Fusarium in Moroccan
wheat and possible
antagonistic relations.
Sarah Vansweevelt
01510577
Promotor(en): Prof. dr. ir. Geert Haesaert, Prof. dr.
Tutor: Dr. Michiel Vandecasteele
Masterproef voorgelegd voor het behalen van de graad inMaster in de biowetenschappen: Landbouw, tropische plantaardige productie.
Fusarium in Moroccan
wheat and possible
antagonistic relations.
Sarah Vansweevelt
01510577
Promotor(en): Prof. dr. ir. Geert Haesaert, Prof. dr.
Tutor: Dr. Michiel Vandecasteele
Masterproef voorgelegd voor het behalen van de graad inMaster in de biowetenschappen: Landbouw, tropische plantaardige productie.
i
Copyright
The author and the promotor give permission to use this thesis for consultation and to copy parts of it for personal use. Every other use is subject to the copyright laws, more specifically the source must be extensively specified when using results from this thesis.
De auteur en de promotor geven de toelating deze masterproef voor consultatie beschikbaar te stellen en delen van de masterproef te kopiëren voor persoonlijk gebruik. Elk ander gebruik valt onder de beperkingen van het auteursrecht, in het bijzonder met betrekking tot de verplichting de bron uitdrukkelijk te vermelden bij het aanhalen van resultaten uit de masterproef.
ii
Foreword
It is no secret that writing a master thesis is a long and challenging task. It would not have been possible for me to do this entirely on my own, so therefore I would like to thank some people. First of all, I would like to thank my promoter, prof. dr. ir. Geert Haesaert for guiding me through this whole process. Not only by reading my work throughout the year, but also by giving constant feedback and useful sources of information. I would also like to show my gratitude towards my supervisor dr. Michiel Vandecasteele for guiding and overseeing the work in the lab. Also for reading my paper and giving me feedback and even finishing my work during the Covid-19 crisis. Also the other lab-staff should not be forgotten for always helping me find the right materials and answering all my questions. The knowledge and skills I gained working on this paper, are all thanks to them.
I would also like to thank my supervisors in Morocco, prof. dr. Hassan Hajjaj and prof. dr. Hamid Mazouz, for taking the time to teach me their skills and knowledge about the subject and making the internship very educational. I would also like to thank my Moroccan colleagues for warmly welcoming me to Morocco, teaching me about the Moroccan customs and making my stay so pleasant. The internship was an experience of a lifetime, all thanks to them.
Last but not least, I would like to thank my sister, my parents and my boyfriend for supporting me this whole year with their patience, their input and a sympathetic ear when I needed one. This support was vital to successfully finish this paper.
iii
Preamble
The original goal of this study was to isolate and identificate Fusarium species present in the grain samples from Morocco. These isolates would further have been used to test the antagonistic effects of some non-pathogenic strains of Fusarium and other biocontrol agents. The identification of the isolates was almost done before the Covid-19 crisis and was finished by dr. Michiel Vandecasteele during the quarantine. The testing for biocontrol agents could not be done and was replaced by a large literature part and a theoretical approach of the experimental set up. Also some assumptions about the results are discussed and an extensive literature study was done on previous research and results.
'Deze preambule werd in overleg tussen de student en de promotor opgesteld en door beiden goedgekeurd.’
iv
Abstract
This study focuses on the isolation and identification of the species present in FHB-infected grains from the region of El Hajeb in Meknès, Morocco. Grains from the field, from storage and processed grains from the market were studied by PCR followed by gel electrophoresis. Five main species: F. avenaceum, F. culmorum, F. graminearum, M. nivale and F. poae were analysed and their relative abundances were examined. This showed that F. culmorum was the most common species, probably due to favourable environmental factors and all other species were also commonly found in the samples. Due to infrastructural and practical issues, mycotoxin contaminations could not be assessed, however, literature studies indicate that DON, ZEN and NIV are probably the most common mycotoxins in all the samples and tests should be performed to know if they exceed the limits set by the government. Some biocontrol agents and alternative methods of controlling FHB were given, including the use of non-pathogenic strains of Fusarium, rotation systems and creating storage environments that limit further Fusarium infection and mycotoxin production.
v
Abstract
Deze paper focust zich op de isolatie en identificatie van de species aanwezig in de FHB-geïnfecteerde granen uit de regio El Hajeb in Meknès, Marokko. Zowel granen van het veld, vanuit opslagruimtes en verwerkte producten van op de lokale markt werden onderzocht door middel van een PCR gevolgd door een gel elektroforese. Vijf belangrijke species werden onderzocht, namelijk F. avenaceum, F. culmorum, F. graminearum, M. nivale en F. poae en hun relatieve aanwezigheid werd bepaald. Dit toonde dat F. culmorum het meest voorkomende species was, waarschijnlijk door gunstige omgevingsfactoren. De vier andere species kwamen ook regelmatig voor. De mycotoxines konden niet bepaald worden door praktische en infrastructurele problemen. De literatuur wijst DON, ZEN en NIV aan als de meest voorkomende mycotoxines en er zou getest moeten worden of deze mycotoxines enige limieten, aangebracht door de overheid, overschrijden. Er worden wat biocontrole mechanismen en alternatieve manieren van FHB-vermindering voorgesteld, onder andere het gebruik van niet-pathogene Fusarium stammen, rotatiesystemen en het opstellen van opslagruimtes met de juiste klimatologische parameters om verdere Fusarium contaminatie te verminderen.
1
Table of content
Copyright i Foreword ii Preamble iii Abstract iv Abstract v Table of content 1 List of abbreviations 4 List of figures 5 List of tables 7 Introduction 8 Literature study 10 1. Fusarium ... 10 1.1. What is Fusarium? ... 101.2. Fusarium head blight... 12
1.3. Mycotoxins ... 15
1.3.1. Production 15 1.3.2. Plant infection 18 1.3.3. Health risk 20 1.4. Control methods of Fusarium diseases ... 21
2. Morocco ... 23 2.1. Wheat production ... 23 2.1.1. Cultivation methods 24 2.1.2. Fertilizers 25 2.2. Challenges ... 26 2.2.1. Water scarcity 26 2.2.2. Pests, diseases and weeds 27 2.3. Fusarium and mycotoxins... 28
2
2.3.1. Fusarium contamination 28
2.3.2. Mycotoxin contamination 29
2.3.3. Situation in Morocco 29
2.4. Contamination of processed wheat products ... 31
2.4.1. Moroccan diet 31 2.4.2. Mycotoxin contamination 32 3. Biocontrol and antagonistic interactions ... 34
3.1. General info ... 34
3.2. Previous research... 36
3.2.1. Bacteria 36 3.2.2. Fungi 38 3.3. Methods of testing antagonistic organisms ... 40
3.3.1. In vitro testing 41 3.3.2. Wheat kernel assay 42 3.3.3. Greenhouse and field trials 42 Materials and methods 44 1. Sample collection ... 44
2. Isolation and purification ... 45
3. Identification ... 46
4. Additional research ... 47
4.1. Mycotoxin contamination ... 47
4.2. Biocontrol of Fusarium ... 48
Results 49 2. Total amount of fungi found in the samples ... 50
3. Contamination of field samples ... 52
3.1. Fusarium contamination of durum wheat ... 52
3.2. Fusarium contamination of bread wheat ... 53
3.3. Fusarium contamination of barley ... 53
3.4. Overall contamination of field samples ... 54
4. Fusarium contamination in storage samples ... 54
5. Fusarium contamination in processed samples ... 55
3
5.2. Fusarium contamination in couscous and flour ... 56
5.3. Fusarium contamination of all processed samples ... 57
Discussion 58 1. Field samples ... 58
1.1. Total amount of fungi isolated ... 58
1.2. Influencing factors on Fusarium species composition... 59
1.2.1. Climatic factors 59 1.2.2. Cultivation method 62 1.3. Fusarium species composition ... 63
1.4. Mycotoxins ... 66
2. Storage samples ... 67
2.1. Total amount of fungi found ... 67
2.2. Fusarium species composition ... 68
2.3. Mycotoxins ... 69
3. Processed samples ... 71
3.1. total amount of fungi found ... 71
3.2. Fusarium species composition ... 72
3.3. Mycotoxins ... 74
4. How to control FHB in Meknès region? ... 75
Conclusion 77
4
List of abbreviations
ADON Acetyl-deoxynivalenol BCA Biocontrol agents BEA Beauvericin
CIPCARF Interdepartmental committee for food control and the repression of frauds DNA Deoxyribonucleic acid
DON Deoxynivalenol
EFSA European Food Safety Authority ELISA Enzyme-linked immunosorbent assay ENs Enniatins
EU European union
FAO Food and agriculture organisation of the United Nations FHB Fusarium head blight
FUS Fusaproliferin
GDP Gross domestic product
HPLC High-performance liquid chromatography ISR Induced systemic resistance
ITC Isothiocyanates
JECFA Joint expert committee on food additives MON Moniliformin
MS/MS Tandem mass spectrometry
NA Nutrient agar
NIV Nivalenol
PCR Polymerase chain reaction PDA Potato dextrose agar PDB Potato dextrose broth RNA Ribonucleic acid
SEM Scanning electron microscope TEM Transmission electron microscope ZEN Zearalenone
5
List of figures
Figure 1: Life cycle of Fusarium (Ma et al., 2013)... 10
Figure 2: Symptoms of FHB in wheat florets, healthy (left) to severe (right) (Haesaert, 2019) ... 14
Figure 3: Symptoms of FHB in wheat kernels, healthy (left) to severe (right) (Freisen, 2015) 14 Figure 4: Chemical structures of DON (left) and DON-3G (right) (Bryla et al. 2018) ... 19
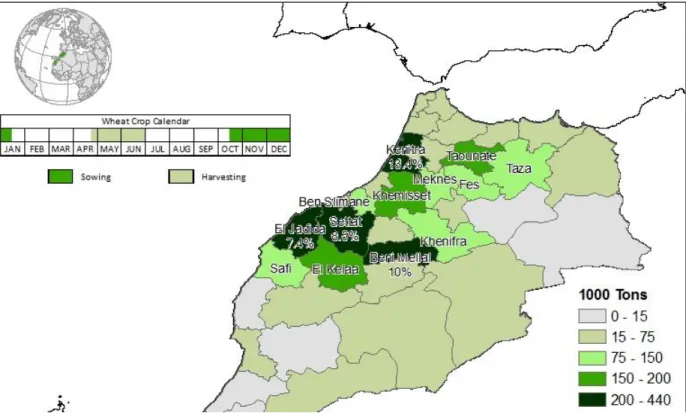
Figure 5: Moroccan wheat production (Purcell, 2011) ... 23
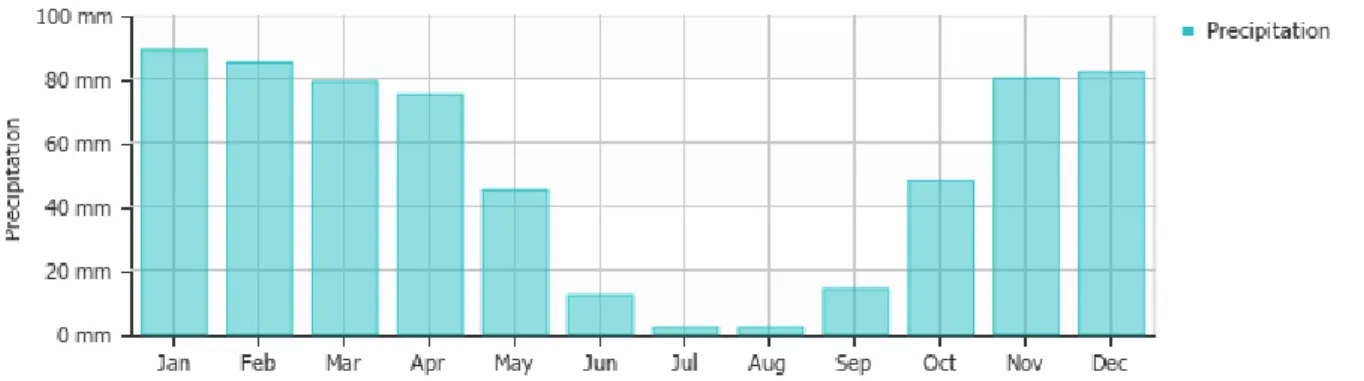
Figure 6: Precipitation (rain/snow) in Meknès, Morocco (Weather & Climate, 2019) ... 26
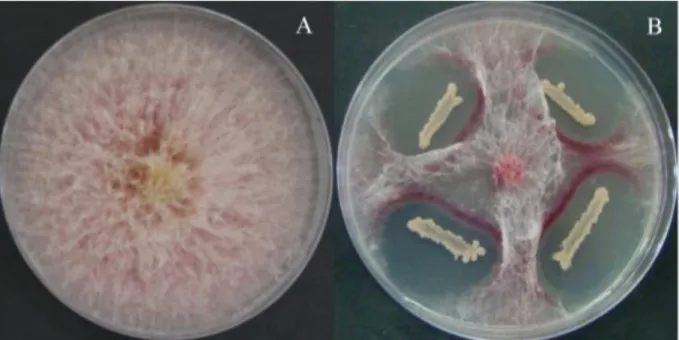
Figure 7: F. graminearum mycelium growth without Bacillus (A) and with Bacillus (B) (Zhao et al., 2014) ... 37
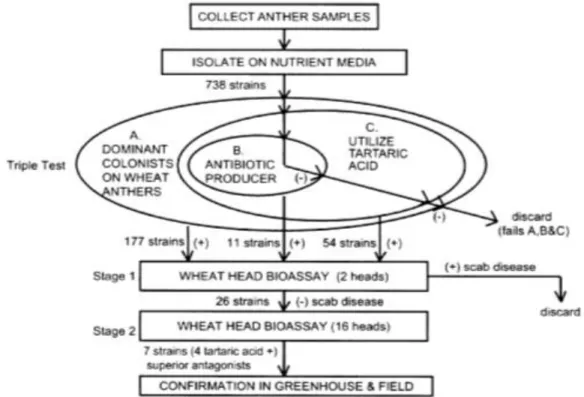
Figure 8: Selection strategy utilized to obtain antagonist organisms against FHB-pathogens on wheat (Schisler et al., 2002)... 40
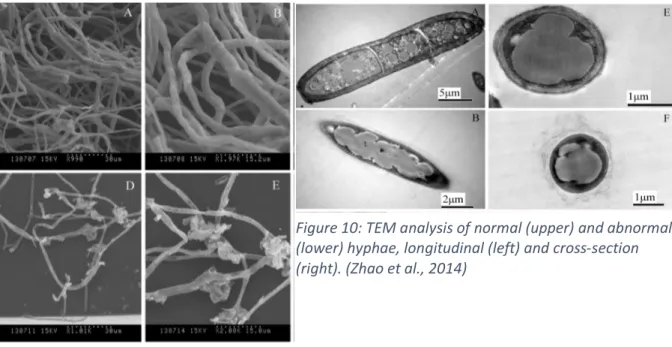
Figure 9: SEM analysis of hyphae of F. graminearum. normal (upper) and abnormal (lower). (Zhao et al., 2014) ... 42
Figure 10: TEM analysis of normal (upper) and abnormal (lower) hyphae, longitudinal (left) and cross-section (right). (Zhao et al., 2014) ... 42
Figure 11: Region of sampling. A: El Hajeb (dark red) in region Meknès-Tafilalet (light red) (Van Zeijst, 2013). B: Gharb region (Wikipedia, 2011). C: Zemmour region (Wikipedia, 2011) ... 44
Figure 12: fungi from grains of bread wheat on petridish. A: mix of fungi found after incubation. B: pure colonies after purification ... 45
Figure 13: Results after gel electrophoresis ... 47
Figure 14: : Amount of fungi isolated, amount of putative Fusarium isolates and fungi determined in Belgium ... 50
Figure 15: Comparison of the three large sample groups of infection levels of the examined fungi ... 51
Figure 16: Infection levels of the five examined fungi in durum wheat ... 52
Figure 17: Infection levels of the five examined fungi in bread wheat ... 53
Figure 18: Infection levels of the five examined fungi in all field samples ... 54
Figure 19: Infection levels of the five examined fungi in all storage samples... 55
Figure 20: Infection levels of the five examined fungi in semolina ... 56
Figure 21: Infection levels of the five examined fungi in couscous and flour ... 57
Figure 22: Infection levels of the five examined fungi in all processed samples ... 57
6 Figure 24: Comparison of infection levels of the examined fungi in field and storage samples ... 69
7
List of tables
Table 1: Environmental impact on fungi development (Ma et al., 2013) ... 11
Table 2: Mycotoxin production by Fusarium species (Stanciu et al., 2015) ... 16
Table 3: Infection stages on floret organs of wheat (Boenisch & Schäfer, 2011) ... 18
Table 4: Field and storage sample information ... 45
Table 5: Primer sequences used in PCR ... 46
Table 6: Climate during March, April and May in Sebt jahjouh and Rass Ijerri (Custom weather, 2019) ... 59
8
Introduction
Wheat production is the backbone of the agriculture in Morocco, therefore it is important that the cultivation is carried out as efficiently as possible. Unfortunately, crop diseases and pests are a large problem in the Moroccan crop production. With a rather humid and warm climate during the growing season, Morocco has opportune climate conditions for a large variety of pathogens including Fusarium spp.. These fungal pathogens can cause major yield losses by causing diseases such as root rot and Fusarium head blight disease (FHB). Approximately71% of the Moroccan durum wheat grains can be contaminated in one season (Ennouari et al., 2018) and large-scale outbreaks can cause economic losses due to lower yield and lower quality up to 3 billion dollars (Ioos et al., 2005). Five of the most common species of the FHB-complex will be studied, namely F. avenaceum, F. culmorum, F. graminearum, F. poae and M. nivale. The variety between the different stages from field to market and the variety between the different crops or regions will be examined with the influences of environmental factors and cultivation methods in mind.
Not only direct losses cause problems but during colonisation Fusarium spp. produce toxic secondary metabolites that, especially in case of FHB, create health issues for humans and animals. These toxic compounds support the fungi during infection and colonisation of the crop and when competition with other pathogens is needed. More importantly, contaminated grains can enter the feed and food chain. In that regard, Zinedine et al. (2016) found that 98% of the couscous semolina samples were contaminated with at least one mycotoxin. Not only in grains, but also in all everyday products, such as milk, bread, fruits, spices, wine… contaminations of more than 50% of the products have been seen. Not all Fusarium species produce the same mycotoxins and not all mycotoxins are equally hazardous to health, which makes it important to know which species are present in cropping systems in order to make the link with the mycotoxins they produce and the risks they can cause.
To combat mycotoxin contamination and direct crop losses, an integrated approach including good cultivation practices, the use of tolerant wheat cultivars and chemical fungicides, is needed. The use of conventional fungicides to control FHB receives increasing pressure due to environmental concerns and official restrictions. The Moroccan farmers lack knowledge of usage of fungicides and these recourses are hard to come by for these farmers. Even if they had the knowledge and the fungicides, a lack of good application techniques still makes it hard to use fungicides properly. For this reason, alternative approaches are getting more attention. One of these alternatives is using biocontrol agents (BCA), which are organisms that can lower
9 the Fusarium infection by competing for space and resources, producing compounds that restrict growth, etc. Worldwide, research is going on to detect the most productive and efficient biocontrol agents.
This research examines the species of Fusarium spp. that are most common in the wheat production cycle in the Fès-Meknès region in Morocco in order to make an association with the potential mycotoxins. After that, saprophytic fungi from the Meknès university collection will be tested on their biocontrol ability against Fusarium spp., because Fusarium head blight (FHB) and mycotoxin contaminations remain a large problem in this region.
10
Literature study
1. Fusarium
1.1. What is Fusarium?
Fusarium is a cosmopolitan genus belonging to the kingdom of Fungi, phylum of the Ascomycota, class of the Sordariomycetes, order of the Hypocreales and family of the Nectriaceae. Fusarium is a filamentous fungus and is often referred to as a part of the larger group of the hyphomycetes. Itis a pathogenic fungus that can often be found in cereals crops including maize (Zea mays), wheat (Triticum ssp.), barley (Hordeum), oat (Avena sativa), rice (Oryza) and millet (Panicum miliaceum). These crops are regularly infected with toxigenic species of Fusarium and this can lead to damage of the plant, yield loss and economic losses (Hassan et al., 2019). The secondary effect of these fungi is the production of mycotoxins that can cause health problems for humans and animals such as digestive and haemolytic disorders, impairment of humoral and cellular immune responses as well as nervous disorders (Isebaert et al., 2009). This study will mainly focus on species affecting wheat, because the research done was mostly concerning wheat samples. These species are mostly part of the
Fusarium graminearum species complex that causes Fusarium head blight (FHB) of wheat and barley, and the Fusarium oxysporum species complex that causes vascular wilt in a numerous variety of crops (Aoki et al., 2014).
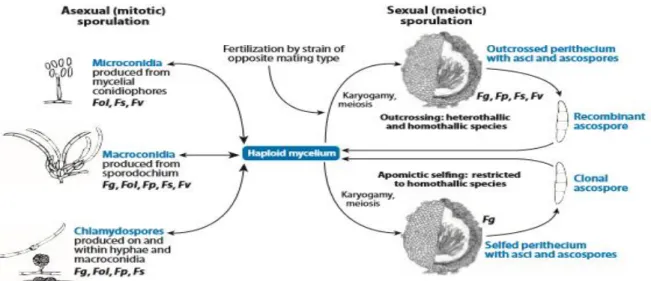
The pathogenesis of these fungi can be different between species, but the overall life cycle is very similar, this is shown in figure 1. Fusarium has a haploid mycelium which forms three different types of mitotic spores in the asexual cycle, namely chlamydospores, micro- and
11 macroconidia. The production of the mitotic spores can vary between species. Only some species, such as F. graminearum, F. avenaceum and Microdochium nivale, also createmeiotic spores, called ascospores and these are produced during the sexual cycle after fertilization of the ascogonium by a opposite mating type. The sexual as well as the asexual spores create haploid mycelium again and can result in airborne spores that cause infection of the floral tissues. The amount of transcription factors affecting conidiation and the asexual cycle were a lot less than the factors affecting the sexual development. This could be related to the more complex development of the sexual cycle, also other research has shown that the regulation of the sexual cycle is complex and that the underlying genetics are not yet completely understood (Ma et al., 2013). The different stages of this cycle are influenced by different environmental circumstances (Table 1).
The sexual reproduction of F. graminearum is optimal around 25-29°C for perithecial and ascospore production. The release of these compounds is triggered by a lower air temperature, with an optimum around 16°C, but is inhibited by rain or a long period of high humidity. The macroconidia are quickly produced at optimum temperatures of 28-32°C, but when the temperature lowers to 20°C, it takes five days until the macroconidia appear. The formation of conidia can be shortened by high humidity. Looking at the water potential of the air it also differs, so can F. avenaceumand F. culmorum already create conidia at -10 to -20 bars, while
F. graminearum had maximal conidia production at -1.4 to -3 bars, so F. graminearum needs a lot more humidity than F. avenaceum or F. culmorum. The light intensity can influence the perithecial initiation, with the most effective wavelengths at 300-320 nm. Raindrops and wind are necessary to spread the Fusarium inoculum. For the germination of Fusarium inoculum, warm and humid conditions are required, with a reduction of the water potential from the air
12 from -1 to -20 bars, the germination levels lower significantly. The growth of the fungi is also depending on temperature and water potential and here the optimums also differ between species. F. culmorum, F. avenaceum and Microdochium nivale arethe fastest growing species, even at the lower temperatures. The optimal temperatures are dependent on the water potential, when lower water potentials are recorded, the optimal temperatures slightly increase (Doohan et al., 2003).
The fungi have different ways of surviving depending on the different stages of the host plant. To persist in the absence of a living host, Fusarium species are able to survive saprophytically on crop residues as mycelium and in the soil as spores. These spores are mostly thick-walled chlamydospores and their survival is highly related to climatic factors (Sangalang et al., 1995). The infection of the crops is initiated by airborne spores landing on flowering ears and entering the plant through natural openings (Trail, 2009). Conidia on the soil, for example are ejected into the air by raindrops, which makes it easier to disperse, find susceptible hosts and infect the spikelets. As the pathogens proliferates, it first grows intercellularly and asymptomatically, spreading through xylem and pith. Later, the pathogens also grow intracellularly, which causes a fast occurrence of the symptoms, such as water soaking and bleaching of the head (Trail, 2009). Invasive mycelia can spread throughout the spikelet, in the node and in the whole rachis. Co-evolution between Fusarium spp. and the host crops has been found, so that the fungi can bypass the resistance barriers by shifting its own virulence by mutation and recombination (Dweba et al., 2017). Fusarium spp. are mostly monocyclic fungi, so the quantity of primary inoculum on crop residues and in the soil is important for infection during the season (Haesaert, 2019). Because Fusarium spp. can not only infect small grain cereals, but also maize, the inoculum can survive on maize debris and disperse to another host later on. These primary inoculum propagules can be found at any time during the adult stages of the susceptible crops which makes it easy to infect at the time when the plant is most vulnerable (Osborne & Stein, 2007). Diseases with Fusarium spp. often include wilts, blights, cancers and blights and occur in both agricultural and natural ecosystems (Ma et al., 2013). While the genus Fusarium
contains a lot of different species, not all species are equally common for important crops or diseases. Some of the most common species and diseases in agricultural important crops will be described further on.
1.2. Fusarium head blight
A common disease found in wheat and other small grain cereals caused by Fusarium spp.is Fusarium head blight, also called Fusarium ear blight disease. This destructive disease is caused by the Fusarium graminearum species complex (Aoki et al., 2014). The species found
13 in infected plants are highly dynamic, mostly depending on climatic circumstances such as moist and temperature, but also agricultural factors, such as soil tillage, previous crops, etc. It is clear that the distribution of Fusarium species varies because of a difference in survival capacities and different ways of infection. This is a consequence of different environmental and biological requirements (Landschoot et al., 2011; Osborne & Stein, 2007), with temperature, humidity, light intensity and wind as critical climatic factors for production and dispersal of spores and conidia, as well as growth and survival of the fungi (Doohan et al., 2003).
Seventeen species have already been proven to be associated with the symptoms of FHB but other species, that were previously not associated with the symptoms, are gaining importance (Landschoot et al., 2011). The most common and important species of Fusarium spp. related to FHB are F. graminearum, F. culmorum, F. poae , F. avenaceum and Microdochium nivale
(formerly Fusarium nivale).The most prevalent species for small grain cereals are F. culmorum
and F. graminearum, but the other species can also have an important economic impact or fasten the colonization (Osborne & Stein, 2007). Some other Fusarium species, such as the members of the Fusarium oxysporum species complex can cause wilt and necrosis but are not especially associated with the head blight disease (Aoki et al., 2014). In an eight-year experiment about head blight disease done by Vogelgsang et al. (2019) mostly F. graminearum
was found; this was also confirmed by other research by Vanheule et al. (2014), Landschoot et al. (2011), Osborn & Stein (2007) and others. Nevertheless F. poae and F. avenaceum were also observed every year. F. graminearum is the major cause of FHB, especially in the warmer and more humid regions of the world. It infects the plant during anthesis using perithecia with ascospores and can later spread to uninfected flowers and damage and contaminate kernels with mycotoxins (Ma et al., 2013). Within this species, the genetic variation is high, and this has an influence on aggressiveness, the production of toxins, reproduction and environmental response (Osborne & Stein, 2007). F. culmorum is a common species that survives mostly in the soil and while the concentration in the soil can be rather high, sometimes it contributes only for a small part in the final population in the kernels. Depending on the year, the amount of F. culmorum detected can differ. It is more prevalent in the cooler regions of the world, but it needs humidity. F. poae mostly works as a secondary pathogen and colonizes the already weakened ears (Landschoot et al., 2011). F. poae is somewhat different from the other species in terms of survival, interactions and symptoms. For example, F. poae is more prevalent in drier areas than F. graminearum that needs more warm and humid climates (Xu & Nicholson, 2009). These differences in environmental niches between F. poae and F. graminearum are used to explain why there is a negative correlation between these two species (Vogelgsang et al.,2019). Other reports have shown that F. poae can grow under a large range of temperatures and has
14 an optimum in the same temperature range in which F. graminearum and F. culmorum can grow, namely 20°C-30°C (Nazari et al., 2018). However, the optimal temperature of F. graminearum is 25-30°C (Xu & Nicholson, 2009). So, when the climate is moderate, for example in Flanders, studies have shown that interactions between and co-occurrence of these two species can be found (Audenaert et al., 2009). Interactions between species are not uncommon. The majority appears as single species on weeds and residues, but on wheat around harvest more interactions are observed (Landschoot et al., 2011). Microdochium nivale
is commonly described as a non-typical Fusarium mycotoxin producer, because this species is not able to produce any of the typical Fusarium mycotoxins. M. nivale can be a leaf parasite and reach the kernels via conidia produced on the leaves instead of through the residues on the field. M. nivale has some different characteristics than the other Fusarium species, so there are also different reactions to some of the control methods, e.g. some triazole fungicides that are very effective against Fusarium do not work against M. nivale, but it can also be the other way around. Some fungicides are very effective against M. nivale but not against the other
Fusarium pathogens. Regardless of these differences, M. nivale can be present in the same regions and on the same crops as the other Fusarium species (Ioos et al., 2005).
The infection in wheat can be seen as blighted head and peduncle tissues, which can turn brown and senesce prematurely, see figure 2. Perithecia or sporodochia on heads and glumes also refer to the presence of FHB. The seeds are often shrunken and bleached, see figure 3 (Freisen, 2015; Osborne & Stein, 2007).
The severity of the symptoms is directly linked to the severity of infection and deoxynivalenol (DON) production by the infecting species (Isebaert et al., 2009). The chance of infection
Figure 3: Symptoms of FHB in wheat kernels, healthy (left) to severe (right) (Freisen, 2015)
Figure 2: Symptoms of FHB in wheat florets, healthy (left) to severe (right) (Haesaert, 2019)
15 depends on different factors. For example, the weather conditions during anthesis have a strong effect on the infection, especially high temperatures and high humidity can increase the chance of infection. This can also be seen in table 1 (Landschoot et al., 2012). In addition, the susceptibility of the hosts differs depending on physiological state, genetics and factors such as adaptation and virulence are of importance for the infection process (Osborne & Stein, 2007). Vogelgsang et al. (2019) has concluded that also the cropping history influences the infection rate in wheat. With maize as the previous crop, the chance of infection increases in comparison to sugar beet and most other previous crops. Especially if there is no or reduced tillage, the chance increases immensely. The highly dynamic nature of FHB disease makes it difficult to control the infection but the effects of the weather, treatments, crop rotations, etc. mostly have an impact on the primary inoculum (Vanheule et al., 2014). This primary inoculum is still infectious even after harvest and at the beginning of the following crop cycle due to the fact that the fungi can survive as saprophytes on crop residues and as spores in the soil. A secondary infection is possible through airborne spores that are deposited during the anthesis inside wheat florets (Audenaert et al., 2009).
1.3. Mycotoxins
1.3.1. Production
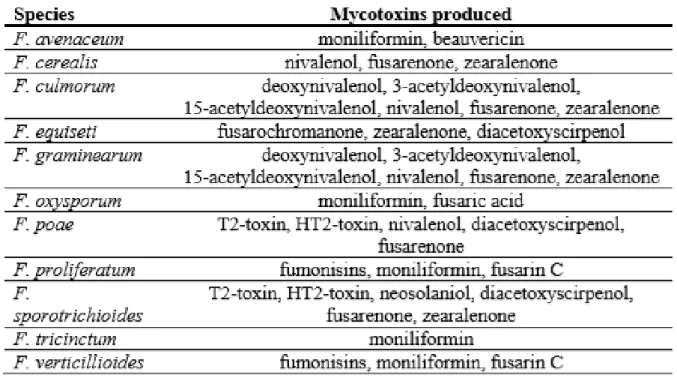
Infection of the plant by Fusarium species is mediated by producing secondary metabolites, called mycotoxins. A lot of research has described different species producing different mycotoxins depending on the geographic area, host species, cropping factors, soils and weather. Because there are a lot of different factors, it is hard to really determine which factors affect the mycotoxin production (Vogelgsang et al., 2019). However, it has been proven that the produced mycotoxins vary, depending on the species of Fusarium that are present. For example F. graminearum and F. culmorum mostly produce trichothecenes B like deoxynivalenol (DON), nivalenol (NIV) and also zearalenone (ZEN). F. poae produces mostly trichothecene A like T-2 and HT-2 toxin but also NIV. F. avenaceum produces more emerging mycotoxins, like moniliformin (MON) and beauvericin (BEA) and some of the species do not produce mycotoxins at all. The diversity in mycotoxins produced by different Fusarium species is illustrated in table 2 (Stanciu et al., 2015).
16 Deoxynivalenol (DON) is the most prevailing and the most economically important of all the mycotoxins produced by Fusarium, especially in small grain cereals. DON is mainly produced by F. graminearum and F. culmorum, but the production is, like the other mycotoxins, influenced by many environmental factors. DON production is increased when a high humidity and intensive rainfall is present during and after the anthesis, but even during the vegetative stage the weather conditions can determine the ultimate DON-concentration. The correlation between the mycotoxins and the species of Fusarium present in the plant are not the same in every crop or equally proven. In small grain cereals the most common mycotoxins are DON, and its conjugates 3-ADON (acetyl-deoxynivalenol), 15-ADON and DON-3G (Audenaert et al., 2013).
DON is the most known representative of the trichothecenes B. The trichothecenes are very stable mycotoxins, even during storage and processing. They are also not degraded by high temperatures. The most important trichothecenes A are T-2 toxin and HT-2 toxin, with the T-2 toxin that can rapidly metabolise to the HT-2 toxin. These toxins will however, in contrast to the other trichothecenes, reduce when the cereals are processed because of redistribution. They can withstand baking and cooking, but malting leads to lower levels in malt. These mycotoxins are produced by F. poae and some other Fusarium species like F. acuminatum, F. langsethiae
and F. sporotrichioides. ZEN is produced by some of the same species that produce DON, these species infect the plant during flowering and produce these mycotoxins in the ear or post-harvest under poor storage conditions. There are different metabolites of ZEN, with α-ZEN and β-α-ZEN as important derivatives, created in mammalian tissue. All these zearalenones
17 are estrogenic compounds. ZEN can survive temperatures of 150°C. Redistribution during milling lowers the concentration of the mycotoxins in some parts of the grains, such as the endosperm, which is used for human consumption, while other parts, such as the bran, contain a higher concentration, which is why these are used for animal consumption. (Marin et al., 2013).
Next to all the well-known mycotoxins, some Fusarium species, such as F. avenaceum and F. oxysporum produce some emergingmycotoxins as well, such as enniatins (ENs), beauvericin (BEA), fusaproliferin (FUS) and moniliformin (MON), see table 2. Emerging mycotoxins are unregulated and more currently discovered or researched, especially their role as mycotoxins has only been recently understood. These mycotoxins have not yet been fully investigated and the effects of the mycotoxins, the maximum concentration to prevent detrimental effects and so on, have not yet been determined (Marin et al., 2013; Sifou et al., 2011).
The mycotoxin production can continue in the storage, also this depends on the temperature and the water availability (aw), but this differs between species and isolates. The parameters causing a larger mycotoxin production are probably related to the fungal growth instead of directly to the mycotoxin production. During storage, type B trichothecenes prefer warm, humid conditions which are the same environmental conditions that are preferred by F. graminearum and F. culmorum for growth. ZEN is produced at the end of the storage period and prefers a warm and humid climate rather than cool and dry. MON produced by F. avenaceum prefers Mediterranean rather than temperate conditions. However, the environmental effects do certainly not outweigh the effect of initial infection levels. The effect of the environment is mycotoxin specific, for example when a moderate initial infection with F. culmorum happens, DON production during storage can increase, whereas when the initial infection is high, the mycotoxin does not increase. Another example is NIV that can increase during storage irrespective of the initial infection levels. As previously said, the mycotoxin concentrations can change during milling, brewing and processing of the grains. Most of the mycotoxins are found on the surface of the grains, which creates a decrease in mycotoxins in the flour after cleaning, sorting, sieving and de-hulling. The cereal by-products, however, can still contain high concentrations of these mycotoxins (Doohan et al., 2003).
Genomic studies can help with identification of the genes and gene clusters involved in the synthesis of mycotoxins. So has Ma et al. (2013) found that the mycotoxins are encoded by clustered genes. These genes encode for successive enzymes in the biosynthetic pathway of the mycotoxins, for transporters and for specific transcription factors that activate expression of genes. Some Fusarium spp. can produce a large variety of secondary metabolites, while
18 others can only produce a few. The genes responsible for producing these secondary metabolites can be general or specialized. General pathogenicity genes occur in Fusarium
species as well as in other pathogenic fungi, while specialized genes are specific to individual
Fusarium species on specific hosts. In the latest case, specialized genes are involved in host-pathogen interactions including mycotoxin contaminations. Trichothecene mycotoxins can promote virulence toward some crops and they probably act through inhibition of protein translation, stimulation of the plant defence and initiation of cell death (Ma et al., 2013).
1.3.2. Plant infection
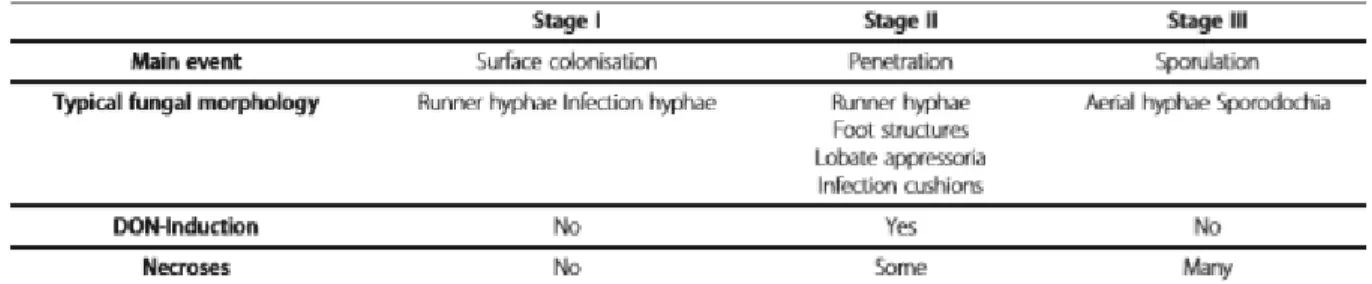
Throughout the different stages of plant infection, the mycotoxins, and especially DON, play an important role in the infection and colonisation process. Species that produce DON can have advantages over the other species during the saprophytic phase. By affecting other organisms with the antimicrobial character of the DON-mycotoxin, they are strong competitors for niches on the crop residues and organic matter sources. Other mycotoxins of the type A and type B trichothecenes can also help in the saprophytic survival, which can explain how F. poae is a great saprophytic survivor, while not being known for its DON-production (Audenaert et al., 2013). During infection, DON can be a real asset to hijack the plant’s defence system. The production of the virulent conidia and ascospores are regulated by some of the same factors that regulate the DON production. This explains why DON is also important in the infection stage of Fusarium (Audenaert et al., 2013). It has been proven that DON is a key virulence factor that causes a ‘stealth’ infection, so by suppressing its biosynthesis, a strong reduction in virulence can be deducted. However, during the initial stage of the infection, DON does not participate in the production of infection features, such as hypha, appressorium, infection cushion… but these structures are more dependent on hydrolytic enzymes. DON plays a part in the initial biotrophic stage of the infection by suppressing the defence systems of the plant and later it controls the necrotrophic stage when nutrient extraction takes place, this is shown in table 3 (Dweba et al., 2017, Boenisch & Schäfer, 2011).
Even though DON is the most prevailing, the other mycotoxins are also important for the virulence of Fusarium spp.. ZEN and NIV are present in a lot of the infected plants but not as abundant as DON. A nuance must be made, because NIV is not as commonly investigated as
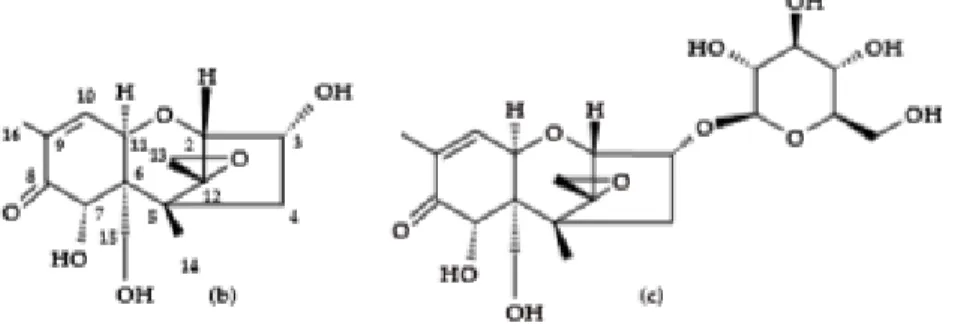
19 DON and ZEN. But when these mycotoxins are all determined, the average ZEN and NIV levels are lower (Vogelgsang et al., 2019). There has been no conclusive research whether ZEN plays a large part in the infection process. The correlations between all these mycotoxins are not equally present in every type of cereal. Vanheule et al. (2014) for example found no correlation between DON and his acetylated forms ADON. Other research however has proven that different chemotypes of Fusarium species correspond with different trichothecene profiles, for example in Fusarium graminearum there are three chemotypes; one producing NIV, one 15-ADON and DON and the last one producing DON and 3-15-ADON. The environment can have an impact on the prevalence of a given chemotype, so not only the species is a determining factor but also the environment determines part of the secondary metabolites production. The explanation for the discrepancy between genotype and chemotype can be found in the dependence of the fungi on the environment for growing and trichothecene production (Ramirez et al., 2019). Vogelgsang et al. (2019) also present the fact that the plant can metabolise ADON to DON rather quickly, as an explanation for a lower ADON concentration. A high correlation between the trichothecenes B and the FHB incidence has been proven by Dweba et al. (2017). The interpretation and prediction of the mycotoxin contamination is hard because of the variability due to detoxifying mechanisms of the plant and the production of ‘masked mycotoxins’, such as DON-3G. These masked mycotoxins are created by the plants that convert the chemical structure of the normal mycotoxins to prevent negative effects on their metabolism. This can be done by two major reactions; first an oxidation or hydrolysation, followed by a further detoxication by a glucosyltransferase-enzymes to glucosides, which are not virulent and do not cause yield loss.
The conversion of DON to DON-3G is more efficient in resistant wheat cultivars and implementing a detoxication mechanism based on glucosylation could increase the level of resistance in susceptible cultivars. These reactions are however not irreversible, so it could go back to their original form during processing or digestion and still cause health hazards (Bryła et al., 2018; Dweba et al., 2017).
20
1.3.3. Health risk
Mycotoxins can transfer to feed and food and are hazardous to the health even at low concentrations, especially DON and ZEN are toxic (Dweba et al., 2017). DON is the main mycotoxin produced by Fusarium complex of FHB and there is a direct relationship between the incidence of FHB and DON contamination of the grains harvested. It is also known as ‘vomitoxin’ and a lot of harm can come from an intoxication. Indeed, for DON, an LD50 value of 78 mg/kg in mice has been demonstrated (Bryła et al., 2018). DON can cause a variety of toxicological effects on the immune system and the gastrointestinal system. At a low dose it causes anorexia and reduced growth, at a high dose it causes vomiting and diarrhoea (Ennouari et al., 2013). Contamination can lead to chronic disease symptoms that can be severe, such as necrosis of the intestinal tract and inhibition of the mitochondrial functions. DON contamination can even be fatal to humans and animals (Audenaert et al., 2013). Although DON is not as toxic as some other mycotoxins like T2-toxin, it can cause a lot of damage because of its high presence rates (Marin et al., 2013). ZEN is also an important contaminant of the mycotoxins produced by FHB-pathogens. ZEN is a non-steroidal oestrogenic mycotoxin. The effects of ZEN on animals and humans are related to its oestrogenic activity and includes alterations in the reproductive tract and decreased fertility (Stanciu et al., 2015). ZEN is also toxic to the liver, kidneys and immune system (Drakopoulos et al., 2020). NIV is not as commonly found in food as DON but it exhibits higher toxicity in animals, with an LD50 of only 39 mg/kg in mice. NIV mostly creates its effect by inhibition of protein, DNA and RNA synthesis (Bryła et al., 2018). From the trichothecene type A, mostly the T2 toxin and HT-2 toxin are known. T2 can inhibit DNA and RNA synthesis (Marin et al., 2013). To decrease ingestion by animals and humans, maximum thresholds were set by the European Food Safety Authority (EFSA) for some of these mycotoxins. In other continents and countries there are also limits but they can differ from the European standard (Landschoot et al., 2012). Some of the emerging mycotoxins are cytotoxic, such as ENs, and BEA. They can insert into cell membranes, where they disturb the ionic homeostasis. ENs are known to have an insecticidal activity and act as an antifungal and antibacterial agent. It can also inhibit the enzyme functions (Zinedine et al., 2011). BEA can harm humans and other mammals by inducing apoptosis of cells and influencing the contraction of muscles. They both can cause mitochondrial dysfunctions. FUS can be phytotoxic and can decrease the chlorophyll content. MON is a very strong acid and is an inhibitor of several enzymes that can lead to the interruption of the gluconeogenesis. There have not been set any thresholds for these mycotoxins, because they have not been as intensively investigated (Marin et al., 2013; Sifou et al., 2011).
21 A good screening of the fungal population is necessary to give valuable insight in the phytopathology of Fusarium spp. and can help the control of the fungal diseases (Vanheule et al., 2014). The screenings gain importance when the interactions between different fungal population become complex and dynamic. Molecular surveillance contributes to the knowledge of the toxigenic potential of Fusarium spp. and can avoid spread of new strains or improve predictions. Genetics play an important role in the screening for genes in varieties that can be used for creating resistant cultivars. Molecular characterization of the fungal populations used in the test is needed to establish molecular markers that can be associated with resistance in genotypes. Quantification of fungal DNA can also be a good marker for the level of fungal and mycotoxin contaminations. With more genetic information, the evaluation of the potential risks during cultivation and to humans and animals could be facilitated. (Morcia et al., 2013).
1.4. Control methods of Fusarium diseases
Managing Fusarium infection and mycotoxin production is complex as there is not one species responsible and different factors should be included in the management, such as weather and agricultural factors. To create an effective management to lower the FHB-occurrence, it is impossible to not take the other Fusarium diseases, such as foot rot and wilt, into account, as they can facilitate the infection with FHB. Only an integrated disease management can be sufficient, because every management factor on its own has its limitations. A combination of cultural, biological, chemical and genetic control is recommended. This integrated disease management affects not only the FHB pathogens, but other Fusarium species and even other pathogens as well. To monitor infection probability, it is important to include all the climatic factors, such as temperatures, humidity, rainfall… These factors are necessary to develop a prediction system to assist farmers in taking strategic measures to minimize the disease and the losses in yield and quality (Dweba et al., 2017). An effective measure is avoiding susceptible plant species as previous crops for wheat, especially in combination with reduced tillage. Changing from maize to potato, canola and pasture can cause a serious reduction in the infection level (Vogelgsang et al., 2019). Reduced tillage leaves a bigger opportunity for
Fusarium to survive, so ploughing is advised. However, studies have shown that frequently ploughing can cause soil compaction and there is a bigger risk of erosion (Drakopoulos et al., 2020). A lower nitrogen fertilization can also have a reducing effect on Fusarium infection. This is not surprising because pathogen nutrition is also necessary to have a successful colonization. Nitrogen can be a limiting factor for the pathogen when the plant is also growing. While they can use different sources of nitrogen, they prefer ammonia and glutamine. They need nitrogen to be able to penetrate, to make hyphae fuse or to adhere to the roots, so this
22 explains that when nitrogen fertilization is not abundant, less infection is found (Divon et al., 2006). Different research groups have shown that there is not one management strategy that has enough effect on the entire Fusarium genus. In that regard, ploughed fields promote F. poae while suppressing F. graminearum. This makes it important to choose the appropriate measures in relation to field characteristics (Vogelgsang et al., 2019). Other control strategies are the use of bio-control agents such as bacteria and fungi, but with these strategies there is never a full eradication of the fungi and further studies are necessary (Dweba et al., 2017). Also the use of plant-based extracts has gained interest in research, as it can serve as an alternative for fungicides mostly in the form of biofumigation. Drakopoulos et al. (2020) has studied mulch from different mustard types because of their ability to release isothiocyanates (ITC) that has a broad biocide spectrum. Clovers contain a lot of antioxidant and antiradical properties and can provide the soil with additional nitrogen. Beside the primary effect of the mulch, there is also a secondary beneficial effect of a more active microbial community that can have a suppressive effect on the pathogens (Drakopoulos et al., 2020).
Chemical control is still commonly used because of the easy use and effectiveness of fungicides. Some other factors like climate impact, economic returns, management and dose should be considered in choosing which fungicide can be used. Some of the most effective fungicides used to protect the plant against Fusarium are triazoles, such as tebuconazole, metconazole and prothioconazole. However, none of these chemicals can realise complete control and there is a high possibility of resistance development (Dweba et al., 2017). Studies have shown that the sensitivity to triazoles differs between species and it also depends on the specific compounds used in the fungicide (Vogelgsang et al., 2019). For example, Ioos et al. (2005) have proven that tebuconazole and metconazole are effective against F. culmorum and
F. graminearum but have almost no effect on M. nivale.
Another way of controlling FHB infection is host plant resistance. Different types of resistance can occur from inhibiting the initial infection to preventing the spreading to tolerance without real yield or quality loss. The combination of the resistance to initial infection and preventing the spreading gives the most stable and durable way of resistance. Resistance can be incorporated in plants by plant breeding and biotechnology. Because resistance is a quantitative trait controlled by different genes, it is a slow process to get full resistance. Success of breeding resistance depends on different factors, such as the nature of the pathogen, the type of genetic resistance, the method of screening… Another important factor to successfully create resistant cultivars is the accessibility of good infrastructure and skilled people, which is not always easy. This is not an ultimate solution because the pathogens can
23 break through the resistance by a shift in virulence (Dweba et al., 2017). While there are cultivars with very high resistance, most of the farmers choose cultivars with an intermediate resistance to FHB, because the cultivars with higher resistance, can be more susceptible to other diseases, lodging or give lower yields (Vogelgsang et al., 2019). As mentioned before, only an integrated disease management is sufficient to lower the infection rate; this can be obtained by using resistant cultivars with the right cultivation methods and chemical control used when necessary (Dweba et al., 2017).
2. Morocco
2.1. Wheat production
Morocco as a country is divided into different topographical regions. In the northwest, the most agricultural rich lands can be found and there is a mild climate. In the South Eastern part there are the less rich soils of the mountains and plateaus and there is a more arid climate. The most southern part is the Sahara Desert, which is agriculturally negligible. Semi-arid lands can be found scattered in the northeast, the southwest and the central-southern parts. The region from which the samples for this study were taken, Fès-Meknès, lies in the part known as the Moroccan Plateau, the soil in this region is a light reddish siliceous soil and the texture is sandy clay loam. This soil allows good cereal yields but has a poor moist retention. The World Bank reported that Morocco has 7.7 million hectares suitable for cropping, from which about 4.2
24 million hectares were used for cereals in the season of 2018-2019 (USDA, 2019). There is another 2 million hectares for grazing and forests. Some land could be useful under permanent irrigation. One third of the agricultural sector is represented by high value products such as citrus, olives and other fruit trees. These crops have grown in numbers due to a better use of agricultural inputs and better irrigation systems (World bank, 2017).
Morocco is a country with a rapid growing population and a high consumption of cereal products, e.g. couscous and pastries. For economic purposes, they try to be as self-sufficient as possible. The most common cereal grown is durum or hard wheat. From the production of wheat at least 80% is rainfed and from this, 40% is grown in the semiarid zone of the country. They plant the seeds in the fall, so they can benefit from the winter rains, because almost 90% of the rainfall in the growing season of wheat occurs from November to March. (Shroyer et al., 1990). The vegetative growth takes place from November to mid-February and the reproductive phase from the beginning of March to the end of April (Mrabet, 2000). Most of the farmers use a high seed rate because they cover them with an offset disk and it becomes a heterogeneous field with unpredictable rates of germination and emergence. The sowing mostly happens by hand, sometimes following a tractor- or animal-drawn tillage (Karrou, 1998). At the end of the growing season the largest part of the cereals is harvested mechanically but afterwards the remaining stubble is cut by hand or used as grazing land. Grain losses in relation to harvest are significant (Shroyer et al., 1990).
2.1.1. Cultivation methods
The climate in Morocco is not ideal for wheat growth, but still around 4 million hectares of the arable land are occupied with dryland cereal. Because the growing conditions are not really suited, the plant grows under constant stress which leads to low yield, even with fertilizers and adapted cultivars. The yield has increased the last 40 years, due to better cultivation methods, and is now at an average of 2.5 ton per hectare. Combinations of tillage systems, crop rotations, sowing rate, etc. help optimize the yield. Some research of Bouzza (1990) concluded that no-tillage systems result in higher yields because of the water saving effect of crop residues that cover the soil while these effects are less pronounced when the residues are incorporated in the soil. The effects of tillage or no-tillage on the wheat yield can differ depending on the soil type, moisture and climate, making the forecast of the effects of a specific method rather difficult. The choice of tillage or no-tillage should always depend on the specific characteristics of a site. In areas with swelling clays, which are very common in Morocco, no-tillage can increase the wheat yield, especially in the dry years. Also deep disking and chisel systems can favour the wheat yield. No-tillage can help in a continuous wheat cultivation to prevent soil
25 degradation (Mrabet, 2000). No-tillage also reduces the drying out of the soil surface and the residues can release nutrients after mineralization. The no-tillage systems even create a build-up of nitrogen and carbon in the surface layers of the soil (Ibno Namr & Mrabet, 2004). Wheat can grow successively but when the rainfall is limited a crop rotation with maize and legumes is advised (Shroyer et al., 1990). Although crop rotations are known to cause higher yields and less diseases, fallowing is obligatory in the semi-arid areas for sustainable yields (Ibno Namr & Mrabet, 2004). A better technique of seed planting, can help reduce the seeding rate and improve emergence, that is why improvements in direct drill design could help (Karrou, 1998; Mrabet, 2000). It was proven by INRA that selecting on early maturing and resistance against diseases, insects, drought and heat, can increase yields immensely (Karrou, 1998). With these factors in mind, higher grain yields can be obtained in high-yield and low-yield environments. Irrigation in combination with more fertilizers and other inputs results in a significant improvement of yield. However, rain fed cropping is still the dominant production system so improving this system could be more useful. The improvements in rainfed cropping have lower costs, a higher economic return and can be applied by the rural poor farmers as well (Karrou, 1998).
Not only the yield is of importance but also the quality of the harvested grains. This includes protein content, gluten strength, weight, virtuousness and yellow pigment content caused by xanthophyll and lutein. These are all important qualities that are meaningful when these grains are used by the industry to make high-quality end-products such as couscous and burghul. These characteristics are influenced by genotype as well as by growing conditions and the interaction between both. Some traits, like weight and pigment are more influenced by genetics than by the environment, while other quality parameters, such as protein content and vitreousness are more influenced by the environment and have less genetic stability (Taghouti et al., 2010).
2.1.2. Fertilizers
The response to nitrogen fertilizers depends on the soil type, but in most cases a higher yield is found, even with low inputs of 40 kg/ha, which is also the current consumption amount and is one third less than the requirements (FAO, 2006). The increase in yield due to fertilizer usage can range from 0.3 ton to 0.7 ton per hectare. The most important factor to determine the amount of N-fertilizer needed, is the previous cropping history. With phosphor fertilizers, the response was not consistent and more depending on the soil type. Potassium deficits are rare and cereals do not have a high demand. Most of the soils are rather calcareous, so deficiencies of magnesium, sulphur and micronutrients are also rare and fertilizing with these nutrients is
26 not needed. Fertilizers are not commonly used in Morocco because the extra investment does not always result in higher yield because of abiotic stress. However, from 1956 to 1978, the total fertilizer consumption increased ninefold, which resulted in higher yields. Morocco is fortunate to have a lot of natural sources for fertilizers (Shroyer et al., 1990).
2.2. Challenges
2.2.1. Water scarcity
The largest part of Morocco has a semi-arid climate, which means that rainfall is limited to 500 mm a year. The seasons are characterized by dry, hot summers and humid winters, thus the precipitation is not evenly spread throughout the year, with most of the precipitation occurring from November through March (figure 6). The overall rainfall is low during the whole year, with not one month exceeding 100 mm precipitation. This indicates why water scarcity is the most limiting factor for yield, especially because even in the rainy season, long periods of drought can occur. During the cropping season, up to 45% of the water is lost through soil evaporation, with a large part of the losses occurring at the early growth stages.
It could help when the plants create a large root system using water from deeper layers of the soil, but even the deeper parts of the soil are too dry during large parts of the growing season. A more realistic solution to yield losses due to drought is to limit the evaporation (Karrou, 1998). When water supply is limited, a small increase in water availability can cause a large increase in yield, so it is important to try to limit drought (Mrabet, 2000).
It has been proven that some specific systems have a positive effect on the water holding capacity of the soil and limit soil evaporation. The first system to reduce water scarcity in the soil is a reduction of the fallow period. This causes less water to evaporate, reduces erosion of the soil and creates less need for herbicides. However, this only gives positive results when precipitation is no less than 300 mm per year, so in the most arid regions of Morocco, this is not advised, but in the Fès-Meknès region this could help. A second way of lowering
27 evaporation losses is by covering the soil with mulch and crop residues. The water storage in the soil can increase with 30 mm by applying plant residues and mulch at the surface instead of incorporating these residues by deep soil tillage. The surface application creates a favourable infiltration and reduces evaporation (Mrabet, 2000). However, there still needs to be attention for the amount of precipitation. When rainfall is divided in small amounts per rainfall event and there is a lot of mulch on the soil, too little water actually reaches the soil, due to interception and evaporation from the mulch layer. The coverage of the soil can also be done by growing fast soil covering cultivars or the covering of the soil can be enhanced by the plant patterns, for example narrowing the row spacing increases yield and the water use efficiency. However, a big problem caused by irregular rainfalls is that when the rain arrives too early, the early plants grow too vigorously and the high biomass production causes soil water depletion in the early stages of the growing season and the grain production will be too low. (Karrou, 1998). This shows that the consequences for yield depend on the development stage of the crop in which drought or rainfall occurs (Mrabet, 2000). No-tillage systems also cause higher water use efficiency, reduce soil moisture loss and keep the organic carbon high in the surface horizons (Ibno Namr & Mrabet, 2004).
2.2.2. Pests, diseases and weeds
Not only the abiotic problems of drought lower the yield of wheat in Morocco. There are also biotic factors that can cause large yield losses. Weeds are normally not a very large problem, however they do compete with the wheat crops for moisture. But because they can be removed by hand and used for livestock, they do not cause a large problem. On bigger farms the use of herbicides is not uncommon. Some of the major weeds are wild jujube (Ziziphus lotus (L.) Lam.), dwarf thesium (Thesium humile Vahl) and silverleaf nightshade (Solanum elaeagnifolium Cav.). More problems are caused by plant diseases, especially because the growth of the wheat crops also needs humid conditions, whether it is by irrigation or rainfall. Humid conditions are ideal for most pathogens to grow. Based on yield losses, some of the most harmful and most common diseases are leaf rust (Puccinia recondita f. sp. Tritici ), stem rust (P. graminis f. sp. tritici), speckled leaf blotch (Zymoseptora tritici), tan spot (Pyrenophora trichostoma), and root rots (Fusarium and Helminthosporium spp.). Pests are very harmful and can be devastating, especially the Hessian fly (Mayetiola destructor) can cause yield reductions up to 100%. The damage can be controlled by crop locations, resistant cultivars and planting dates. Pesticide use is limited, only on irrigated fields with a good microclimate is it useful to use crop protection products (Shroyer et al., 1990).
28
2.3. Fusarium and mycotoxins
2.3.1. Fusarium contamination
A large problem in wheat cultivation in Morocco are fungi of the genus Fusarium. They cause not only direct yield losses through plant diseases, but also indirectly due to mycotoxin contamination; mycotoxins can transfer to the feed and food chain and are a threat to animal and human health. The genus Fusarium is large and the different species can cause different diseases, such as root rot, vascular wilt and head blight disease.
Root rot in wheat plants, a disease mostly caused by Fusarium species in West Morocco, can cause immense damage to yield but also make the plant more vulnerable to other diseases such as FHB. Lyamani (1988) found that Fusarium culmorum was most often detected on plants with root rot while in the soil F. equiseti, F. solani and F. oxysporum were the most common species. On the seeds, it was also F. culmorum and F. equiseti that were mostly present but also F. verticilioides species were isolated. Which species were prevalent really depends on the growing season because in other years F. graminearum, F. oxysporum, F. solani and F. poae could also be isolated from the seeds. When seeds and soil were inoculated with F. culmorum there was almost no emergence of the seedlings. When only the soil was infected with Fusarium species, there was an increase of root rot, but the seedlings did emerge and only the grain yield was slightly reduced. Overall root rot occurs at low severities in most of the fields in West central Morocco. F. culmorum was present in a quarter of the fields and it caused most damage to wheat plants in comparison to all the other pathogens(Lyamani, 1988; Qostal et al., 2019).
The main problem of root rot is not only yield loss but also the impairment of the plant which brings an opportunity for other pathogens to utilize the plants weakened state to cause more infections, such as Fusarium head blight disease. Previously, the infection cycle of FHB was explained including the most common species belonging to the Fusarium complex. Most of these species, like F. graminearum and F. culmorum have an optimum growth temperature of 20°C to 25°C and because Morocco also has a rather humid climate, with an average of 71% in the Fès-Meknès region, Morocco is an ideal environment for the pathogen to survive and to grow. Whether or not the infection in the ears takes place, is depending on the rainfall during flowering, but the environment of Morocco gives the fungi all the compounds for a successful infection (Ennouari et al., 2013).
29
2.3.2. Mycotoxin contamination
Mycotoxins are abiotic hazards and the production is highly influenced by the environment. When wheat ears get infected by Fusarium, it can lead to FHB and the production of mycotoxins and the Fusarium species are known for producing a wide variety of different mycotoxins (Blesa et al., 2014). The most frequent mycotoxins produced by Fusarium spp.are trichothecenes B, such as deoxynivalenol (DON) and nivalenol (NIV), mostly produced by F. graminearum and F. culmorum, but also F. poae can produce NIV. Also trichothecenes A, such as T2-toxin and HT-2 toxin, are produced by F. poae and some other mycotoxins like zearalenone (ZEN), produced by F. graminearum, F. culmorum and fumonisins, produced by F. verticillioides. Not only these well-known mycotoxins occur but they also produce emerging mycotoxins such as enniatins (ENs), beauvericin (BEA) and fusaproliferin (FUS), these are also produced by several species, such as F. poae and F. avenaceum. This is also shown in table 1. Not all mycotoxins are produced by all species of Fusarium, but they can all occur in Morocco (Marin et al., 2013; Sifou et al., 2011). Previous studies have proven that in Morocco most of the DON producing species are F. graminearum and F. culmorum but in most cases there are different toxigenic species present resulting in a mix of mycotoxins (Ennouari et al., 2018). In a study performed by Serrano et al. (2012) on different mycotoxins, 50% of the samples from Morocco were contaminated with a mix of mycotoxins. The samples were taken from the field or from processed foodstuff and in both cases about half were contaminated.
Because a mix of mycotoxin producing fungi can be present in one crop, it is important to look which mycotoxins are most likely to appear in case of Fusarium head blight disease because when the grains are contaminated, the transfer of these mycotoxins to humans and animals is a big risk. The most common mycotoxins that occur when the plants have FHB are DON, ZEN and NIV, all three produced by two very common species of the FHB-complex, namely F. graminearum and F. culmorum. F poae is also a part of the FHB-complex, so mycotoxins like T2 an HT-2 can also appear. F. avenaceum is also known for its part in the FHB-infection so some emerging mycotoxins like enniatins, beauvericin and moniliformin could also be present. The risks of these mycotoxins when entering the food and feed chains were previously described (Nicholson et al., 2004; Vanheule et al., 2014).
2.3.3. Situation in Morocco
The fact that the Moroccan public is rather ignorant to the existence of these mycotoxins, makes it difficult to set up adequate prevention actions to avoid the presence in food and feed. When looked at durum wheat samples from the field and on markets, Ennouari et al. (2013) found 11% of the samples contaminated and a large range of toxin levels between the different
30 samples, but no sample exceeded the maximum value set by the EU authorities. The amounts found in Morocco were on average lower than the other countries in the Mediterranean area. The different areas where DON was found in Morocco, were concentrated in 4 of the 7 areas investigated. This confirms the strong influence of the environment in the occurrence of infection. The ultimate level of contamination can be affected by the species of Fusarium that occurred because some of them are DON-producing while others do not produce DON (Ennouari et al., 2013). In an investigation by Zinedine et al. (2011) 42% of the wheat samples, sampled at markets were contaminated by ENs, BEA was found in 28% of the samples and FUS was hardly found in any sample. Compared to other countries as Italy, Austria, South Africa and the USA, the amount of BEA and FUS found in Morocco is rather low. The ENs levels found, however, are a lot higher than in other studies. In the study of Zinedine et al. (2011) the co-occurrence of the emerging mycotoxins ENs, BEA and FUS has been seen. 20% of the samples were contaminated with at least two mycotoxins. It ranged from two mycotoxins up to four mycotoxins in one sample. When a plant is infected by different species of Fusarium, ENs and BEA are generally detected. Blesa et al. (2014) found 68% of the wheat samples from different markets contaminated with Fusarium mycotoxins. The most abundant mycotoxin was ENB, which was present in more than 61% of the samples. The differences in contaminations levels among the enniatins was high. BEA was present in 10% and had rather low contamination levels. 5% of the samples were contaminated with DON and these levels also varied a lot. Half of the samples were contaminated with more than one mycotoxin, and the co-occurrence of these mycotoxins ranged from two to six in one sample. With fungi of the
Fusarium genusproducing more than one mycotoxin, it is no surprise that in infected kernels, more than one mycotoxin could be found. Morocco was no exception and multi-mycotoxin presence was found (Blesa et al., 2014).
With the climate of Morocco rather humid and hot, it favours the growth of moulds and unfortunately, there are not a lot of the food processing units in Morocco that have a good risk management system to detect mycotoxin contamination. Moreover, a lot of the food is still home-made. While other countries already have regulation and maximum limits for the mycotoxins, there were no regulations or maximum limits set in Morocco up to 2016. In 2016 a joint decree of the Moroccan minister of agriculture and marine fisheries and the minister of health was set up with maximum permitted levels for contaminants of primary products and foodstuffs. This should be implemented in 2020. In this decree there are differences made between different unprocessed cereals, like durum wheat and oats have a higher alert threshold than the others and for unprocessed maize there is only a maximum level. Also cereals for direct human consumption, such as flour, bran and germ, are a category on its own.