroxide and other strong bases are typically employed as the standard catalyst for the industrial aldol reaction13. As a replacement for these homogeneous catalysts, several types of heterogeneous catalysts have already been investigated, with acid as well as base and amine active sites. Related advantages are a reduced energy cost for separating the catalyst from the product stream, a longer catalyst lifetime, and reduced equipment corrosion28,29. An overview of the different types of heterogeneous catalysts is given below.
2.2.1 Zeolites
Zeolites are a family of microporous crystalline aluminosilicates, composed of AlO4 and
SiO4 tetrahedra, with oxygen atoms connecting the neighbouring tetrahedra. These materials
present a rich ion-exchange chemistry and are able to catalyze a wide variety of reactions30. Zeolites are widely applied on an industrial scale for numerous acid-catalyzed reactions in the petrochemical industry31,32, but have not yet found widespread application in the fine chemical and pharmacological industry33. This might be due to the fact that the typical organic molecules that are synthesized are too bulky for the microporous zeolite34.
Zeolites have already been investigated as heterogeneous catalysts for both the liquid- and vapor-phase aldol reaction35–39. Various structural morphologies have been studied, such as MFI35 and BEA37. However, it was reported that catalytic activity decreased rapidly due to the formation of large condensation products inside the zeolite pores, which caused pore-blocking and subsequent deactivation. Enlarging the pores of the zeolites slightly reduced the catalyst’s susceptibility to coking, but also resulted in a decrease in acid strength and therefore catalytic activity36,37. Thus, it can be concluded that zeolites appear not to be ideal as stable heterogeneous catalysts for the aldol reaction.
Literature Study 12
2.2.2 Layered double hydroxides
Layered double hydroxides (LDHs), or anionic clays, are a wide family of natural and synthetic layered materials of which hydrotalcite, with the general formula Mg6Al2(OH)16CO3.4H2O, is the most well-known example (Figure 2-1). Essentially, the
structure can be described as a cadmium-iodide type layered hydroxide (e.g. Mg(OH)2, brucite)
where a partial Mg2+/Al+3 substitution has taken place. The electric charge is balanced by the presence of anions (such as CO3-2) in the interlayer space, where they co-exist with water
molecules40. Because of their good anion-exchange capacity and their solid base properties,
hydrotalcite materials are applied as adsorbants41, sensors42, and as heterogeneous catalysts in a wide variety of reactions, such as transesterification in the production of biodiesel43,44, steam reforming of methanol45,46, and the production of syngas47. The anionic clays can be used as catalysts either in the form of mixed oxides Mg(Al)O with Lewis basicity obtained by calcination of the precursor, or in the form of reconstructed hydrotalcite with OH- counter ions as Brønsted basicity in its rehydrated form48.
Figure 2-1. Schematic representation of the structure of hydrotalcite materials. Bivalent and trivalent
cations (e.g. Mg2+ and Al3+) are sixfold coordinated to form octahedra that share edges to constitute
infinite layers. Small spheres drawn in the interlayer region represent the compensating anions (e.g., CO32−). Adopted from Debecker et al48.
Catalysts derived from LDHs have already been frequently investigated for the liquid-phase aldol reaction49–53. These materials, however, typically suffer from catalyst deactivation by strong adsorption of reagents and products. In addition, ambient CO2 adsorbs on the basic
sites during exposure to atmospheric conditions54. It is possible to regenerate the basic character
of the catalyst through calcination, but this is never complete55.
2.2.3 Functionalized silicas
Silica comprises a large class of materials with the general formula SiO2 or SiO2.xH2O.