CERENAL
as
novel
prognostic
biomarker for patients diagnosed with
brain metastases
Charlotte Van Parijs
01503963Supervisors: Prof. Dr. Sylvie Rottey, Dr. Tijl Vermassen
A dissertation submitted to Ghent University in partial fulfilment of the requirements for the degree of Master of Medicine in Medicine
Deze pagina is niet beschikbaar omdat ze persoonsgegevens bevat.
Universiteitsbibliotheek Gent, 2021.
This page is not available because it contains personal information.
Ghent University, Library, 2021.
III
PREFACE
This dissertation was written in order to achieve a degree of Master of Medicine in Medicine at the Ghent University. This grateful, but sometimes difficult work would not have been possible without the help of a few important people, whom I would like to thank.
First, I would like to express my special appreciation to my promotor Prof. Dr. Sylvie Rottey, for giving me the chance to work on this interesting topic. Whenever a problem would appear, she was there to search for a solution, despite of her busy schedule. She was a real motivation for me to do my utmost and for this I would like to express my gratitude to her.
Dr. Vermassen, to guide me through the process of writing this dissertation from start to finish. He gave me useful feedback, which made it possible for me to improve this dissertation in a matter I am very proud of. Without his persistent help, I would not have been able to reach the aim of this dissertation.
Finally, I would like to thank my lover Julien for his encouragements. His support was of great importance to be able to finish this dissertation in a good manner. He was there to encourage me every time I faced an obstacle, read this dissertation with utmost concentration and guided me through the stressful times. I am really grateful for his everlasting support.
Charlotte Van Parijs
IV
PREFACE ... III
1.
Abstract ... 1
1.1. English abstract ... 1 1.2. Dutch abstract ... 22.
Introduction ... 4
2.1. Brain metastasis ... 42.2. Brain metastasis treatment ... 4
2.2.1. Overall survival and local control ... 5
2.2.2. Quality of life ... 8
2.2.3. Cost-effectiveness ... 9
2.2.4. Which treatment to choose? ... 9
2.3. Prognostic scoring systems ... 9
2.3.1. Recursive Partitioning Analysis ... 10
2.3.2. Score Index for Radiosurgery ... 10
2.3.3. Basic-Score for Brain Metastases ... 10
2.3.4. Graded Prognostic Assessment ... 11
2.3.5. Golden Grading System ... 11
2.3.6. Comparison of prognostic scoring systems ... 11
2.3.7. Primary site-specific prognostic scoring systems ... 12
2.3.8. CERENAL ... 14
2.4. Objective ... 14
3.
Patients and methods ... 15
3.1. Literature study ... 15
3.2. Design and setting ... 15
3.3. Database and data extraction ... 15
3.3.1. Inclusion and exclusion criteria ... 15
3.3.2. Data collection ... 16
3.4. Statistical analysis ... 16
V
4.
Results ... 18
4.1. Patient characteristics ... 18
4.2. Brain metastasis characteristics ... 18
4.3. Statistical analysis ... 18
4.3.1. Prognostic scoring systems ... 18
4.3.2. Univariate statistical analysis ... 19
4.3.2.1. Overall survival (OS) ... 19
4.3.2.2. Disease-specific survival (DSS) ... 22
4.3.2.3. Progression-free survival (PFS) ... 25
4.3.2.4. Overall survival (OS) – sub-analysis lung ... 26
4.3.2.5. Disease-specific survival (DSS)– sub-analysis lung ... 30
4.3.2.6. Progression-free survival (PFS) – sub-analysis lung ... 33
4.3.3. Multivariate statistical analysis ... 34
5.
Discussion ... 36
5.1. General discussion and limitations ... 36
5.2. Statistical analysis ... 39
5.2.1. Survival analysis and prognostic factors for the whole population ... 40
5.2.2. Survival analysis and prognostic factors for the lung-subpopulation ... 44
6.
Conclusion ... 47
7.
References ... 48
1
1. A
BSTRACT
1.1.
E
NGLISH ABSTRACT
INTRODUCTION: For many years, brain metastases (BM) have been associated with a very poor survival. Treatment of BM can prolong survival, but it is still unclear which treatment modality is preferable. Whole-brain radiotherapy (WBRT) has been the widely used standard of care for patients with BM but concerns regarding neurocognitive deterioration arise. Stereotactic radiosurgery (SRS) may form a solution in terms of neurotoxicity but is not applicable for all cases. Many studies have tried to determine a significant survival difference for SRS, but only for patients with 1 BM, a significant survival advantage with SRS was established. For patients with more than one BM, the survival advantage still remains unclear. Prognostic scoring systems have been designed to help clinicians determine patients with a good prognosis, for who intensive treatment of BM could be useful. Most frequently used prognostic scoring systems are RTOG RPA, BS-BM, GPA, SIR and GGS. RTOG RPA is seen as the standard prognostic scoring system. Lately, interest in primary site-specific prognostic factors has increased and primary site-specific prognostic scoring systems have been developed.
The aim of our study is to investigate whether CERENAL (a new prognostic scoring system, established in RCC patients by our group) can be used as a prognostic factor in a population with mixed primary tumors, and in a lung cancer subpopulation.
METHODS: One hundred and eighty patients receiving cranial radiotherapy for BM between January 2010 and December 2017 at Ghent University Hospital have been included in our retrospective, single-center study. For those patients, the RPA, BS-BM and CERENAL score were determined when possible. Patients and BM demographics were established. Associations between the prognostic scoring systems and different parameters were determined using Fisher exact or Chi square test. Univariate, multivariate and survival analysis were performed to determine prognostic factors and the relevance of the three prognostic scoring systems.
RESULTS: Of the 180 BM patients, 71 (39.4%) were lung cancer patients. Median OS for all patients was 6.3 months and median OS for the lung cancer patients was 5.4 months. Median DSS for all patients was 6.1 months, and median DSS for the lung cancer patients was 5.8 months. Following prognostic factors associated with OS in the whole population could be determined in univariate analysis: KPS, age at diagnosis of primary tumor, nodal stage of primary tumor, age at
2
moment of BM diagnosis, time between primary tumor and BM, presence of liver metastases at moment of BM diagnosis, brain surgery, WBRT, total WBRT dose and SRS. All three prognostic scoring systems were significantly associated with OS in the whole population (RPA: p < 0.001, BS-BM: p < 0.001, CERENAL: p < 0.001), as well as in the lung cancer population (RPA: p < 0.001, BS-BM: p = 0.005, CERENAL: p = 0.005). The three prognostic scoring systems were also significantly ? zin klopt hier niet associated with DSS in the whole population (RPA: p = 0.002, BS-BM: p < 0.001, CERENAL: p < 0.001) and in the lung cancer population (RPA: p < 0.001, BS-BS-BM: p = 0.001, CERENAL: p = 0.004). After multivariate analysis, only CERENAL remained significantly associated with OS (p < 0.001) and DSS (p < 0.001) in the whole population. In the lung cancer population, only RTOG RPA class III (p < 0.001) was significantly associated with OS, and RTOG RPA class III (p < 0.001) and BS-BM (p = 0.025) were significantly associated with DSS in multivariate analysis.
CONCLUSION: We can state that CERENAL is a reliable prognostic scoring system for a mixed population of primary cancers with brain metastases in our hospital, even better than the widely used RTOG RPA and BS-BM. For a lung cancer sample size, CERENAL may be an accurate prognostic scoring system, but further investigation is necessary to confirm the value of CERENAL in a larger cohort.
1.2.
D
UTCH ABSTRACT
INLEIDING: Hersenmetastasen zijn geassocieerd met een slechte prognose. Goede behandeling kan de prognose verbeteren, maar het is nog onduidelijk welke behandeling de beste is. De meeste ervaring is er met WBRT (whole-brain radiotherapy), maar WBRT kan schadelijk zijn voor het hersenweefsel en neurologische bijwerkingen geven. SRS (stereotactic radiosurgery) kan hiervoor een oplossing bieden, al is het nog onduidelijk of SRS zorgt voor een goede lokale controle en eveneens een overlevingsvoordeel biedt ten opzichte van WBRT. Er zijn prognostische scoresystemen ontwikkeld om patiënten in te delen naargelang prognose, om te bepalen of intensieve behandeling nog een voordeel kan bieden. De meeste gekende zijn RTOG RPA, BS-BM, SIR, GPA, GGS. Er zijn ook prognostische scoresystemen per type primaire tumor. Het doel van deze studie is om te bepalen of CERENAL, een nieuw prognostisch scoresysteem ontwikkeld door onze groep, kan gebruikt worden in een populatie van gemengde primaire tumoren, en bij longkankerpatiënten met hersenmetastasen.
3
METHODE: Honderdtachtig patiënten met hersenmetastasen die in het UZ Gent behandeld werden met hersenbestraling, werden in deze studie geïncludeerd. Bij wie mogelijk werden de RPA, BS-BM en CERENAL score bepaald. Demografische gegevens (patiënt- en ziektekenmerken) werden berekend. Met Fisher exact of Chi square werden associaties tussen verschillende parameters en de prognostische scoresystemen bepaald. Univariate en multivariate analyse werden uitgevoerd om prognostische factoren en de relevantie van elk scoresysteem te bepalen.
RESULTATEN: Eenenzeventig van de 180 patiënten (39.4%) waren longkankerpatiënten. Mediane OS (overall survival, algemene overleving) voor alle patiënten en voor de longkankerpatiënten waren respectievelijk was 6.3 en 5.4 maanden. Mediane DSS (disease-specific survival, ziekte-specifieke overleving) waren respectievelijk 6.1 en 5.8 maanden. Deze prognostische factoren konden bepaald worden in univariate analyse: KPS, leeftijd bij diagnose van primaire tumor, N-stadium van de primaire tumor, leeftijd bij diagnose van hersenmetastasen, tijd tussen diagnose van primaire tumor en van hersenmetastasen, aanwezigheid van levermetastasen bij diagnose van hersenmetastasen, hersenchirurgie, WBRT, totale dosis WBRT en SRS. De 3 prognostische scoresystemen waren significant geassocieerd met OS in de volledige populatie (RPA: p < 0.001, BS-BM: p < 0.001, CERENAL: p < 0.001) en in de longkanker populatie (RPA: p < 0.001, BS-BM: p = 0.005, CERENAL: p = 0.005), alsook met DSS in de volledige populatie (RPA: p = 0.002, BS-BM: p < 0.001, CERENAL: p < 0.001) en in de longkanker populatie (RPA: p < 0.001, BS-BM: p = 0.001, CERENAL: p = 0.004). In de multivariate analyse bleef enkel CERENAL significant geassocieerd met OS (p < 0.001) en DSS (p < 0.001) in de volledige populatie. In de longkanker groep bleef enkel RTOG RPA klasse III (p < 0.001) significant geassocieerd met OS, en RTOG RPA klasse III (p < 0.001) en BS-BM (p = 0.025) met DSS. CONCLUSIE: CERENAL is een goed prognostisch scoresysteem in een populatie met verschillende primaire tumoren met hersenmetastasen, het lijkt zelfs een betere prognostische waarde te hebben dan de vaak gebruikte RTOG RPA en BS-BM. Voor de longkankergroep zou CERENAL ook nuttig kunnen zijn, maar om deze te bevestigen in multivariate analyse is nog verder onderzoek met grotere populaties nodig.
4
2. I
NTRODUCTION
2.1.
B
RAIN METASTASIS
Brain metastases (BM) are frequently observed in patients with solid tumors. They are estimated to occur in 10 to 20% of these patients during the course of their disease, although reported incidences sometimes rise up to 45% (1-3). The increasing incidence can be explained by the rising incidence of primary malignancies and improvement in systemic therapies. This leads to more long-term survivors and a prolonged survival. Metastatic tumors can be controlled for years, which means BM have more chances to develop. Besides, better visualization techniques, such as MRI, can contribute to BM being detected more frequently (4, 5). The most frequently associated primary malignancies are lung cancer (incidence of 16.3%), breast cancer (5.0%), melanoma (7.4%), renal cancer (9.8%) and colorectal cancer (1.2%) (2, 5-8). Incidences may be underestimated due to asymptomatic patients. In adults, BM are the most common intracranial neoplasms (4, 5, 9). They are best visualized with MRI, which is a sensitive but non-specific technique to detect brain metastases. BM can present with fatigue, headache, seizures, focal weakness or numbness and cognitive impairments (10-12). BM can also be asymptomatic in leading to accidental discovery on MRI for other reasons or during screening when starting a certain systemic therapy. BM have to be distinguished from primary tumors of the brain, abscesses, vascular and inflammatory lesions (10).
2.2.
B
RAIN METASTASIS TREATMENT
Historically, BM are associated with a very poor survival. Without treatment, patients succumb to the disease in a few weeks. Use of corticoids can prolong the survival to 10 weeks after diagnosis. It should be noted that the association between the poor median overall survival (OS) and the use of corticoids, is because of its use as a palliative treatment, to comfort the patient (13). Apart from corticoids to alleviate the symptoms, there are a variety of treatment modalities that can be used to treat brain metastases. Most commonly used are whole-brain radiation therapy (WBRT), stereotactic radiosurgery (SRS), a combination of WBRT and SRS, surgery and more recently also systemic therapies. There is no clear evidence on which treatment modality is best for maximizing overall survival (OS), although there have been subgroups identified with a better prognosis when being treated with a certain therapy.
5
WBRT has been the widely used standard of care for BM. WBRT irradiates the whole brain. Median OS is 4 to 6 months (6). It has been the standard treatment strategy because of the hematogenous dissemination to the brain. This means that the entire brain may be seeded with micro-metastases, which are (possibly) not visible on MRI, and are also targeted via WBRT (14). However, there are concerns regarding the neurocognitive deterioration caused by WBRT, as a result of late radiation toxicity (15). As patients with brain metastasis live longer due to a better systemic disease control, the importance of this problem rises (6, 15).
SRS is an alternative to WBRT. It is a high precision localized irradiation with high doses on the tumor and low risk of damage to surrounding brain tissue (4, 6). There is a rapid dose fall in order to minimize the risk of damage to the surrounding tissue. The role of SRS is expanding due to the neurocognitive deterioration caused by WBRT (16). Historically, SRS was primarily used to treat a single brain metastasis, whereas WBRT was used to treat multiple brain metastases. Another treatment modality is WBRT in combination with a SRS boost, to improve the local control and to minimize the risk of local recurrence.
Surgical resection is another local therapeutic option that can result in a reduction of neurological deficits and seizures and in immediate relief of symptoms of intracranial hypertension. An important advantage is the possibility of histological diagnosis, which is important if the primary tumor is unknown or uncertain (17). Possible complications are bleeding and leptomeningeal dissemination (4). However, major complications have shown to be extremely rare. Incomplete resection leads to a high number of local recurrences and must be avoided (18).
More recently, there has come interest in systemic therapies to treat BM. Chemotherapy, targeted agents and immunotherapy have shown promising efficacy against BM but are not yet used routinely as a treatment for BM (4, 19).
2.2.1.
O
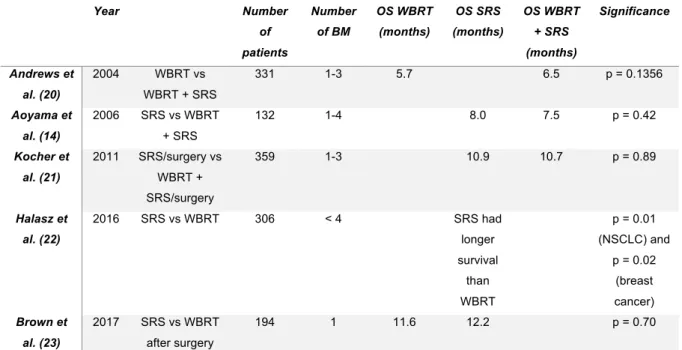
VERALL SURVIVAL AND LOCAL CONTROLThere is no clear evidence that one strategy may be preferred over another, although many researchers tried to define the best treatment for brain metastases. Several large randomized trials have been performed in order to determine the most optimal treatment modality. An overview is given in Table 1 (see addendum).
6
In 2004, Andrews et al. (20) published the results of the RTOG 9508 randomized phase III trial. From January 1996 to June 2001, 331 patients were assigned to either WBRT and SRS or WBRT alone. A survival advantage was shown for patients with a single BM in the WBRT + SRS group. Patients in the WBRT + SRS group were also more likely to have a better Karnofsky Performance Status (KPS) and a better local control than patients treated with WBRT alone. Nevertheless, there was no significant survival benefit for all patients with brain metastases treated with WBRT + SRS versus WBRT alone (median OS of 6.5 months versus 5.7 months, respectively) (20).
In 2006, a randomized controlled trial involving 132 patients with 1 to 4 BM compared treatment with WBRT + SRS versus SRS alone. The respective median OS were 7.5 months and 8.0 months, however this survival benefit of SRS alone was not found to be significant. Moreover, in this trial, intracranial relapse occurred less frequently in the WBRT + SRS group (p < 0.001) (14).
A phase III trial in 2011 failed to show a significant survival benefit for patients treated with WBRT + SRS versus observation after SRS. This trial assessed 359 patients with 1 to 3 BM from solid tumors with a good performance status. The median OS was 10.9 months in the SRS + WBRT group, and 10.7 months in the SRS + observation group (p = 0.89). However, it has been shown in this work that WBRT added to SRS may reduce the relapse rate at initial brain sites (31% to 19%, p = 0.040) and at new sites (48% to 33%, p = 0.023) (21).
Based on these trials, the American Society for Radiation Oncology developed guidelines for clinical practice in 2012. They stated that the management of patients with BM depends on the number of BM and the estimated prognosis, emphasizing the importance of good prognostic factors. They do not state one particular prognostic scoring system to assess the prognosis of BM patients, but mostly propose the diagnosis-specific Graded Prognostic Assessment (ds-GPA), as clinicians should consider histology-specific prognostic factors. Their conclusion was that any radiotherapeutic intervention, whether it is WBRT or SRS, is associated with an improved brain control. Only in single BM patients, SRS or surgery has been found to improve survival compared to WBRT alone and must have priority (13).
In 2016, Halasz et al. (22) conducted a large study involving 400 non-small cell lung cancer patients (NSCLC) and 387 breast cancer patients in a prospectively collected longitudinal database, to determine if there was any difference in treatment with SRS versus WBRT. For both NSCLC (p = 0.01) and breast cancer (p = 0.02) patients, patients with less than 4 BM, < 4 cm in size, treated
7
with SRS had a significant better survival compared to patients treated with WBRT. Thus, their main finding was that SRS is associated with an improved survival (22).
The review of Khan et al. (9) published in 2017 stated an improvement in survival for patients with a single BM treated with WBRT and SRS compared to WBRT only. However, in patients with multiple BM, no significant better survival was found comparing SRS only, WBRT only and SRS + WBRT. In all patients with BM, the addition of SRS to WBRT resulted in a better local tumor control rate when compared to WBRT alone. SRS alone resulted in a higher local tumor control failure and more distant brain recurrences as compared to WBRT + SRS (9).
Another phase III trial including patients with resected BM treated with WBRT or SRS showed no difference in overall survival, however decline in cognitive function occurred more frequently with WBRT. They stated that SRS should be considered as standard of care after resection of BM, as a less toxic alternative to WBRT (23).
Based on a review on the different treatment modalities described in literature, the European Association of Neuro-Oncology (EANO) published guidelines for treatment of BM from solid tumors in 2017. Two phase III trials reported a survival benefit for patients treated with surgery following WBRT compared to WBRT alone. A longer time of functional independence and lower rate of intracranial relapse was achieved in patients who received surgery. In conclusion, they stated that patients with good performance status and controlled systemic disease, benefit from a survival advantage when surgery is added to WBRT.
SRS on the other hand, yielded a local control at one year of 80-90% with symptom improvement in the treatment of a limited number (1 to 3) of recently diagnosed BM. SRS can also be used for the treatment of elderly patients (≥ 80 years), as they respond as well as younger patients. Three phase III trials and a meta-analysis compared the omission versus the addition of WBRT to surgery or SRS. When adding WBRT to surgery or SRS in patients with a limited number of BM, there is a better local and distant control, however, there is no better overall survival.
A recent meta-analysis of 3 randomized controlled trials comparing SRS + WBRT to SRS alone stated a survival advantage of SRS alone in patients under 50 years. The addition of WBRT did not diminish the risk of developing new BM. On the contrary, in patients over 50 years, the addition of WBRT diminished the risk of developing new BM but did not affect overall survival. Adjuvant WBRT following surgery decreased local and distant recurrences in patients with BM > 3 cm and/or active systemic disease.
8
When comparing surgery with or without postoperative SRS, there is an advantage for postoperative SRS due to decreased local relapse while avoiding the cognitive sequelae of WBRT. One prospective phase II trial and various retrospective trials reported local control rates around 80% at one year. This suggests postoperative SRS is as effective as postoperative WBRT in achieving local control.
Nowadays, there is a tendency of treating BM more with chemotherapy and targeted therapies. There are high response rates in small-cell lung cancer (SCLC) as primary tumor to chemotherapy, intermediate response rates in breast cancer and NSCLC as primary tumor and low response rates in melanoma as primary tumor. The response rate of BM to chemotherapy reflects this sensitivity of the primary tumor, however, the response in the brain is not always comparable to the extracranial response due to minor changes in genetics of the tumor cells. The association of chemotherapy and radiotherapy does not improve survival but may improve response rates compared to radiotherapy alone. The response rates of BM to targeted agents seem to be higher than those observed after chemotherapy. Yet, most targeted agents are small molecules with a limited penetration to the blood-brain barrier. To overcome this limitation, second- and third-generation small-molecule inhibitors are being investigated (4).For melanoma patients with BM, combination of nivolumab and ipilimumab is seen as a promising treatment option. Forty-six per cent of the patients with asymptomatic BM who had not yet received local brain treatment, had a good intracranial response to combination therapy of nivolumab and ipilimumab (24). Another study investigated nivolumab or ipilimumab in combination with SRS as a treatment option for melanoma patients with BM. Six-months intracranial progression-free survival respectively was 69% and 48% (25). These results show that immunotherapy may become an important treatment option for BM, with or without local brain therapy.
2.2.2. Q
UALITY OF LIFERegarding quality of life, SRS can offer an advantage. Because of earlier detection due to MRI, some patients still have a very good performance status and a favorable prognosis. WBRT may have a negative impact on quality of life and on neurocognitive function (16). Moreover, a greater risk of decline in learning and memory function is observed when treating patients with SRS + WBRT (26). A randomized controlled trial has compared SRS + WBRT versus SRS alone in patients with 1 to 3 BM. The decline of neurocognitive functioning was more frequent after SRS + WBRT versus SRS alone. There was more deterioration in verbal fluency, immediate recall and delayed recall (4). Another phase III trial compared adjuvant WBRT versus observation after surgery or radiosurgery in patients with a limited number of BM, with health-related quality-of-life
9
(HRQOL) as endpoint. Patients in the observation arm had significant better HRQOL scores, which were clinically relevant. WBRT after surgery or radiosurgery may have a negative impact on some aspects of quality of life (27). A prospective study showed that there is a neurocognitive functioning impairment due to the BM themselves, but SRS does not have an additional unfavorable effect on neurocognitive functioning (11).
2.2.3. C
OST-
EFFECTIVENESSSRS alone has been proven to be cost-effective for up to 10 BM compared to SRS + WBRT. SRS may be cost-effective relative to WBRT, but this depends on the cost of SRS, the willingness-to-pay and the patient preferences (28).
2.2.4. W
HICH TREATMENT TO CHOOSE?
There is clearly still some discussion about when to treat patients and which treatment to choose. We can state that patients with a single BM have a survival benefit when treated with SRS only. For patients with multiple BM, it is not clear which treatment modality is most preferable. The combination of WBRT and SRS may be better in terms of reducing local recurrence. For improving OS, we cannot state that one treatment modality is superior to another. Surgery when possible might improve local control and functional independence, but it should be followed by WBRT or SRS. More recently, they started treating BM with chemotherapy and targeted therapy, but more research is needed to investigate the possible advantages. More research has also to be done for maximizing OS. In terms of quality of life and neurocognitive functioning, SRS is considered to be less harmful. There should be noted that most of the clinical trials did not use prognostic scoring systems to determine patients with best or worst survival. Analyses were performed on the whole BM patient population, while there can be a difference in treatment need according to the prognosis of the patient.
2.3.
P
ROGNOSTIC SCORING SYSTEMS
Over the last 20 years, several prognostic factors have been developed and investigated for patients with BM. Prognostic scoring systems are necessary to inform patients about their prognosis, but even more to make an adequate patient selection for treatment. Clinicians are frequently faced with the dilemma of which treatment is the most appropriate for which patient. Patients with a good performance status and limited disease are more likely to benefit from more aggressive approaches, while symptom control and palliative treatment is recommended for patients with advanced disease. Various prognostic scoring systems have been developed to help clinicians choose the right therapy in an evidence based manner (8, 29). Several scoring systems
10
have been listed in Table 2 (see addendum) with an overview of all prognostic factors included in each prognostic scoring system.
2.3.1. R
ECURSIVEP
ARTITIONINGA
NALYSISThe Recursive Partitioning Analysis (RPA) was developed in 1997 in three Radiation Therapy Oncology Group brain metastases trials. At that moment, new approaches such as SRS yielded promising results. Developers needed to identify if this was due to the therapy alone, or if part of the result could be attributed to patient selection. 1200 patients were enrolled in these trials, all of them were treated with WBRT. They were able to identify 4 prognostic factors positively associated with survival: Karnofsky Performance Status (KPS) of 70-100, absent/controlled primary tumor, age less than 60 years and no extracranial metastases. This led to 3 classes: RPA class I (patients with KPS ≥ 70, < 65 years of age with controlled primary disease and no extracranial metastases), RPA class III (KPS < 70) and RPA class II (all patients not included in class I or class III) (30). One problem with the RPA score is that most of the patients are subdivided in class II, which means that class II is a very heterogeneous population with varying clinical factors. This is a problem that most of the prognostic scoring systems share. Because of this problem, class II was redefined into three subclasses, after determining 4 factors favoring longer survival in a study of 2000 patients treated with Gamma Knife radiosurgery: KPS (90-100% versus 70-80%), tumor number (solitary versus multiple), extracranial metastases and primary tumor status (controlled versus uncontrolled). The subclass is defined by the sum of this prognostic factors: RPA class II-a has a score of 0 or 1, RPA class II-b has a score of 2 and RPA class II-c has a score of 3 or 4 (31).
2.3.2. S
COREI
NDEX FORR
ADIOSURGERYIn 2000, researchers designed another prognostic score system, the Score Index for Radiosurgery in Brain Metastases (SIR). Data of 65 patients treated with radiosurgery between 1993 and 1997 were retrospectively analyzed. SIR is obtained by the summation of 5 prognostic factors: age, KPS, extracranial disease status, number of brain lesions and largest brain lesion volume. Each prognostic factor is assigned a score between 0 and 2. The SIR may range from 0 to 10, with a prolonged expected survival for the higher scores (32).
2.3.3. B
ASIC-S
CORE FORB
RAINM
ETASTASESThe Basic-Score for Brain Metastases (BS-BM) was proposed in 2004, after analyzing 110 patients treated with radiosurgery between 1999 and 2003. It is composed of three prognostic factors: KPS, primary tumor control and presence of extracranial metastases. The BS-BM gives the patient a score between 0 and 3, 0 standing for worst expected survival and 3 standing for best expected survival (33).
11
2.3.4. G
RADEDP
ROGNOSTICA
SSESSMENTThe Graded Prognostic Assessment (GPA) was developed in 2008, to overcome limitations of the previously developed prognostic factors, as the authors stated. They analyzed the outcome of 1960 patients treated with WBRT or SRS. The GPA is composed of the prognostic factors age, KPS, extracranial metastases (none versus present) and number of BM (one, two to three or more than three). The score ranges between 4.0 for patients with best prognosis, and 0.0 for patients with worst prognosis (29).
2.3.5. G
OLDENG
RADINGS
YSTEMAnother frequently used prognostic score system is the Golden Grading System (GGS), which has also been developed in 2008. Researchers analyzed the outcome of 479 patients with one or multiple brain metastases treated with radiosurgery or radiosurgery + WBRT. The GGS divides patients into 4 groups (score 0-3), based on age (≥ 65 years), KPS (< 70) and presence of extracranial metastases (34).
2.3.6. C
OMPARISON OF PROGNOSTIC SCORING SYSTEMSAlthough many prognostic scoring systems have been developed, it remains unclear for clinicians which scoring system is best to use. Defining the most appropriate scoring system has been a topic of research since long time. In 2004, RPA, SIR and BS-BM were compared in a cohort of 110 patients treated with Gamma Knife radiosurgery. SIR and BS-BM were found to be significantly associated with survival (p = 0.031 and p = 0.043 respectively), whereas RPA was not. They proposed BS-BM for clinical use, because of its simplicity (33). In 2008, when the GPA was proposed, Sperduto et al. (29) revealed some limitations of the previously described prognostic indices: RPA and BS-BM do not include number of BM and SIR requires volume of the largest lesion at the time of radiosurgery, which is a treatment factor. After comparing the four prognostic scoring systems, they concluded that RPA and BS-BM are very similar in their capability of predicting prognosis. Consecutive levels of SIR failed to show survival differences. Their newly proposed GPA was as prognostic as the RPA and more prognostic than the other indices, therefore claiming the GPA to be the least subjective, most quantitative and easiest to use prognostic index (29). In a validation study of the GPA, the GPA (p = 0.03) was proven to have an equally good prognostic prediction performance as the BS-BM (p = 0.001) and as the RPA (p = 0.02) (35). When testing RPA, SIR, BS-BM, GPA and modified RPA in stereotactic radiosurgery, there were significant differences between each two adjacent classes, except for GPA score 3.0 and 3.5-4.0 SIR showed best results for predicting preservation of neurological function. RPA, SIR and GPA had large discrepancies in patient numbers among groups, which makes it more difficult to use in
12
clinical practice whereas the modified RPA has a well-balanced patient distribution (36). In 2013, clinical utility of prognostic scoring systems in patients with BM treated with radiosurgery has been reinvestigated. The RPA, SIR, BS-BM, GPA, DS-GPA and GGS were all found to correlate highly with survival. The unfavorable classes of the SIR, BS-BM and GGS performed best in predicting early death. The favorable classes of the GGS performed best in predicting long term survival, although the differences between prognostic indices are modest. All prognostic scoring systems had the limitation of an unbalanced number of patients within each prognostic class. In conclusion, they stated that the RPA remained the standard (37). One reason for these different outcomes might be that some studies used rather small patient populations. Also, there were differences in patient population, which may affect the outcomes (35). After many studies, it is still not clear which is the most optimal prognostic index to use. All have their benefits and limitations, but the perfect prognostic index has not been developed yet.
2.3.7. P
RIMARY SITE-
SPECIFIC PROGNOSTIC SCORING SYSTEMSMore recently, prognostic grading systems varying by primary site have been developed. Primary tumors have different factors determining prognosis, due to different behavior of BM depending on primary site. That means no prognostic scoring system would be applicable to all BM patients. For this reason, different prognostic scoring systems are needed. At first, researchers defined the Diagnosis-Specific GPA (DS-GPA). They defined the significant prognostic factors for NSCLC, SCLC, melanoma, RCC, gastro-intestinal and breast cancer. NSCLC and SCLC had four significant prognostic factors: age, KPS, presence of extracranial metastases and number of BM. RCC and melanoma had two significant prognostic factors: KPS and number of BMs. For both gastro-intestinal and breast cancer, KPS was the only significant prognostic factor. This confirms that the original GPA is a good prognostic score system for NSCLC and SCLC, as it contains all 4 of the prognostic factors. The DS-GPA for RCC, melanoma, breast and gastro-intestinal cancer consist of all their respective significant prognostic factors (38).
NSCLC, breast cancer, melanoma and RCC are the four most common cancers that tend to metastasize to the brain (2, 5-8). More research has been done for those cancers to find adequate prognostic scoring systems.
Most of the prognostic factors, like RPA, GPA and BS-BM have been confirmed for NSCLC (39). One reason might be that, however those prognostic factors were developed in a general population of patients with BM, in most studies, NSCLC patients were relatively more represented than other primary tumors (40). Besides that, most of the prognostic significant factors for survival in NSCLC patients (KPS, age and extracranial metastases) were are incorporated in most scoring
13
systems (38, 41). Histology type was also found to be a significant prognostic factor. Adenocarcinoma histology was confirmed in two studies to be favoring longer survival, in patients treated with WBRT as well as in patients treated with SRS (42, 43). This has led to the investigation of a new prognostic index, the lung-molGPA. This prognostic scoring system incorporates EGFR and ALK alterations in patients with adenocarcinoma, plus the four original factors used in the DS-GPA (age, KPS, extracranial metastases and number of BM) (44, 45). For non-adenocarcinomas, mutation status is not routinely tested but the four original prognostic factors of the DS-GPA could be confirmed for non-adenocarcinomas (44).
For BM of breast cancer, the originally developed prognostic scoring systems seem not to be sufficient, most likely due to the rather small percentage of breast cancer patients in those trials (46). The RPA index was based on 1200 patients from three RTOG trials, but only 12% of them were patients with breast cancer (30), and a comparison of four prognostic indices on 1960 patients from five RTOG trials included only 11% patients with brain metastases from breast cancer (29). However, in one group of 83 patients with BM from breast carcinoma, the prognostic value of RPA and SIR was confirmed. Other factors found to be prognostic for poor survival were KPS < 70, presence of extracranial metastases, presence of more than one BM and interval from diagnosis of primary tumor to diagnosis of BM < 38 months. They did not find significance for hormone receptor status and age (47). Conversely, HR-negative status has been proven to be an independent prognostic factor for poor survival in a study with 117 patients exclusively treated with WBRT. Other prognostic factors for poor survival were RPA class III and lymphopenia (≤ 0.7 x 109/L). Those 3 prognostic factors were constructed into a 3-tiered prognostic scoring system,
which confirmed the prognostic value of the RPA (48). Another study with 120 BM breast cancer patients confirmed the prognostic value of performance status (PS) and lymphopenia (49). Among 125 breast cancer patients, poor PS, HER2-positivity and no additional systemic treatment have been found to be significant risk factors for poor outcome. Those 3 prognostic factors were made into a 4-tiered prognostic scoring system (50). However, the best prognostic scoring system remains uncertain and a trial with a bigger amount of breast cancer patients with BM is needed (40, 46-50).
In 2018, a study of 422 melanoma patients with BM treated with SRS, compared the RPA, SIR, BS-BM and the original DS-GPA in their ability of predicting prognosis for melanoma patients. They faced the problem that RPA, SIR and BS-BM had large discrepancies in their patient numbers assigned to each score group. 82% of the patients were classified as RPA class II, 76% as SIR 4-7 and 64-7% as BS-BM 1. When comparing pairs of adjacent classes, RPA, SIR and BS-BM were found to be significant, except from BS-BM 3 versus BS-BM 2. The DS-GPA had the advantage of
14
a better distribution of patients into each score group, and was found to be more predictive of OS (51). The original DS-GPA, consisting of the prognostic factors KPS and number of BM, has been reviewed in a larger cohort of 823 patients treated with different therapies. Five prognostic factors have been identified as significant in predicting survival: age, KPS, extracranial metastases, number of BM and BRAF status. They were formed into the melanoma-molGPA (52).
The original DS-GPA for RCC consisted of two prognostic factors: KPS and number of BM (38). In 2017, there has been added one prognostic factor to the score: cumulative intracranial tumor volume (CITV) seemed to be significant for predicting survival. Increased CITV is not exactly the same as the number of BM, since the researchers found that increased CITV was correlated with decreased KPS, but they could not find any association between number of BM and KPS. One disadvantage of CITV as prognostic factor, is that the volumes of BM are usually calculated during dosimetric planning of SRS (53). However, prognostic indices are necessary to predict outcome before, and not after treatment decisions are made (29). The DS-GPA for RCC has also been reviewed. In 2018, Sperduto et al. (54) selected a cohort of 711 RCC patients with BM, to form the renal-GPA: KPS, number of BM, extracranial metastases and hemoglobin (54).
2.3.8. CERENAL
Recently, a new prognostic scoring system has been developed in a cohort of 75 RCC patients treated with targeted therapy in our centre. CERENAL is based on the prognostic factors used for the RPA and BS-BM score and findings in literature showing that SRS and a low number of BM may prove a survival benefit for metastatic RCC patients. Table 2 shows the included prognostic factors. It is a score ranging between 0 and 6, with a score of 0 associated with the best survival and a score of 6 associated with the worst survival. Statistical analysis revealed CERENAL, RPA and BS-BM to be comparable to each other in this RCC population. Despite the similarity, CERENAL showed better prognostic potential in this RCC patients population when compared to RPA and BS-BM (55).
2.4.
O
BJECTIVE
The aim of this study is to investigate whether CERENAL, next to RTOG RPA and BS-BM, can be recommended as a reliable prognostic scoring system in a mixed population of patients with BM. In addition, the prognostic value of these prognostic biomarkers will be determined in a subgroup of lung cancer patients with BM.
15
3. P
ATIENTS AND METHODS
3.1.
L
ITERATURE STUDY
A comprehensive literature search was performed to identify articles related to treatment and prognostic factors of brain metastases. A search of the database PubMed was conducted between September and December 2018, using the following Mesh terms: ‘brain neoplasms/radiotherapy’, ‘brain neoplasms/secondary’, ‘prognosis’, ‘radiosurgery’, ‘cranial irradiation’, ‘carcinoma, Non-Small-Cell Lung’, ‘carcinoma, Renal Cell’, ‘melanoma’, ‘breast neoplasms’. These terms were combined to maximize the results. Particular attention was dedicated to reviews and larger randomized clinical trials. Additional searches of the cited references from primary articles were conducted to expand the search. Google Scholar was used to include recent articles that cited the primary articles.
3.2.
D
ESIGN AND SETTING
This 8-year single center retrospective study was conducted at Ghent University Hospital. The main objective was to confirm the prognostic scoring system CERENAL for a mixed population of primary tumors. For this, data extraction from electronic patient files was needed to create a new database. Approval from the Ethical Committee of the Ghent University Hospital was obtained prior to this data extraction.
3.3.
D
ATABASE AND DATA EXTRACTION
Data on patients with BM undergoing therapy for their BM in the Ghent University Hospital have been collected retrospectively. Possible treatment modalities were surgery, whole brain radiation therapy and stereotactic radiotherapy. The database includes all patients who started local therapy for the BM between January 2010 and December 2017
3.3.1. I
NCLUSION AND EXCLUSION CRITERIAInclusion criteria
Patients with confirmed BM undergoing whole brain radiotherapy and/or stereotactic radiotherapy in the University Hospital of Ghent in the period between January 2010 and December 2017 were included. This was done independently from the type of primary tumor.
Exclusion criteria
Patients undergoing treatment for brain diseases other than BM, such as meningioma and neuroblastoma, were excluded. Patients who underwent systemic treatment in hospitals other than Ghent University Hospital, of whom we could not collect enough data, were also excluded.
16
3.3.2. D
ATA COLLECTIONFollowing data were collected:
- Patient characteristics: Gender, date of birth, age at diagnosis of primary tumor, KPS and ECOG PS at diagnosis of BM, KPS and ECOG PS at start of systemic treatment, date of last follow-up, time diagnosis primary tumor to last follow-up, date of death and disease-specific death
- Tumor-related characteristics: Date of diagnosis of primary tumor, histology and type of primary tumor, tumor grade differentiation, tumor stage, nodal stage, surgery of primary tumor, type of surgery and metastasis at diagnosis of primary tumor
- Metastasis-related characteristics: Liver metastases, bone metastases, adrenal metastases, lung metastases, lymph nodes and other metastases
- Brain metastasis-related characteristics: Date of first BM, age, time primary tumor to first BM, neurologic symptoms, number and localization
- Biochemical data at diagnosis of BM and before start of systemic treatment (fist-line,
second-line and third-line): Hemoglobin, lymphocyte count, neutrophil count, CRP,
lactate dehydrogenase, calcium, alkaline phosphatase and albumin
- Systemic treatment-related characteristics: First-line, second-line, third-line, type of treatment, start and stop date, duration, progressive disease (PD) at stop and PD at BM at stop
- Local therapy for BM: Brain surgery, WBRT, SRS, dose, start date, device, number of lesions treated and dose/fraction
3.4.
S
TATISTICAL ANALYSIS
3.4.1. SPSS
All patients were analyzed as one group, as well as a sub-analysis of patients with lung cancer, representing the most common tumor causing brain metastasis in our population, was conducted. Other common tumors (breast, melanoma, RCC) were not analyzed as a subgroup due to the limited sample size for statistical analysis. To avoid small groups, BS-BM and CERENAL were dichotomized for statistical analysis, as previously described in literature (55). The low BS-BM score contains the BS-BM scores 0 and 1; and the high BS-BM scores contains the BS-BM score 2 and 3. The low CERENAL score contains the CERENAL scores 0, 1, 2 and 3; and the high CERENAL score contains the CERENAL scores 4, 5 and 6.
Chi square en Fisher’s Exact test were used to determine significance between the three prognostic factors and the clinical parameters. The hazard ratio (HR) of each prognostic score on overall
17
survival (OS), disease-specific survival (DSS) and first-line progression-free survival (PFS) was determined by a 2-sided log-rank (Mantel–Cox) test. PFS was calculated from time from systemic treatment initiation until radiographic progression or discontinuation due to adverse events whereas OS and DSS were calculated from time from brain metastasis diagnosis until day of death or last follow-up. Data was reported as survival time (95% confidence interval = 95%CI) unless otherwise stated. Patients that were lost to follow up were censored in the survival analysis. Survival curves were plotted using the Kaplan–Meier method. Univariate analysis using 2-sided log rank (Mantel-Cox) was used to determine prognostic factors associated with OS and DSS. The covariate effect of the survival risk factors (reaching a P value < 0.05 on univariate test), was determined via Cox proportional hazard model (backward method). P values < 0.05 were considered statistically significant. Analyses were performed with SPSS v25.0.
18
4. R
ESULTS
4.1.
P
ATIENT CHARACTERISTICS
A total of 180 patients receiving radiotherapy for brain metastasis from 2010 up to 2018 were included. Median (range) age at diagnosis of primary tumor was 59 years (20-80). Seventy-one patients (39.4%) were suffering from lung cancer, 30 (16.7%) from breast cancer, 27 (15.0%) from melanoma, 20 (11.1%) from RCC and 29 (16.1%) patients suffered from a different primary tumor. In 3 patients (1.7%), the primary tumor could not be determined. A total of 99 patients (55.0%) were metastasized at moment of primary tumor diagnosis, with lymph nodes being the most important site of metastasis (21.7%). An overview of patient characteristics is given in table 3 and an overview of characteristics of lung cancer patients is given in table 4 (see addendum). The patient characteristics of the other primary tumor groups were not determined separately due to the limited subgroup sample size.
4.2.
B
RAIN METASTASIS CHARACTERISTICS
Median (range) age at diagnosis of BM was 60.5 years (21-81). Median (range) time between diagnosis of primary tumor and BM diagnosis was 14.5 months (0-247.1). Most patients were also suffering from extracranial metastases at the moment of BM occurrence, with lung being the most frequent metastatic site (79 patients, 43.9%). One hundred and four patients (57.8%) had 3 or more BM at diagnosis of the BM, causing important consequences concerning treatment options. In 72 patients (40.0%) metastases occurred both supratentorial and infratentorial. Almost every patient (n = 171, 90.0%) received WBRT as a local therapy for the BM, with a median (range) total dose of 20 Gy (4-60 Gy). More details about brain metastasis characteristics are shown in table 5 and in table 6 (for lung cancer subgroup) (see addendum).
4.3.
S
TATISTICAL ANALYSIS
4.3.1. P
ROGNOSTIC SCORING SYSTEMSThe prognostic scoring systems RTOG RPA, BS-BM and CERENAL were calculated for every patient when possible, using the prognostic factors indicated in table 2. Due to missing data in the electronic patient record, not all prognostic scoring systems could be established for every patient. The RTOG RPA score could be calculated in 140 patients, BS-BM in 111 patients and CERENAL in 146 patients. The association between the prognostic scoring systems and different clinical parameters is shown in table 7 (see addendum). Very strong association was found between CERENAL and the following parameters: liver, lung and bone metastases, all at moment of BM
19
diagnosis, number of BM and use of SRS. This might be explained by the criteria used for calculation of CERENAL. Extracranial metastases, number of BM lesions and received SRS are included in the CERENAL score, which may cause the very strong association. With RPA, a strong association was found with liver, lung, bone, lymph node and other metastases, all at moment of BM diagnosis. With BS-BM, a strong association was found with liver, bone and lung metastases at moment of BM diagnosis. Associations with both RPA and BS-BM might be explained by the importance of extracranial metastases as a criterium for RPA and BS-BM score calculation. More associations can be found in table 7.
4.3.2. U
NIVARIATE STATISTICAL ANALYSISSummary of clinical parameters significantly linked with OS and DSS can be found in table 8 for the whole population and table 9 for the lung cancer patients. The most important results of the univariate analysis are summarized in table 10 (whole population) and table 11 (lung cancer patients) (tables: see addendum).
4.3.2.1. O
VERALL SURVIVAL(OS)
Mean (95%CI) OS of all 180 patients was 14.0 (9.91-18.02) months and median (95%CI) OS of all 180 patients was 6.3 (5.16-7.44) months.
CLINICAL PARAMETERS
Using the univariate Log Rank test, clinical parameters of the sample significantly linked with OS were determined. Relevant clinical parameters positively linked with OS were: KPS at moment of BM diagnosis (p < 0.001), brain surgery (p = 0.001), WBRT (p = 0.010), total WBRT dose (p < 0.001) and SRS (p < 0.001). Relevant clinical parameters negatively linked with OS were: age at diagnosis of primary tumor (p = 0.007), nodal stage (p = 0.040), age at diagnosis of BM (p = 0.006), time between diagnosis of primary tumor and diagnosis of BM (p < 0.001) and presence of liver metastases at moment of BM diagnosis (p = 0.002). All other parameters did not appear to be significantly linked with OS.
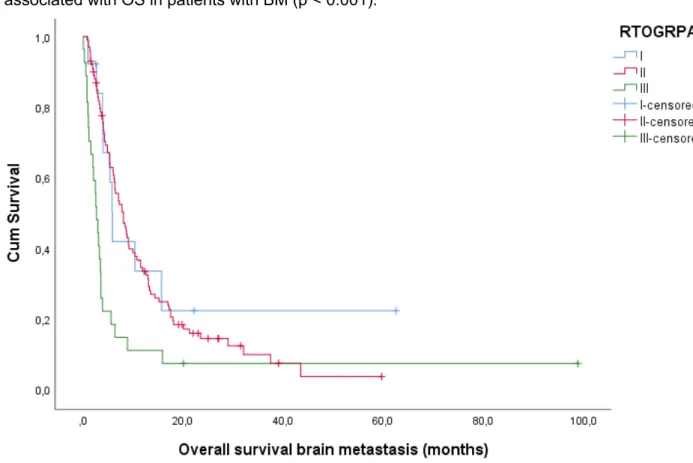
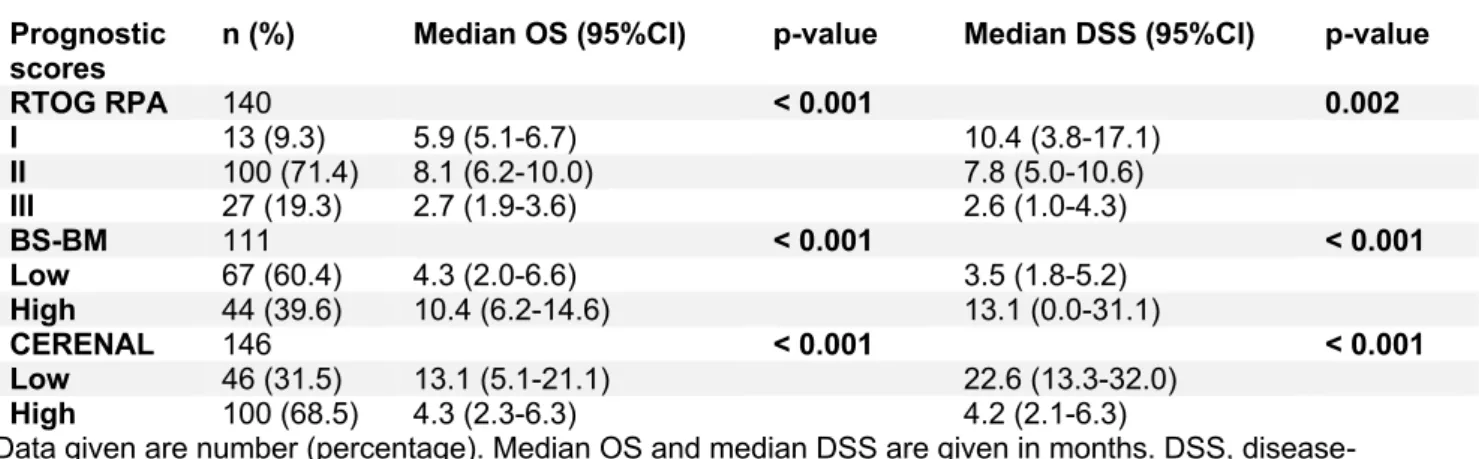
RTOG RPA
In 140 out of 180 patients, calculation of the RTOG RPA score was possible (13 were divided in RTOG RPA class I, 100 in RPA RTOG class II and 27 in RTOG RPA class III). Overall, mean survival time (95%CI) was 14.9 (10.15-19.71) months, while the median overall survival time was 6.3 (4.95-7.65) months. In class I, II and III, respectively 9 (69.2%), 85 (85.0%) and 25 (92.6%) patients died during follow up. Mean survival time was 19.0 (4.96-33.12), 13.0 (9.96-15.99) and
20
10.3 (0.77-19.82) months respectively, while median survival time was 5.9 (5.07-6.73), 8.1 (6.20-10.01) and 2.7 (1.85-3.55) months respectively. Log Rank confirmed RTOG RPA to be significantly associated with OS in patients with BM (p < 0.001).
Figure 1: Kaplan-Meier plot for OS according to RTOG RPA. Log Rank test (Mantel-Cox) is significant with p < 0.001.
BS-BM
In 111 out of 180 patients, calculation of the BS-BM score was possible. Sixty-seven patients had a low BS-BM score (BS-BM 0 or 1) and 44 patients had a high BS-BM score (BS-BM 2 or 3). The mean OS for all 111 patients was 16.5 (10.78-22.12) months. Median OS was 6.7 (5.20-8.20) months. In the low and high BS-BM score group, 61 (91.0%) and 32 (72.7%) patients died during follow up. In the low BS-BM score group, mean and median survival time were 11.6 (5.98-17.28) and 4.3 (1.96-6.64) months respectively. In the high BS-BM score group, mean and median survival time were 20.7 (14.33-27.16) and 10.4 (6.21-14.59) months respectively. Log Rank confirmed a significant association between BS-BM and OS in patients with BM (p < 0.001).
21 Figure 2: Kaplan-Meier plot for OS according to BS-BM. Log Rank test (Mantel-Cox) is significant with p < 0.001.
CERENAL
In 146 out of 180 patients, calculation of the CERENAL score was possible. Forty-six patients were found to have a low CERENAL score (CERENAL 0-3). One hundred patients had a high CERENAL score (CERENAL 4-6). Mean and median overall survival were 14.0 (9.60-18.33) and 6.2 (5.28-7.12) months respectively. In the low CERENAL score group, 34 patients died (73.9%). Mean survival time was 20.3 (14.08-26.44) months and median survival time was 13.1 (5.06-21.14) months. In the high CERENAL score group, 93 patients died (93.0%). Mean survival time was 9.7 (5.91-13.59) months and median survival time was 4.3 (2.29-6.31) months. Log Rank confirmed CERENAL to be significantly associated with OS in patients with BM (p < 0.001).
22 Figure 3: Kaplan-Meier plot for OS according to CERENAL. Log Rank test (Mantel-Cox) is significant with p < 0.001.
4.3.2.2. D
ISEASE-
SPECIFIC SURVIVAL(DSS)
Mean DSS of the 117 patients who died from a disease-specific death was 19.4 (12.54-26.32) months and median DSS was 6.1 (3.89-8.31) months.
CLINICAL PARAMETERS
As well as for OS, clinical parameters significantly linked with DSS were determined using the univariate Log Rank test. Relevant clinical parameters positively linked with DSS were: immunotherapy as second-line therapy (p = 0.039), KPS at moment of BM diagnosis (p = 0.001), brain surgery (p = 0.003), WBRT (p = 0.001), total WBRT dose (p < 0.001) and SRS (p < 0.001). Relevant clinical parameters negatively linked with DSS were: age at diagnosis of primary tumor (p = 0.006), nodal stage (p = 0.042), liver metastases at moment of primary tumor diagnosis (p = 0.005), adrenal metastases at moment of primary tumor diagnosis (p = 0.023), lymph node metastases at moment of primary tumor diagnosis (p = 0.041), age at diagnosis of BM (p < 0.001), time between diagnosis of primary tumor and BM diagnosis (p < 0.001), also liver metastases at moment of BM diagnosis (p = 0.001) and also adrenal metastases at moment of BM diagnosis (p = 0.002). All other parameters did not appear to be significantly linked with DSS.
23
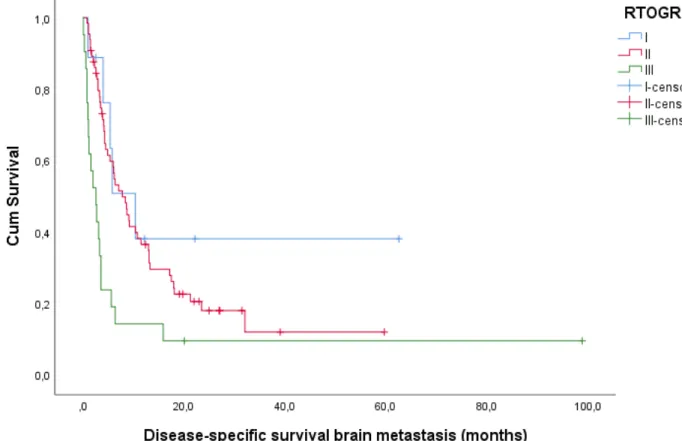
RTOG RPA
Mean and median disease-specific survival were 20.2 (12.44-27.89) and 5.8 (4.07-7.53) months respectively. In class I, II and III; 5, 50 and 19 patients died from a disease-specific death during follow up (55.6%, 76.9% and 90.5%). Mean DSS in class I, II and III were 27.2 (7.88-46.54), 15.4 (10.23-20.61) and 12.1 (0.00-24.23) months respectively. Median DSS were 10.4 (3.76-17.05), 7.8 (5.00-10.60) and 2.6 (0.96-4.25) months. Log Rank confirmed RTOG RPA to be significantly associated with DSS in patients with BM (p = 0.002).
Figure 4: Kaplan-Meier plot for DSS according to RTOG RPA. Log Rank test (Mantel-Cox) is significant with p = 0.002.
BS-BM
Mean and median DSS for all patients were 21.5 (12.94-30.11) and 6.4 (4.33-8.48) months. In the low BS-BM score group, 45 patients (88.2%) died from a disease-specific death, versus 16 patients (57.1%) in the high BS-BM score group. Mean and median DSS in the low BS-BM score group were 13.2 (5.69-20.63) and 3.5 (1.81-5.19) months, versus a mean and median DSS of 27.07 (16.73-37.40) and 13.1 (0.00-31.10) months in the high BS-BM score group. A significant association between BS-BM and DSS in patients with BM was confirmed by Log Rank (p < 0.001).
24 Figure 5: Kaplan-Meier plot for DSS according to BS-BM. Log Rank test (Mantel-Cox) is significant with p < 0.001.
CERENAL
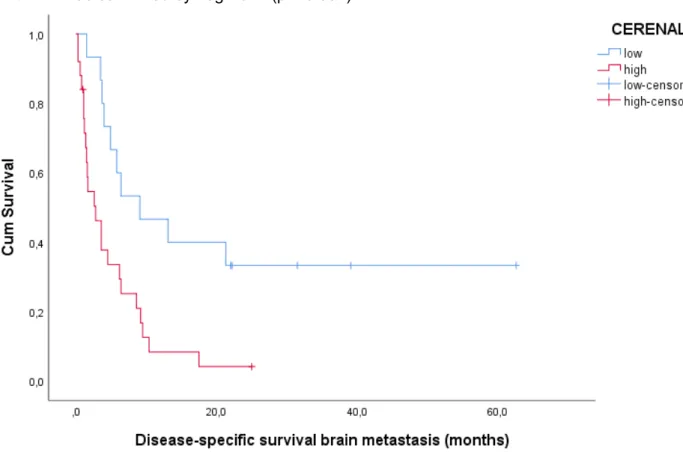
The mean and median DSS were 19.2 (11.92-26.54) and 6.2 (4.21-8.19) months. In the low and high CERENAL score group, respectively 15 (55.6%) and 60 (89.6%) patients died from a disease-specific death during follow-up. Mean and median DSS were 28.2 (17.66-38.67) and 22.6 (13.25-31.95) months in the low CERENAL score group, and 11.6 (5.83-17.35) 4.2 (2.06-6.34) months in the high CERENAL score group. Log Rank confirmed CERENAL to be significantly associated with DSS in patients with brain metastasis (p < 0.001).
25 Figure 6: Kaplan-Meier plot for DSS according to CERENAL. Log Rank test (Mantel-Cox) is significant with p < 0.001)
4.3.2.3. P
ROGRESSION-
FREE SURVIVAL(PFS)
RTOG RPA
For 34 patients receiving first-line therapy after the diagnosis of BM, calculation of the RTOG RPA score was possible. Five patients were divided in class I, 25 patients in class II and 4 patients in class III. For all patients, mean PFS time was 8.0 (2.74-13.13) months and median PFS time was 4.6 (3.43-5.86) months. In class I, II and III; 3 (60.0%), 19 (76.0%) and 3 (75.0%) patients showed progressive disease at the end of first-line therapy. Mean PFS time was 2.0 (1.50-2.47), 9.3 (3.06-15.59) and 2.5 (1.71-3.29) months and median PFS time was 1.9 (1.26-2.54), 4.9 (3.05-6.75) and 2.5 (1.38-3.62) months. Log Rank confirmed RTOG RPA to be significantly associated with PFS in patients with BM (p < 0.001).
26 Figure 7: Kaplan-Meier plot for PFS of first-line therapy according to RTOG RPA. Log Rank test (Mantel-Cox) is significant with p < 0.001.
BS-BM
For 40 patients receiving first-line therapy after the diagnosis of BM, calculation of the BS-BM score was possible. Nevertheless, Log Rank showed no significant association between BS-BM and PFS in patients with BM (p = 0.314).
CERENAL
For 43 patients receiving first-line therapy after the diagnosis of BM, calculation of the CERENAL score was possible. Nevertheless, Log Rank showed no significant association between CERENAL and PFS in patients with BM neither (p = 0.342).
4.3.2.4. O
VERALL SURVIVAL(OS)
–
SUB-
ANALYSIS LUNGMean OS of all 71 lung cancer patients was 12.5 (8.24-16.86) months and median OS of all 71 patients was 5.4 (3.69-7.11) months.
27
CLINICAL PARAMETERS
Using the univariate Log Rank test, clinical parameters of the lung cancer patients sample significantly linked with OS were determined. Relevant clinical parameters positively linked with OS in lung cancer patients were: KPS at moment of BM diagnosis (p < 0.001), type of surgery of primary tumor (p = 0.046) and total WBRT dose (p < 0.001). Relevant clinical parameters negatively linked with OS in lung cancer patients were: age at diagnosis of primary tumor (p < 0.001), grade of differentiation of primary tumor (p < 0.001), tumor stage (p = 0.003), nodal stage (p = 0.004), presence of liver metastases at diagnosis of primary tumor (p = 0.004), presence of adrenal metastases at diagnosis of primary tumor (p = 0.019), age at diagnosis of BM (p < 0.001), time between diagnosis of primary tumor and diagnosis of brain metastasis (p < 0.001), presence of liver metastases at moment of BM diagnosis (p = 0.001), presence of adrenal metastases at moment of BM diagnosis (p = 0.017) and progressive disease of BM at stop of first-line therapy (p = 0.036). All other parameters did not appear to be significantly linked with OS in lung cancer patients.
RTOG RPA
In 60 out of 71 lung cancer patients, calculation of the RTOG RPA score was possible. Ten patients were divided in RTOG RPA class I, 42 patients were divided in RTOG RPA class II and 8 patients were divided in RTOG RPA class III. The mean OS for all 60 patients was 13.7 (8.71-18.65) months and median OS for all 60 patients was 5.4 (3.58-7.22) months. In class I, II and III, respectively 7 (70.0%), 36 (85.7%) and 8 (100%) patients died during follow up. Mean survival time of lung cancer patients in class I was 22.3 (6.30-38.35) months and median survival time of lung cancer patients in class I was 5.9 (0.00-13.03) months. Mean survival time of patients in class II was 11.2 (7.62-14.76) months and median survival time was 6.2 (2.21-10.19) months. Mean survival time of lung cancer patients in class III was 2.6 (0.60-4.56) months and median survival time was 1.2 (0.09-2.31) months. Log Rank confirmed a significant association between RTOG RPA and OS in lung cancer patients with BM (p < 0.001).
28 Figure 8: Kaplan-Meier plot for OS in lung cancer patients according to RTOG RPA. Log Rank test (Mantel-Cox) is significant with p < 0.001)
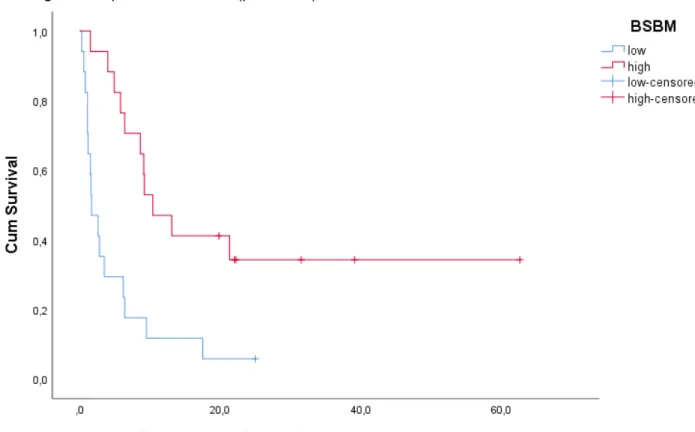
BS-BM
In 43 lung cancer patients out of 71, calculation of the BS-BM score was possible. Twenty-one patients were divided in the low BS-BM score group (BS-BM score 0 or 1) and 22 patients were divided in the high BS-BM score group (BS-BM score 2 or 3). Mean overall survival for all 43 lung cancer patients was 15.7 (9.33-22.00) months and median overall survival was 6.4 (2.80-10.00) months. In the low BS-BM score group, 20 lung cancer patients died (95.2%) during follow-up. Mean OS time in the low BS-BM score group was 6.1 (3.29-8.96) months and median OS time was 2.8 (0.11-5.49) months. In the high BS-BM score group, 16 lung cancer patients died during follow up (72.7%). Mean OS time in the high BS-BM score group was 23.0 (12.81-33.25) months and median OS time was 9.2 (4.03-14.37) months. A significant association between BS-BM and OS in lung cancer patients with BM was confirmed by Log Rank (p = 0.005).
29 Figure 9: Kaplan-Meier plot for OS of lung cancer patients according to BS-BM. Log Rank test (Mantel-Cox) is significant with p = 0.005.
CERENAL
In 58 out of 71 lung cancer patients, calculation of the CERENAL score was possible. Twenty-one patients were divided in the low CERENAL score group (CERENAL score 0, 1, 2 or 3), and 37 patients were divided in the high CERENAL score group (CERENAL score 4, 5 or 6). Overall, mean OS was 11.9 (7.18-16.56) months, while the median OS time was 4.9 (3.15-6.66) months. In the low and high CERENAL score group, respectively 16 (76.2%) and 35 (94.6) lung cancer patients died during follow-up. Mean OS in the low CERENAL score group was 20.7 (10.45-30.90) months and median OS time was 6.4 (0.72-12.08) months. Mean OS time in the high CERENAL score group was 5.7 (3.83-7.54) months and median OS time was 3.6 (2.08-5.12) months. Log Rank confirmed CERENAL to be significantly associated with OS in lung cancer patients with BM (p = 0.005).
30 Figure 10: Kaplan-Meier plot for OS in lung cancer patients according to CERENAL. Log Rank test (Mantel-Cox) is significant with p = 0.005.
4.3.2.5. D
ISEASE-
SPECIFIC SURVIVAL(DSS)–
SUB-
ANALYSIS LUNGMean and median OS for all 48 lung cancer patients who died from a disease-specific death, were 15.4 (9.01-21.70) months and 5.8 (3.83-7.77) months.
CLINICAL PARAMETERS
As well as for OS, clinical parameters significantly linked with disease-specific survival in lung cancer patients were determined using the univariate Log Rank test. Relevant clinical parameters positively linked with DSS in lung cancer patients were: KPS (p < 0.001), SRS (p = 0.050) and total WBRT dose (p < 0.001). Relevant clinical parameters negatively linked with DSS in lung cancer patients were: age at diagnosis of primary tumor (p < 0.001), grade of differentiation of primary tumor (p < 0.001), tumor stage (p = 0.002), nodal stage (p = 0.007), liver metastases at diagnosis of primary tumor (p < 0.001), adrenal metastases at diagnosis of primary tumor (p = 0.003), lymph node metastases at diagnosis of primary tumor (p = 0.017), age at moment of BM diagnosis (p < 0.001), time between diagnosis of primary tumor and BM diagnosis (p < 0.001), also liver metastases at moment of BM diagnosis (p < 0.001), also adrenal metastases at moment of BM
31
diagnosis (p = 0.003), also lymph node metastases at moment of BM diagnosis (p = 0.039) and progressive disease of BM at stop of first-line therapy (p = 0.011). All other parameters did not appear to be significantly linked with DSS in lung cancer patients.
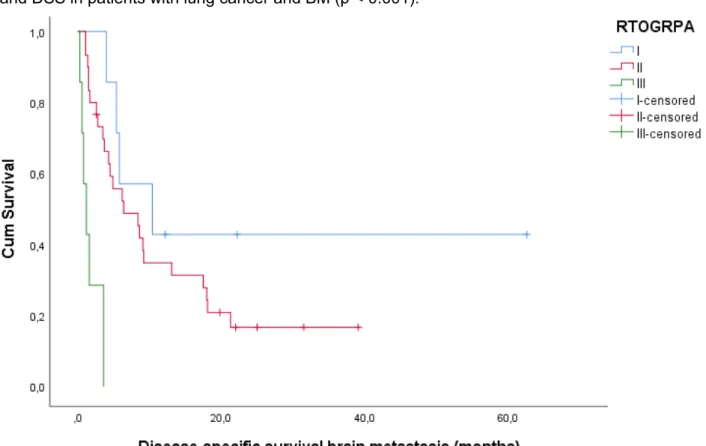
RTOG RPA
The mean and median DSS time of all 44 lung cancer patients were 16.0 (9.30-22.75) months and 5.4 (2.98-7.82) months. In class I, 4 patients died from a disease-specific death (57.1%). Mean DSS time in class I was 30.49 (9.84-51.13) months. Median DSS time was 10.4 (0.00-22.21) months. In class II, 24 lung cancer patients died a disease-specific death (80.0%). Mean DSS and median DSS for lung cancer patients in class II were 12.6 (7.80-17.43) months 6.4 (1.58-11.22) months. In class III, all 7 patients died a disease-specific way (100.0%) during follow-up. Mean DSS time for lung cancer patients in class III was 1.7 (0.65-2.70) months and median DSS time was 1.2 (0.17-2.23) months. Log Rank confirmed a significant association between RTOG RPA and DSS in patients with lung cancer and BM (p < 0.001).
Figure 11: Kaplan-Meier plot for DSS in lung cancer patients according to RTOG RPA. Log Rank test (Mantel-Cox) is significant with p < 0.001).
32
BS-BM
For all 34 patients, mean DSS time was 17.2 (9.32-25.09) months and median DSS time was 6.2 (4.06-8.34) months. In the low and high BS-BM score group, 16 (94.1) and 11 (64.7) lung cancer patients died from a disease-specific death (94.1%) during follow-up. Mean DSS time in the low BS-BM score group was 4.9 (1.79-8.02) months and median DSS time was 1.7 (0.22-3.18) months. Mean DSS time in the high BS-BM group was 27.2 (14.77-39.70) months and median DSS time was 10.4 (5.02-15.78) months. Log Rank confirmed BS-BM to be significantly associated to DSS in lung cancer patients with BM (p = 0.001).
Figure 12: Kaplan-Meier plot for DSS in lung cancer patients according to BS-BM. Log Rank test (Mantel-Cox) is significant with p = 0.001.
CERENAL
For all 40 patients, mean DSS time was 14.0 (7.35-20.69) months and median DSS time was 4.5 (1.82-7.18) months. In the low CERENAL score group, 10 lung cancer patients died from a disease-specific death (66.7%) during follow-up. Mean DSS time in the low CERENAL score group was 25.8 (12.37-39.14) months and median DSS time was 9.1 (0.00-18.32) months. In the high CERENAL score group, 23 patients died from a disease-specific death (92.0%). Mean DSS time in the high CERENAL score group was 5.1 (2.74-7.46) months and median DSS time was 2.8
33
(0/90-4.70) months. A significant association between CERENAL and DSS in lung cancer patients with BM was confirmed by Log Rank (p = 0.004).
Figure 13: Kaplan-Meier plot for DSS in lung cancer patients according to CERENAL. Log Rank test (Mantel-Cox) is significant with p = 0.004.
4.3.2.6. P
ROGRESSION-
FREE SURVIVAL(PFS)
–
SUB-
ANALYSIS LUNGRTOG RPA
For 17 lung cancer patients receiving first-line therapy after the diagnosis of BM, calculation of the RTOG RPA score was possible. Five patients were divided in class I, 13 patients in class II and 1 patient in class III. For all patients, mean PFS time was 4.2 (3.20-5.21) months and median PFS time was 4.1 (2.66-5.54) months. In class I, 2 patients showed progressive disease at the end of first-line therapy (66.7%). Mean PFS time in class I was 2.0 (1.56-2.51) months and median PFS time was 1.9 (1.42-2.38) months. In class II, 9 patients showed progressive disease (69.2%). Mean PFS time was 4.7 (3.59-5.77) months and median PFS time was 4.8 (3.18-6.42) months. In class III, that 1 patient showed progressive disease after 3.2 months. Log Rank confirmed RTOG RPA to be significantly associated with PFS in lung cancer patients with BM (p = 0.037).