UNTARGETED CHEMOMETRIC FINGERPRINTING OF
BIOTRANSFORMATION PRODUCTS FROM FERMENTED
MAIZE USING GC-HRTOF-MS AND LC-HRTOF-MS:
ENHANCING THE NUTRITIONAL QUALITY OF AN
IMPORTANT AFRICAN STAPLE FOOD
Hanna Vankelst
Supervisors: Prof. Sarah De Saeger, Prof. Patrick Njobeh
Commissioners: Dr. Marthe De Boevre, Prof. Patrick Njobeh
A Master dissertation for the study program Master in Pharmaceutical Care
Academic year: 2019 - 2020
UNTARGETED CHEMOMETRIC FINGERPRINTING OF
BIOTRANSFORMATION PRODUCTS FROM FERMENTED
MAIZE USING GC-HRTOF-MS AND LC-HRTOF-MS:
ENHANCING THE NUTRITIONAL QUALITY OF AN
IMPORTANT AFRICAN STAPLE FOOD
Hanna Vankelst
Supervisors: Prof. Sarah De Saeger, Prof. Patrick Njobeh
Commissioners: Dr. Marthe De Boevre, Prof. Patrick Njobeh
A Master dissertation for the study program Master in Pharmaceutical Care
Academic year: 2019 - 2020
Deze pagina is niet beschikbaar omdat ze persoonsgegevens bevat.
Universiteitsbibliotheek Gent, 2021.
This page is not available because it contains personal information.
Ghent University, Library, 2021.
PREAMBLE
This preamble was drawn up in consultation with supervisors Professor De Saeger and Professor Njobeh, which was approved by both. Part of this Master dissertation took place at the Department of Biotechnology and Food Technology of the University of Johannesburg, South Africa (11/02/2020 - 12/03/2020). The objective of this research was to optimize the Gas Chromatography High Resolution Time-Of-Flight Mass Spectrometry (GC-HRTOF-MS) extraction method, detect and identify possible health-promoting compounds in fermented maize samples. This analysis proceeded according to plan. On return to Belgium (12/03/2020), the coronavirus (COVID-19) preventive measures were announced, which made further planned analyses and laboratory work no longer possible. The process of obtaining the results of GC-HRTOF-MS analysis from the University of Johannesburg, South Africa, was also delayed because of the COVID-19 pandemic and thereby, resorted to working from home.
Originally, two more analyses were to take place at the Centre of Excellence in Mycotoxicology and Public Health (CoEMPH), Ghent University, which are: (1) determination and quantification of mycotoxins present in fermented maize and (2) detection and identification of degradation products formed by mycotoxins present in maize. In order to overcome this lab work that didn’t take place, a Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS) analytical method for the determination of mycotoxins in maize was explained and clarified by employees of the CoEMPH lab through skype meetings. In this way, the “Materials and Methods’ section for the LC-MS/MS analysis could be fully written out. For the untargeted screening of degradation products formed by mycotoxins, there was not yet a predefined protocol. Therefore, this analysis could not be written out in the ‘Materials and Method’ section, but was mentioned in the ‘Introduction’ (by description of the degradation products) and ‘Discussion’ section. The Master dissertation was completed with the available data obtained during the GC-HRTOF-MS analysis in South Africa.
SUMMARY
Mycotoxins are toxic secondary metabolites produced by fungi that can contaminate all kind of foods like maize. These toxins cause carcinogenic, teratogenic and immunotoxic effects in both humans and animals. In Africa, a continent where maize is used in several regions as a staple food, the prevalence of aflatoxin (AF) and fumonisin (FUM) (types of mycotoxins) is at its highest. This dissertation, therefore, provided a further insight into this mycotoxin problem and investigated a possible method to reduce exposure of mycotoxins among African populations. Fermentation as a method to reduce the concentration of mycotoxins in maize was investigated via Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS) analysis. The fermented maize were compared with untreated maize using the 'Sorghum method', a Standard Operating Procedure (SOP) established and validated by the Centre of Excellence in Mycotoxicology and Public Health at the University of Ghent. Also, the formed biodegradation products of AF and FUM were investigated. Detection and identification was done using an Liquid Chromatography High Resolution Time-Of-Flight Mass Spectrometry (LC-HRTOF-MS) analysis.
The influence of fermentation as a food-processing technique on the nutritional quality of maize was investigated by a Gas Chromatography High Resolution Time-of-Flight Mass Spectrometry (GC-HRTOF-MS) analysis via an untargeted screening at the University of Johannesburg. The identification of health-promoting compounds formed after maize fermentation was done via an Orthogonal Partial Least Square Discriminant Analysis (OPLS-DA). The fermented whole maize samples contained, based on the measured peak area, a significant higher amount of phenolic compounds, fatty acid derivatives, phytosterols, fatty acids and tocotrienols than the raw whole maize material. Analogue, a significant increase in peak area was reported for tocotrienols, ketones and esters in dehulled fermented maize. All these metabolites can be considered as health-promoting compounds since they improved the nutritional quality of fermented maize. Also, the influence of the type of fermentation (traditionally or controlled) and the grain form (whole grain or dehulled) on the formation of health-promoting compounds were investigated. Vitamin E, together with some fatty acid derivatives, occurred in higher amounts in controlled fermented samples than in traditionally fermented samples. The grain form influenced the formation of phenols and vitamin E, where phenols did not occur in dehulled fermented maize and the amount of vitamin E was higher in fermented whole maize.
It can be concluded that fermentation has positive effects on the nutritional quality of maize by reducing harmful mycotoxins and by the formation of health-promoting compounds. Mytox-South and LEAP-Agri are projects
SAMENVATTING
Mycotoxines zijn toxische secundaire metabolieten geproduceerd door bepaalde schimmels die verschillende soorten voedingsmiddelen zoals mais kunnen contamineren. Deze toxines veroorzaken carcinogene, teratogene en immunotoxische effecten in zowel mensen als dieren. In Afrika, een continent waar in verschillende regio’s mais gebruikt wordt als dagelijkse basis voeding, is de prevalentie van aflatoxine (AF) en fumonisine (FUM) (twee types mycotoxines) het hoogst. In deze master thesis werd daarom verder inzicht verschaft in de mycotoxine problematiek en werd een mogelijke methode onderzocht om de blootstelling van mycotoxines aan de Afrikaanse bevolking te verlagen. Fermentatie als methode om de concentratie aan mycotoxines in mais te reduceren werd onderzocht via een vloeistofchromatografie-tandem massaspectrometrie (LC-MS/MS) analyse. De gefermenteerde mais stalen werden vergeleken met onbehandelde mais via de ‘Sorghum methode’, een Standaard Procedure die opgesteld en gevalideerd is door het “Centre of Excellence in Mycotoxicology and Public Health” aan de Universiteit van Gent. Ook de gevormde biodegradatie producten van AF en FUM werden onderzocht. De identificatie en detectie van deze biodegradatie producten werd uitgevoerd via een LC-HRTOF-MS analyse.
De invloed van fermentatie op de nutritionele kwaliteit van mais is onderzocht door middel van een gaschromatografie-vliegtijd massaspectrometrie (GC-HRTOF-MS) via “untargeted screening’” aan de Universiteit van Johannesburg. De identificatie van gezondheid-bevorderende componenten gevormd na maisfermentatie, werd uitgevoerd door middel van een orthogonale gedeeltelijke kwadraat discriminant analyse (OPLS-DA). De gefermenteerde mais stalen bevatten een hogere hoeveelheid aan fenolverbindingen, vetzuurderivaten, fytosterolen, vetzuren en tocotriënolen dan het niet-gefermenteerd mais. Analoog werd een aanzienlijke toename in piekoppervlakte gerapporteerd voor tocotriënolen, ketonen en esters in gepeld gefermenteerde mais. Al deze metabolieten worden beschouwd als gezondheid-bevorderende componenten aangezien zij de nutritionele kwaliteit van gefermenteerd mais verbeteren. Ook de invloed van het type fermentatie (traditioneel of gecontroleerd) en de graanvorm (ongepeld of gepeld) op de vorming van gezondheid-bevorderende componenten werd onderzocht. Vitamine E en enkele vetzuurderivaten kwamen in grotere hoeveelheden voor in gecontroleerde gefermenteerde stalen dan in de traditioneel gefermenteerde stalen. De graanvorm beïnvloedde de vorming van fenolen en vitamine E, waarbij fenolen niet voorkwamen in gepeld gefermenteerde mais en de hoeveelheid vitamine E hoger was in ongepeld gefermenteerd mais.
Als conclusie kan worden gesteld dat fermentatie de nutritionele kwaliteit van mais positief beïnvloed door enerzijds het verminderen van schadelijke mycotoxines en anderzijds de vorming van gezondheid-bevorderende componenten. Mytox-South en LEAP-Agri zijn projecten die verder onderzoek verrichten naar de ontwikkeling van mycotoxine reductie en preventie methodes voor het verbeteren van de voedingskwaliteit en -veiligheid.
DEDICATIONS
First of all thank you to prof. Sarah De Saeger and Dr. Marthe De Boevre for reaching me this interesting master thesis and giving me the opportunity to explore and experience the University at Johannesburg,
thank you for all your support. Thank you to all the lovely people at the University of Johannesburg for giving me such a warm welcome,
for guiding me around and letting me explore your city. To Prof. Patrick Njobeh for your hospitality, guidance, advice and support,
thank you for welcoming me at your University. To Tshepiso, for the practical organisation of our stay. To PhD student Julianah, for guiding me through my thesis, for your tips and advice, thank you for the nice company. To PhD students Sefater and Rumbi for assisting and accompanying me. To Dr. Adebo for his advice, support and assistance, thank you for bringing out the statistician in me. To Prof. Siska Croubels, Prof. Gunther Antonissen, Prof. Siegrid De Baere, Prof. Sarah De Saeger and PhD student Kaat Neckermann for your company at the beginning of our stay, thank you for letting us experience a lot of interesting excursions organised by Prof. Patrick Njobeh. Thank you to the people of the CoEMPH lab at the University of Ghent for your support during this virtual times.
To PhD student Celine, for your tips and advice, for your good guidance. To Christ’l and Fré for all the explanation through skype calls and always taking time to answer questions.
To Nina for joining me in this incredible adventure, for all your support and the great moments together in South Africa. To my family, for the nice Sunday walks and the tasty Saturday evening food.
To Jens for being such a big support and help during my thesis adventure, thank you for dealing with me in stressful situations. To my mum and dad for supporting me at all times, thank you for giving me sometimes that necessary ‘Duwtje in de rug’.
TABLE OF CONTENTS
1. INTRODUCTION ... 1
1.1 MYCOTOXINS ... 1
1.1.1 Mycotoxin contamination in maize in Sub-Saharan Africa ... 1
1.1.2 Aflatoxins ... 2
1.1.3 Fumonisins ... 4
1.1.4 Impact of mycotoxins on human and animal health ... 4
1.1.5 Reduction of mycotoxin occurrence in maize ... 5
1.2 FERMENTATION ... 6
1.2.1 Benefits of fermentation ... 6
1.2.2 Types of fermentation ... 6
1.2.3 Cereal fermentation ... 7
1.2.4 Starter cultures used for cereal fermentation ... 7
1.3 FERMENTATIONANDNUTRITIONALQUALITYOFCEREALS ... 7
1.3.1 Nutritional value of cereals ... 7
1.3.2 Influence of fermentation on the nutritional value of cereals ... 8
1.3.3 Biodegradation products formed by fermentation ... 8
1.4 ANALYTICALMETHODS ... 11
1.4.1 LC-MS/MS ... 11
1.4.2 GC-HRTOF-MS ... 12
2. OBJECTIVES... 15
3. MATERIALS AND METHODS ... 16
3.1 CHEMICALS,REAGENTSANDINSTRUMENTS ... 16
3.1.1 In-house validated method (University of Ghent) ... 16
3.1.2 In-house validated metabolimic method (University of Johannesburg) ...17
3.2 EXTRACTIONMETHODSORGHUM(LC-MS/MS) ... 17
3.2.1 Sample preparation and spiking ...17
3.2.2 Mycotoxin standard mixture ... 18
3.2.3 Extraction ... 20
3.2.4 Defatting ... 20
3.2.5 Liquid-liquid extraction/precipitation ... 21
3.2.6 Purification on an amino SPE-column ... 21
3.2.7 Evaporation and reconstitution ... 21
3.3 LC-MS/MS ... 22
3.3.1 Data interpretation ... 24
3.4.1 Raw material and fermentation of maize ... 25
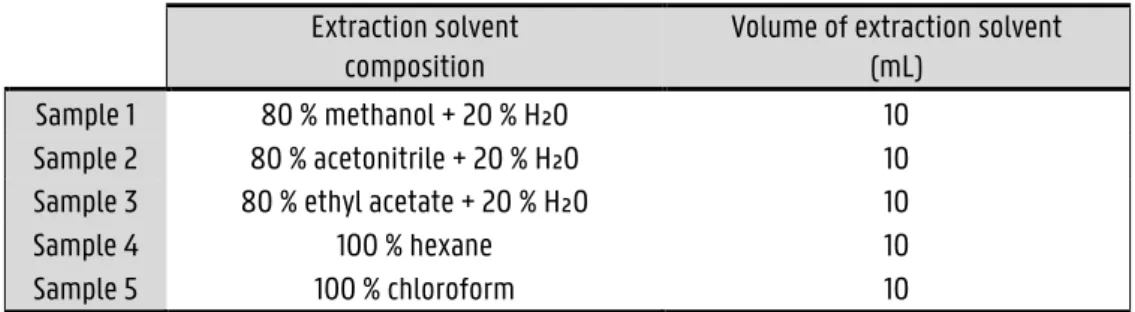
3.4.2 Preparation extraction solvent ... 25
3.4.3 Extraction ... 25
3.4.4 Evaporation and reconstitution ... 26
3.4.5 Data interpretation and evaluation ... 26
3.5 EXTRACTIONMETHODFORSORGHUM(GC-HRTOF-MS) ... 26
3.5.1 Sample preparation ... 26
3.5.2 Extraction ... 27
3.5.3 Evaporation and reconstitution ... 27
3.6 GC-HRTOF-MS ... 27
3.6.1 Data processing and statistical analysis ... 28
4. RESULTS ... 29
4.1 GC-HRTOF-MSANALYSIS ... 29
4.1.1 Comparison of metabolites in raw whole maize grain and obtained fermented whole maize... 29
4.1.2 Comparison of metabolites in raw dehulled maize grain and obtained fermented dehulled maize………32
4.1.3 Comparison of metabolites in traditionally fermented maize and controlled fermented maize………..34
4.1.4 Comparison of metabolites in whole maize fermented grain and dehulled fermented maize grain……..35
5. DISCUSSION ... 37
5.1 DETERMINATIONOFMYCOTOXINSINFERMENTEDMAIZE(LC-MS/MS) ... 37
5.2 DEGRADATIONPRODUCTSOFMYCOTOXINSINFERMENTEDMAIZE(LC-HRTOF-MS) ... 37
5.3 FERMENTEDDEGRADATIONPRODUCTS(GC-HRTOF-MS) ... 38
5.3.1 Comparison of metabolites in raw whole maize grain and obtained fermented whole maize... 38
5.3.2 Comparison of metabolites in raw dehulled maize and obtained fermented dehulled maize... 40
5.3.3 Comparison of metabolites in traditionally fermented maize and controlled fermented maize………..41
5.3.4 Comparison of metabolites in whole fermented maize and dehulled fermented maize ... 41
6. CONCLUSION ... 42
7. REFERENCES ... 43
LIST OF ABBREVIATIONS
AF Aflatoxin
AFB₁ Aflatoxin B₁
AFB₂ Aflatoxin B₂
AFB₂a Aflatoxin B₂a
AFG₁ Aflatoxin G₁ AFG₂ Aflatoxin G₂ AFL Aflatoxicol AFM₁ Aflatoxin M₁ AFM₂ Aflatoxin M₂ AFR₀ Aflatoxin R₀
API Atmospheric Pressure Ionization
C Control sample
CF Controlled Fermented
DG Dehulled Maize Grain
DON Deoxynivalenol
EI Electron Ionization
EPS Exopolysaccharides
ESI Electrospray Ionization
FA Fatty Acid
FAE Fatty Acid Ester
FAEE Fatty Acid Ethyl Ester
FAME Fatty Acid Methyl Ester
FB₁ Fumonisin B₁
FB₂ Fumonisin B₂
FB₃ Fumonisin B₃
FLf Fermented with L. fermentum
FLp Fermented with L. plantarum
FUM Fumonisin
GC-HRTOF-MS Gas Chromatography High Resolution Time-Of-Flight Mass Spectrometry
GRAS General Regarded As Safety
HBV Hepatitis B virus
HPLC High Performance Liquid Chromatography
HRMS High Resolution Mass Spectrometry
IARC International Agency for Research on Cancer
JEFCA Joint food and agriculture organization Expert Committee on Food Additives
KE Kinetic Energy
LAB Lactic Acid Bacteria
LC Liquid Chromatography
LC-HRTOF-MS Liquid Chromatography High Resolution Time-Of-Flight Mass Spectrometry
LC-MS Liquid Chromatography-Mass Spectrometry
LC-MS/MS Liquid Chromatography Tandem Mass Spectrometry
LDL Low Density Lipoprotein
LEAP Long-term EU-Africa research and innovation Partnership
LRMS Low Resolution Mass Spectrometry
m/z Mass to Charge ratio
MC Metabolite Class
MF Molecular Formular
MS Mass Spectrometry
MVDA Multivariate Data Analysis
OPLS-DA Orthogonal Partial Least Square Discriminant Analysis
OT Ochratoxin
PCA Principal Component Analysis
PMTDI Provisional Maximum Tolerable Daily Intake
Q Quadrupole
RF Radio Frequency
Rt Retention Time
S/N Signal to Noise ratio
SOP Standard Operating Procedure
SSA Sub-Saharan Africa
TCA Tricarballylic acids
TOF Time-Of-Flight
UPLC Ultra-High Performance Liquid Chromatography
WG Whole Maize Grain
WHO World Health Organization
1.
INTRODUCTION
1.1
MYCOTOXINS
1.1.1 Mycotoxin contamination in maize in Sub-Saharan Africa
Maize contributes to 40% of the total cereal production and is an important source in the daily diet of the Sub-Saharan African (SSA) population. (1) However, cereals are very susceptible to mycotoxin contamination, either before or post-harvest. (2) Mycotoxins are toxic metabolites produced by fungi that can contaminate maize and can cause carcinogenic, teratogenic and immunotoxic effects in both humans and animals. (3) The investigation of these food-born secondary metabolites started in 1960 because of a mycotoxin (aflatoxin) crisis in turkey poults in the United Kingdom, where it had a major economic impact. (4) The mycotoxins involved in most food safety issues are called the ‘Big Five’. These five main types are aflatoxin (AF), fumonisin (FB), ochratoxin (OT), deoxynivalenol (DON) and zearalenone (ZAN). (5)
Although the problem of mycotoxins is a global concern, it is seen that tropical regions like SSA have a higher level of exposure to mycotoxins. An explanation for this higher occurrence can be found in the characteristics of the climate of SSA. Humidity and high temperatures are perfect environmental factors for fungal growth. Also, the lack of dietary diversity in developing countries is a risk factor, as maize is a staple food in these regions. (2)
In the European Union, tolerable daily intake (TDI) levels formulated by the World Health Organization (WHO) are legislated and well followed-up. However, in regions like SSA, there is a lack of legislation and if it does exist, implementation and follow-up are insufficient. Because of food scarcity, food quality became a side issue. (6) In Table 1.1, the provisional maximum tolerable daily intake (PMTDI) per mycotoxin is provided based on the Joint Food and Agriculture Organization (FAO) Expert Committee on Food Additives (JEFCA). (46)
Table 1.1. Provisional maximum tolerable daily intake (PMTDI) for five major mycotoxins, i.e. fumonisin, aflatoxin, zearalenone, deoxynivalenol and ochratoxin A. (46)
Mycotoxin (Food Contaminant) PMTDI (µg/kg body weight per day) Evaluation Year
Fumonisin 2 2016
Aflatoxin Not established, genotoxic carcinogen 2016
Zearalenone 0,5 2000
Deoxynivalenol 1 2011
1.1.2 Aflatoxins
The fungi that are mainly responsible for the production of AF in maize are Aspergillus parasiticus and
Aspergillus flavus. The group of aflatoxins (AFs) consists of 18 types, all difuranocoumarin metabolites. Aflatoxin B₁ (AFB₁), aflatoxin B₂ (AFB₂), aflatoxin G₁ (AFG₁) and aflatoxin G₂ (AFG₂) are the four main types where AFB₁ has the highest toxicity and occurs most frequently. (7) In the literature, AFB₁ and AF are often used interchangeably. Aflatoxin M₁ (AFM₁) and aflatoxin M₂ (AFM₂) are hydroxylated metabolites of AFB₁ and AFB₂, respectively. These components are formed in the liver of animals that are exposed to AF-contaminated feed and can be carried over in milk. (8)
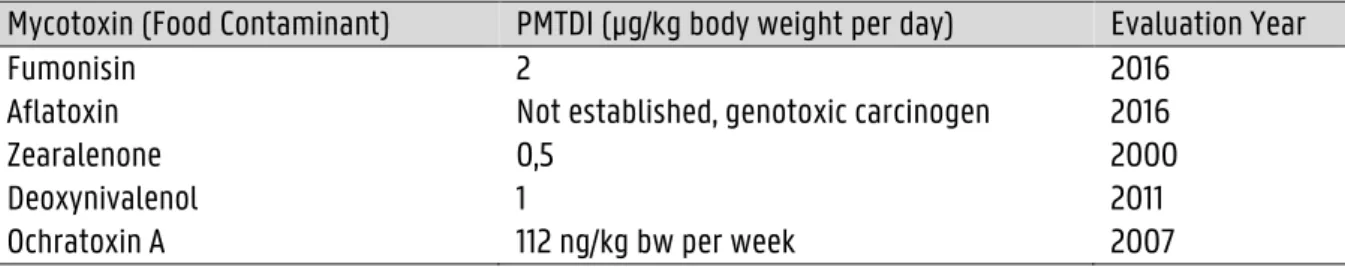
The lactone ring, as well as the difuran ring, contributes to the toxicity. Some types, such as AFB₁ have an extra double bond in the terminal furan ring (Figure 1.1). This double bond explains why AFB₁ is much more toxic than AFB₂. (9) The toxicity and impact on human health will be discussed further in Section 1.1.4.
Figure 1.1. Chemical structure of aflatoxin B₁ (L) and aflatoxin B₂ (R). (47) Degradation products of aflatoxin
Degradation implements a modification in the chemical structure of a compound. Specifically for AF, the modification leads to a change in the difuran ring and the lactone ring, the two key sites of toxicity as mentioned before. (9) A list of the most reported degradation products in the literature can be found in Table 8.1 in the addendum.
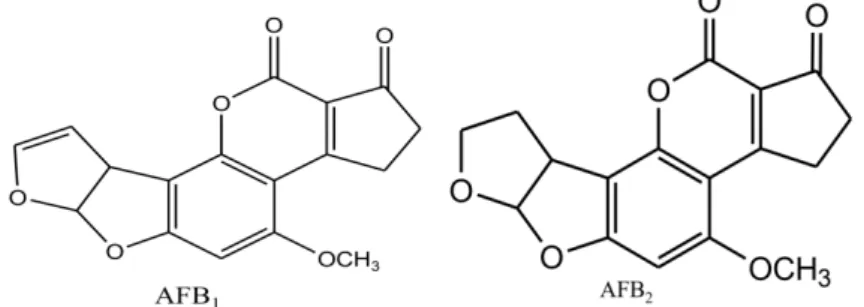
Pseudomonas putida can transform AF into AFD₁, AFD₂, AFD₃. In AFD₁ and AFD₂, only the lactone ring is cleaved, while for AFD₃, the lactone ring as well as the difuran ring are modified (Figure 1.2). (10) Aflatoxicol (AFL) and
aflatoxin R₀ (AFRo) are two degradation products formed by two species of the Corynebacteria.
3,4-1-carboxyethylphenyl-2-methylpropanoic acid (C₁₃H₁₆O₄) is an aromatic compound transformed by Rhodococcus
Figure 1.2. Scheme of AFB₁ degradation by Pseudomonas putida. (48)
This Master Dissertation focuses on microbial degradation. It is important to keep in mind that yeast, protozoa, enzymes and fungi can degrade AF as well. Fungi produce AFs, but certain fungal species can also degrade AFs by the acidic environment they create. (8) Pleurotus ostreatus can convert AF into Aflatoxin B₂a (AFB₂a), which is a hydrolyte of AF (Figure 1.3). (9)
Figure 1.3. Conversion of AFB₁ to AFB₂a by pleurotus ostreatus. (49)
Enzymes playing an important role in the degradation of AF are lactases, oxidases, peroxidases and reductases. (8) AFB₁-8,9-epoxide is the only degradation product that is even more carcinogenic than AFB₁ itself and is transformed by a manganese peroxidase. (11) Enzymes of the cytochrome P450 system in the human body also convert AFB₁ into AFB₁-8,9-epoxide, which can be further hydrolysed into AFB₁-8,9-dihydrodiol. (8)
1.1.3 Fumonisins
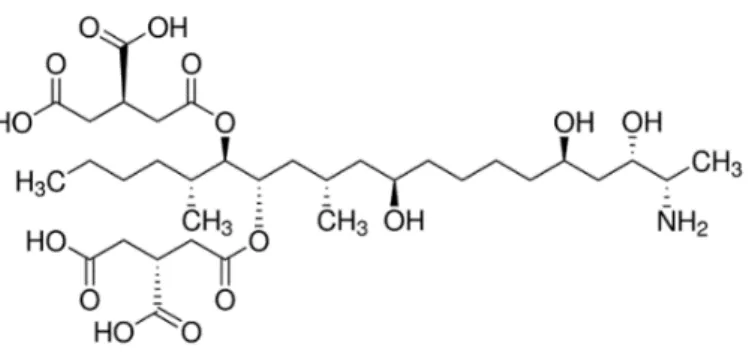
Several Fusarium species produce fumonisins (FUMs). Fumonisin B₁ (FB₁) is the most common type found in processed maize (Figure 1.4). Chemically, FUMs consist of 22 carbon aminopentol with 2 tricarballylic acid (TCA) side chains. (2) FUMs have structural similarities with the class of sphingolipids. The two structural groups that play an important role in FUM toxicity are the free amino group and the 2 TCA chains. The primary amino group is a competitive inhibitor of the ceramide synthase. This inhibition involves a disruption in the biosynthesis of ceramide and the metabolism of sphingolipid. (9)
Figure 1.4. Chemical structure of fumonisin B₁. (47) Degradation products of fumonisins
Detoxification of FUMs is associated with the modification of the free amino group and the 2 TCA chains. (12) The degradation products are less toxic than the original FB₁. (9) A list of the limited degradation products formed by microorganisms is provided in Table 8.2 in the addendum.
Sphingomonas and Sphingopyxis species are bacteria that can degrade FB₁ with the help of 2 enzymes: carboxylesterase and aminotransferase. Exophiala species transform FB₁ into N-acetylated-aminopentol-backbone-N-acetylAP1 and 2-oxo-12,16-dimethyl-3,5,10,14,15-icosanepentol hemiketal by the help of oxidative
deaminase as well as carboxylesterase. Delftia and Comamonas species can convert FUMs into 4 degradation
products, i.e. heptadecanone, eicosane, octadecenal and isonadecene. (9)
1.1.4 Impact of mycotoxins on human and animal health
Exposure to mycotoxins has a major impact on human and animal health. The most common adverse health effects associated with mycotoxins are cancer, stunting and acute poisoning. Every type of mycotoxin can be linked to certain harmful effects. AFB₁ plays a significant role in the development of liver cancer in developing countries and works synergistically with hepatitis B virus (HBV) infection. Since the prevalence of HBV infection is higher in developing countries, the risk of liver cancer increases simultaneously. (6)
By the International Agency for Research on Cancer (IARC), AFs are classified under Group 1 carcinogens. Agents in Group 1 are certainly carcinogenic to humans. (13) FB₁ is linked to a higher risk of oesophageal cancer and it is for that reason that it is classified as a Group 2B carcinogen by IARC, meaning it is possibly carcinogenic in humans. (6) (13) Stunting is a significant problem occurring in children mainly because of malnutrition. WHO defines children to be stunted if their height-for-age is lower than two standard deviations of the WHO height-for-age median. Stunting has a negative impact on the overall development of an infant. (14)
Mycotoxin contamination is the main reason for food loss in SSA. Although human health costs are not yet studied, but the costs related to liver cancer and the indirect associated pain and anxiety as a result of mycotoxin exposure may have a significant socio-economic impact. (14)
1.1.5 Reduction of mycotoxin occurrence in maize
Prevention and curative techniques both play a significant role in the reduction and control of mycotoxin contamination in maize. Insect prevention, biocontrol strategies and cultivation techniques are all pre-harvest preventive measures. Furthermore, good storage facilities are necessary to prevent the growth of potential mycotoxin producing fungi. (11) When field control measures failed and high contamination of mycotoxins are present, the best option is to discard the affected maize crops. (7)
For mildly contaminated maize there are physical, chemical and biological methods employed for its decontamination. (11) Nixtamalization is a physical food process, in which cereals are soaked and cooked in an alkaline solution, followed by washing and hulling. (50) This process still needs to be introduced in Africa, in contrast to the Mexican population where the method is generally used. The main advantages of this technique are the enrichment of the nutritional quality of maize and the simplicity of use. Through a combination of hydrolysis, extraction and the removal of the pericarp, nixtamalization is a preferable physical strategy to mitigate mycotoxin contamination in maize and other grains. (1) Ammonia, methanol and ozonation can remove mycotoxins by binding to them, but because of safety problems they are of limited use in food. (15) This Master Dissertation focuses on microbial degradation as a biological method to reduce mycotoxins in maize and thereby reducing associated toxic effects. Specific microbial degradation is caused by bacteria. The ability of lactic acid bacteria (LAB) to degrade mycotoxins is based on the mechanism of binding, as LAB have strong affinity for mycotoxins. They are used in food processes such as fermentation.
1.2
FERMENTATION
Fermentation is a biotechnological food changing process, which is influenced by the activities of microorganisms and enzymes. These microorganisms can be bacteria, fungi or yeast. (16) This process is traditionally implemented as a form of biopreservation in several communities in Africa, but it is nowadays also highly appreciated for multiple other applications. Fermentation can lead to changes in the nutritional quality of food. (17) It affects the acidity, flavour, organoleptic properties, digestibility and causes an extension of shelf life by converting and breaking down the raw material. (18)
1.2.1 Benefits of fermentation
The goals and benefits of fermentation reviewed in literature are variable and differ in value. Two important advantages are mentioned. First, the improvement of the nutritional value of foods (17) by the formation of several bioactive microbial metabolites and the elimination anti-nutritive factors. (19) The second significant benefit is the improvement in food safety through the detoxification of mycotoxins. The control of mycotoxins can be established by different mechanisms. Microbial degradation can lead to a change in the chemical structure, that can cause a reduction in toxicity. (11) Another mechanism is the adhesion of mycotoxins to the cell wall of the bacterium used during the fermentation process. (20) In addition to these two benefits with a significant effect on food quality and safety, there are several other advantages related to fermentation. For example preservation through metabolic activities. Antimicrobial molecules such as hydrogen peroxide, bacteriocins and organic acids are produced by bacteria linked to the fermentation process. The presence of antimicrobial molecules formed in the fermented products explains their preservative properties. (18) The process also causes an enhanced digestibility by an increment of the enzymatic activity and proteolytic activity of the microorganisms presented. (19) Finally, it improves organoleptic properties such as flavour, aroma and texture. (21)
1.2.2 Types of fermentation
Two types of fermentation are known, i.e. traditional and controlled fermentation. Traditional fermentation is the conversion of raw material by microorganisms, which are present in the raw substance and the environment. (16) In other words, the natural microorganisms present on the fermenting substrates trigger the natural fermentation process. (22) During this spontaneous fermentation process, there is no control on the possible presence of unspecified microorganisms. For this reason, there are growing concerns on food safety. (22) Controlled fermentation is the second type of fermentation. The raw material is inoculated with starter cultures. A starter culture is a microbiological culture that will support or assist the initiation of the fermentation process. This method provides a more controlled environment for fermentation and thereby enhances the food safety. (23)
1.2.3 Cereal fermentation
Fermented foods and beverages are products that underwent biochemical changes caused by the presence of microorganisms. The preparation of fermented food differs between African regions. (17) The sequence of steps may differ or other parameters are used. (24) The first step always consists of washing the grain with water. Then, the grain is dried and immersed in water. (22) This process is called soaking or steeping and lasts for at least 48 to 72 hours. (17) Soaking reduces the hardness of the grain, which makes it easier to later wet mill the maize. After water removal, wet milling can take place followed by an optional sieving step. (24) Subsequently, the fermentation starts with or without the use of a starter culture. (25) Next, sedimentation takes place, which gives tartness to the fermented product. (26) Finally, another dewatering and dry milling step are applied, ending up with freeze-drying of the product. (27) Soaking and sedimentation are considered to be the two most important phases because they have a major influence on the quality and physical properties of fermented food. (28)
1.2.4 Starter cultures used for cereal fermentation
The type of microorganism that is associated with a certain fermentation process will depend on certain parameters: pH-value, water activity, the food matrix and the temperature. (16) Generally, a mixed microorganism group is inoculated. (18) LAB are the most prominent used microorganisms for the creation of a starter culture in cereal fermentation. LAB is a wide-ranged group of gram-positive bacteria with a high occurrence of Bifidobacterium, Lactococcus and Lactobacillus spp. These bacteria are either rod shaped (bacilli) or spherical (cocci) and characterized as non-sporulating, non-respiring, acid tolerant and catalase negative. (19) LAB convert sugars into antimicrobial agents such as lactic acid and acetic acid. They are characterized as probiotics, which are living microorganisms with a beneficial effect on the intestinal microbial balance of the host. LAB have a ‘Generally Regarded As Safe’ (GRAS) status. (18) Fermentation can also get triggered by different kinds of fungi such as Aspergillus, Penicillum, Fusarium and Cladosporidium. (18)
1.3
FERMENTATION AND NUTRITIONAL QUALITY OF CEREALS
1.3.1 Nutritional value of cereals
Cereals are rich in dietary fibre, vitamins, carbohydrates, minerals and phytochemicals. (29) As an important source of dietary nutrients, it is not surprising that cereal grains account for more than 60% of world food production. (17) Nevertheless, the nutritional quality of cereals is compromised due to a deficiency of some essential amino acids and lower levels of proteins. Further to that is the presence of anti-nutritive factors that
have a detrimental effect on the nutritional value of food as they have an indirect negative impact on the bioavailability of minerals. (30)
1.3.2 Influence of fermentation on the nutritional value of cereals
Fermentation has several beneficial effects on the nutritional value of food. In short, it can break down the anti-nutritive factors and produce health-promoting compounds. (31) The process leads to a change in nutrient content as well as their availability. (25) The molecular composition of the formed fermented biodegradation products influences their nutritional health benefits. (32) It is important to mention that both increases and decreases of components as well as the formation of new components are completely depending on the nature of the raw material, the microflora used and fermentation conditions. (25) Therefore, variations can be observed in several reports in the literature. Characterization and description of possible health-promoting compounds are discussed in the section subsequent on this paragraph.
1.3.3 Biodegradation products formed by fermentation
Vitamins
Cereals are an important source of vitamins. It is reported that vitamins may be formed or increased in their availability as a result of fermentation. (31) The most common reported vitamins are vitamin E, folic acid, thiamine, riboflavin and niacin. (25) All of them are vital and essential components, necessary for several biochemical processes in the human body. (33)
Minerals and anti-nutritive factors
Fermentation reduces the content of anti-nutritive factors, which has a positive influence on the bioavailability of minerals such as iron, zinc, magnesium and calcium. (1) Anti-nutritive factors include phytic acid and tannins. (19) Phytic acid as an example, is a strong chelating agent that can bind with different minerals and thus have a negative effect on their bioavailability. (17) Many microorganisms used for fermentation contain the enzyme phytase. This enzyme can hydrolyse phytic acid resulting in a reduction of phytic acid and hence an increase in the bioavailability of minerals. (25) Tannins are polyphenols (phytochemical). Besides the various positive effects polyphenols have on the flavour, colour and structure of food, it also can have negative impact on human health when consuming it. Tannins can cause stomach irritation, vomiting and liver damage. (34) Fermentation can reduce the amount of polyphenols in food through polyphenol oxidase, an enzyme present in the microflora used for the fermentation process. (17)
Proteins
Proteins are vital for the growth of the human body and they are essential in the production of enzymes, blood cells and hormones. They are also involved in repairs and maintenance of body tissues. (35) Bioactive molecules that may be produced by microorganisms are amino acids, the key elements of proteins. An increase in the amino acids, i.e. lysine, methionine and tryptophan has been reported in the literature. (26) They belong to the group of essential amino acids as the human body is not able to synthesize them, but is depending on the intake of food to be able to absorb them. (35)
Carbohydrates
Fermentation causes a decrease in the number of carbohydrates in fermented food. More specifically, the number of indigestible poly- and oligosaccharides decreases. This results in a reduction of symptoms related to flatulence or a swollen abdomen. (17) The microorganisms present can also form polysaccharides such as exopolysaccharides (EPS). (31) Exopolysaccharides improve sensory properties, but also have possible health-promoting effects. These bioactive compounds have potential probiotic properties, which means they can have a positive influence on the intestinal health of the human body. (36) An example of an exopolysaccharide produced by LAB is dextran. (37)
Dietary fibres belong to the group of carbohydrates. When consumed by humans, they reach the colon intact, since they are not absorbed at the level of the small intestines. Some examples of dietary fibres are hemicellulose, lignin, B-glucan, cellulose and some types of oligosaccharides. (38) The consumption of fibres is associated with several health-promoting effects. In addition to having a positive effect on intestinal function, fibres also lower cholesterol levels in the blood, ensure a more stable sugar level and are important in the prevention of obesity. (39)
Phytochemicals
Phytochemicals can be defined as bioactive compounds derived from plant-based foods. They are substances with certain biological or physiological functions and thus have health-promoting effects. (40) Food processing such as fermentation can increase or decrease the levels and bioavailability of phytochemicals. (26)
There are different types of phytochemicals, divided into 6 groups including phenolics, lipids, alkaloids, carbohydrates, terpenoids and other nitrogen-containing compounds. (41) Phenolics, carotenoids, vitamin E, dietary fibre and phytosterols represent the most important groups of phytochemicals in grains. (39) The phenolic group consists of phenolic acids, flavonoids and alkylresorcinols. The most occurring phenolic acid in cereals is ferulic acid, a derivative of cinnamic acid, bounding to cell wall structures. (39) Bioprocessing can be used to increase the bioavailability of ferulic acid. (26) Ferulic acid can be converted to vanillin and other aromatic compounds. It contributes to the sensory properties of food and may have some antioxidant activities. (42) Flavonoids in this case, are also reported as substances with strong antioxidant properties and linked to reduced risk for developing several chronic diseases. (43)
Carotenoids are the natural pigments of cereals. This group is characterized by a long chain of alternating single and double bonds. This unique composition makes them good antioxidants. They can stay stable by forming free radicals themselves or deactivate other free radicals. (44) Phytosterols have been demonstrated to lower blood cholesterol levels, through the absorption of cholesterol in the guts, which lower the risk of cardiovascular diseases. (40) This claim is mentioned in the ‘Warenwet’ and thus confirmed. (45) Several of these phytochemical compounds in addition to phytosterols are also claimed to potentiate some antioxidant activities. Such a health-promoting claim is only officially awarded when approved by the European Union regulation and mentioned in the ‘Warenwet’. (45)
Flavour components
Amino acids are potential precursors of flavour components. After a decarboxylation or deamination into aldehyde they can further oxidize into acids or can be reduced to alcohols. (17) The aromatic compounds diacetyl, acetic acid and butyric acid are responsible for the presence of aromas that make the fermented cereal more appetizing. (1)
1.4
ANALYTICAL METHODS
1.4.1 LC-MS/MS
Liquid chromatography-mass spectrometry or LC-MS is used as an analytical technique to determine mycotoxins in maize as well as their degradation products. The objective of LC as a separation method is to retain the individual components of interest, by separating the components from each other and from undesired components based on their retention time. (51) Depending on the application, high-performance liquid chromatography (HPLC) or ultra-high performance liquid chromatography (UPLC) can be used. MS is used as a detection method for those components of interest by identifying them based on their mass to charge ratio (m/z-ratio). (52)
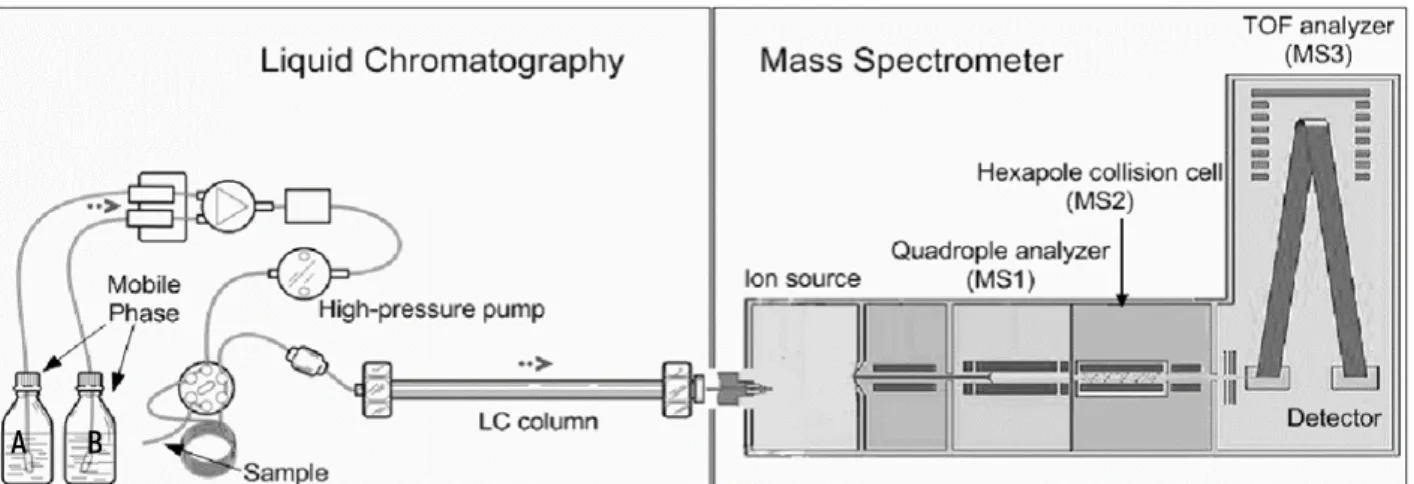
A mass spectrometer consists of three main compartments: an ion source, mass analyzer and a detector (Figure 1.5). The technique used in this Master Dissertation for the generation of charged ions is electrospray ionization (ESI), which is an example of atmospheric pressure ionization (API). (53) The ESI ion source consists of a narrow metal capillary with a high voltage. The LC eluant is pumped from the needle (0 kV) into the capillary (3-5 kV). (54) The potential difference produces a strong electrical field and together with the help of a nebulizer gas (N₂) and a desolvation gas (heated N₂) a spray of charged droplets is formed. The small droplets are attracted to the cone because of the ion charge and the suction effect due to the prevalence of vacuum in the cone. (55)
Figure 1.5. Drawn scheme of a Liquid Chromatography coupled to a High-Resolution Mass Spectrometer with a TOF mass analyzer. (51)
The mass analyzer selects and separates ions based on their m/z-ratio by applying magnetic or electric fields. The selection of the precursor ion takes place in the first quadrupole (MS₁ or Q1). Only the components with desired m/z-ratios will continue to the second department, the collision cell. In the collision cell, the fragmentation of the precursor ion takes place. In this cell, a different voltage and gas (argon) is applied than in the previous department. Argon gas is responsible for the formation of fragment ions by colliding with the incoming ions in the opposite direction. (53) The collision cell consists of a step wave in the SYNAPT and a travelling-wave (T-wave) in the Premier. In a step wave, a wave of potentials is created where undesired components such as matrix particles are being
deflected while the ions of interest continue.T-wave guides the desired components through the sequence of
electrodes by using a direct current. (56) This reduces possible signal interference, which improves the sensitivity of the analysis. (57)
As final step fragment ions are selected, the way of selection is based on the mass analyzer used. In case of low resolution MS (LRMS), a quadrupole is chosen. The selection of fragment ions takes place in a second quadrupole or MS₂. Such a tandem mass spectrometer is the go-to device if high levels of sensitivity are required. (58) A time of flight (TOF) mass analyzer can be used in case of high resolution MS (HRMS). An explanation of the principle of TOF can be found in Section 1.4.2. Because of its high mass resolution, high levels of selectivity can be reached. High selectivity means less signal interference, which can be translated into higher sensitivity. HRMS can discriminate molecules of similar mass and is usually applied for untargeted or de novo screenings. (52) When the ions hit the detector, an electric current is produced and sent to the computer software (Massalynx) to convert the measured response into a mass spectrum. (59)
In this Master Dissertation, the Premier (LRMS) was used for the determination and quantification of mycotoxins in maize samples and the SYNAPT (HRMS) was used for an untargeted screening of the biodegradation products formed by AFs and FUMs.
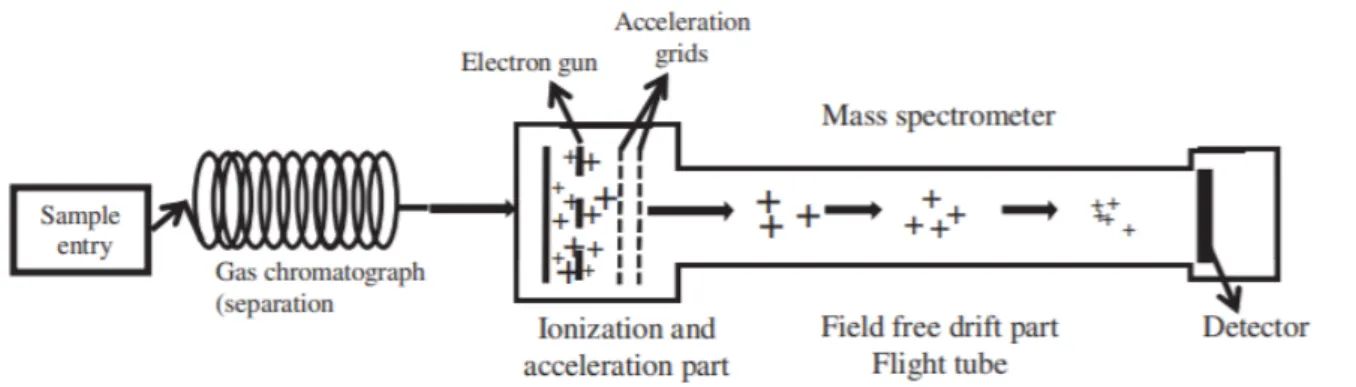
1.4.2 GC-HRTOF-MS
The applied analytical technique used for untargeted screening of metabolic molecules formed during fermentation is Gas Chromatography-High Resolution Time of Flight-Mass Spectrometry (GC-HRTOF-MS). (60) The GC is responsible for the separation of volatile components within the sample over time. HRTOF-MS is used as a detection method for the formed ions. Their m/z-ratio and relative abundance are measured and assembled in a mass spectrum. Structure identification, based on their m/z-ratio, is possible since every spectrum is a unique
fingerprint for a specific component or compound. (61) A combination of GC and HRTOF-MS is used to achieve higher sensitivity and resolution. (62)
A sample (0.2 - 3 µL) is injected through a heated injection port to allow evaporation of the components (Figure 1.6). This evaporation is necessary for the components being able to move from the inlet into the column with the help of a carrier gas (helium, hydrogen, nitrogen and argon) present in a pressurized cylinder or gas generator. (61) The capillary column is surrounded by a temperature-controlled oven and contains a highly viscous liquid coated inner wall, the stationary phase. Separation to individual components is based on the difference in boiling point and affinity of the components for the stationary phase on the column. (63)
Figure 1.6. Schematic diagram of GC-HRTOF-MS. A sample is injected through a heated injection port into the capillary column of the GC. The individual components enter the HRTOF-MS where ionization occurs. The ions reach the flight
tube. The time to reach the detector depends on the velocity of the ion and, thereby indicating it's mass. (64) Electron Ionization (EI) is used as an ion source for the ionization of vaporized components once entering the HRTOF-MS system. The positive ions are accelerated by passing negatively charged plates. When entering the ion drift room or flight tube, all ions have the same kinetic energy. By converting Equation 1, the velocity (v) can be written as Equation 2. Since all ions have the same kinetic energy, the velocity of each ion depends on its mass. Particles with a higher mass have a lower velocity compared to those having a lower mass. (59)
𝐾𝐾𝐾𝐾 =12 𝑚𝑚 ∗ 𝑣𝑣² 𝑣𝑣 = �2𝐾𝐾𝐾𝐾𝑚𝑚
In which: m: Mass (kg)
v: Velocity (m s⁄ )
TOF is used as a mass analyzer that measures the time needed for an ion to fly through the drift room before entering the detector. The time of flight is given by Equation 4 (using Equations 2 and 3) and shows that the time is proportional to the square root of the mass of the ions. Particles with a higher mass travel longer in the flight tube and reach the detector slower than those having a lower mass. (63)
𝑡𝑡 = 𝑣𝑣𝑙𝑙 𝑡𝑡 = 𝑙𝑙 ∗ � 𝑚𝑚
2𝐾𝐾𝐾𝐾
In which: l: Length of the flight tube (m)
v: Velocity (m s⁄ )
t: Time of flight (s) m: Mass (kg)
KE: Kinetic Energy (J)
When the ions enter the detector, the individual chemical or physical properties generate an electric signal measured by a computer software program. The identification of the component can be determined by their peak area, peak height, peak width, m/z-ratio and retention time. This information is all assembled in a mass spectrum. (59)
2.
OBJECTIVES
Mycotoxin contamination in food and related risks for animal and human health are a worldwide problem. Cereals are very susceptible to mycotoxin contamination and are used in several regions in Africa as a staple food. Therefore, maize grain samples originating from Kenya were used in this Master’s project as a matrix to gather further knowledge about the extent of exposure to African populations of AFs and FUMs produced by fungi. A better understanding of this problem remains necessary to develop methods to prevent or reduce the concentrations of mycotoxins in food. Fermentation as a method to reduce the levels of mycotoxins in grains as well as the formation of possible health-promoting compounds during this process was investigated and reported in this Master Dissertation.
To extract and determine mycotoxins present in fermented maize, a validated in-house method was used. To verify the reduction in the concentrations of mycotoxins caused by fermentation, levels of AFs and FUMs in fermented maize and a control sample were compared using an LC-MS/MS analysis. To detect and identify the biodegradation products formed by AFs and FUMs, an untargeted screening was carried out using Liquid Chromatography High Resolution Time-of-Flight Mass Spectrometry (LC-HRTOF-MS).
To optimize the GC-HRTOF-MS analysis, a validated in-house extraction method (South Africa) was used. The optimal solvent mix was determined for the detection and identification of health-promoting compounds present in fermented maize. To extract and detect possible health-promoting compounds formed during the fermentation food process, a validated in-house method (South Africa) was used. The GC-HRTOF-MS analysis was carried for the untargeted screening of biodegradation products formed during the fermentation process in maize. Improvement in the nutritional quality of fermented maize was investigated by a statistical analysis, Orthogonal Partial Least Square Discriminant Analysis (OPLS-DA), that detected significant metabolites contributing to the differences between fermented maize and a control sample.
This Master Dissertation was made possible by LEAP-Agri, a long-term EU-Africa research and innovation partnership on food and nutrition security sustainable agriculture. The results will contribute to the Mytox-South project, an intercontinental partnership striving to solve mycotoxin problems.
3.
MATERIALS AND METHODS
3.1
CHEMICALS, REAGENTS AND INSTRUMENTS
3.1.1 In-house validated method (University of Ghent)
The following materials and reagents were used during the in-house validation experiment. Plastic extraction tubes of 50 ml with a screw cap, SOL17102, Novolab, Geraardsbergen, Belgium; Latex disposable gloves, Novolab; IKA-WERKE grinder, Stranger Germany; Vortex mixer, Labinco L46, VWR; Plastic tube stoppers, diameter 16, Novolab; Arium® pro UV, SARTORIUS LAB INSTRUMENTS, Göttingen, Germany; Glass beaker 100 mL, Egilabo; Fridge, Liebherr, Geeroms; Aluminium foil, Reynolds, Novolab; Centrifuge, Sigma 3-16 PK SET3, Sigma, Eeklo, Belgium; Durapore®
membrane filter, 0,22 𝜇𝜇m GV, cat.nr. GVWP04700, Merck Millipore, Molsheim, France; Milli-Q system, Millipore;
Plastic Pasteur pipettes, SOL2600111, Novolab; Graduated pipette 5 mL, 10 mL, VWR, Egilabo; Nigrogen spin, glassworks, UGhent; HPLC vial caps, WAT058875, Waters; Schott Duran flask of 100 mL, 1 L, VWR, Novolab;
Ultrafree-MC centrifuge filter 0,5 ml, Durapore PVDF, 0,22 𝜇𝜇𝑚𝑚, UFC30GVNB, Merck Millipore, Molsheim, France; Vial + insert,
0,3 ml PP short thr micro-vial, 32 mm × 11,6 mm, LP11190932, La-pha-pack, Filter Service, Eupen, Belgium; Cap + septa, 9,5 mm septa, silicone white/PTFE blue, 1,5 mm, slitted, LP09021327, La-pha-pack, Filter Service, Eupen, Belgium; Volumetric flask of 1 L or 0.5 L + glass stopper, VWR, Egilabo; Electronic analytical balance, Sartorius BP210S, n° 40703609; precision pipettes 3.00 mL, Egilabo; Plastic syringes of 20 mL, Becton Dickinson, BD300613, Novolab; Micromass Quattro Premier XE triple quadrupool mass spectrometer, Waters, Zellik, Belgium; Shaker AF 6A, exacta; Acquity UPLC console, Waters, Zellik, Belgium; Edwards rotary pump E2M28; vacuum elution manifold with waterflow pump, J.T. Baker Inc, Boom; Sovirel tubes 10 mL with fitting screw cap and Teflon ring, VWR;
Symmetry® C18 column, 5 𝜇𝜇m 2,1 × 150 mm, Waters part. n° WAT056975, Zellik, Belgium = Guard column; Symmetry®
C18 column, 3,5 𝜇𝜇m 2,1 × 10 mm, Waters part. n° WAT106127, Zellik, Belgium; Tips for automatic pipettes HP15/HP16,
VWR, Eppendorf; Zearalanon (ZAN), Sigma, Z0167-1MG, Sigma-Aldrich, Overijse, Belgium; Disolol® - C2H5OH, CL00.1807.2500, reagent, denaturated with 1% IPA and 1% MEK + DBN 2,5 l, Chemlab, Zedelgem, Belgium; Methanol HiperSolv CHROMANORM, VWR International, 208640320, Leuven, Belgium; Aflatoxin AFG2 (AFG018), AFG1 (AFG018 A), AFB2 (AFB008), AFB1 (AFG025 A), Oskar Tropitzsch, FERMENTEK, Jeruzalem, Israel; Fumonisin FB1 (FB015A), FB2 (FB015A), Oskar Tropitzsch, FERMENTEK, Jeruzalem, Israel; FB3, Medical Research Council, FERMENTEK; n-Hexane BDH HiperSolv CHROMANORM, Prolabo, VWR International, 24575.320, Leuven, Belgium; Acetic acid glacial ULC/MS, 100 ml, 0001074131BS, Biosolve, Valkenswaard, The Netherlands; Methanol absolute LC/MS, Biosolve, cat.no. 13687802, Valkenswaard, The Netherlands; Denaturated ethanol, Chem-Lab, CL00.1807.2500; Dichloromethane, Acros, 1011197; Acetonitrile Absolute LC/MS (A180), Biosolve, VWR, 01207802, Valkenswaard, The Netherlands; Ammonium acetate (A74), UCB, 1136; Dichlormethane (D79), Acros, 10111197; Nitrogen, central supply, Air Liquide; Formic acid (M15), Merck, 1.00264.1000.
3.1.2 In-house validated metabolomic method (University of Johannesburg)
Plastic centrifuge tubes of 50 mL, A602002; Latex disposable gloves, Novalab; Ultrasonic Bath, Scientech 704, Labotech, South Africa; Centrifuge, Eppendorf 5702R, Merck Millipore, South Africa; Clear vials 2 mL, Merck; Rotavapor, EV311PLUS, LabTech, South Africa; Laboratory Mill 3600, Perten, Instruments, Sweden; Analysette 3 spartan, Fritsch, Germany; Pipettes 1 mL; Glass beaker 100 mL, Egilabo; Plastic Pasteur pipettes, Novolab; LECO Pegasus GC-HRTOF-MS system, LECO corporation, St Joseph, MI, USA; Cellulose acetate Durapore membrane filter (0.45µm), PVDF-L; Concentrator, Eppendorf Plus, South Africa; Gas chromatograph, Agilent Technologies, Inc., Wilmington, DE, USA; Syringe 12 mL, NORM-JECT Luer Lock, MPS multipurpose autosampler, Gerstel Inc. , Germany; Rxi®-5ms column, 30 m × 0.25 mm ID × 0.25 µm, Restek, Bellefonte, USA; Graduated cylinder 10 mL, 20 mL, VWR, Egilabo; Round bottom flask, VWR, Egilabo; Electronic analytical balance; Freezer; Tips for automatic pipettes, Eppendorf, South Africa; Pipette 100 – 1000 µL, Eppendorf, South Africa; Rotavapor, EV311PLUS, LabTech; Acetonitrile, Sigma-Aldrich, South Africa; N-Hexane, Merck; Chloroform, Riedel de Haën; Ethyl acetate, Sigma-Aldrich, South Africa; Methanol, Sigma-Aldrich, South Africa.
3.2 EXTRACTION METHOD SORGHUM (LC-MS/MS)
3.2.1 Sample preparation and spiking
After homogenizing the dried maize samples, 3 g were weighed in an extraction tube of 50 mL of every unknown sample. The blank sample had a similar matrix compared with unknown samples that were analyzed. With this blank sample, 7 extraction tubes were filled (3 g). These tubes represented the 5 spikes for the calibration curve, 1 control spike and 1 blank. Spikes are blank samples where standard components are added to obtain a matrix-matched calibration curve. The use of a matrix-matched calibration curve will reduce possible matrix effects and will give a better estimation of the concentrations found in the unknown samples. The calibration curve consists of 5 spikes (Spike1 = ¼ X, Spike2 = ½ X, Spike3 = X, Spike4 = 2 X, Spike5 = 4 X) where X represents the concentration (µg/kg) of mycotoxin in the middle concentration of the calibration curve. So to all the samples, 60 µL of zearalenone (ZAN) of 2.5 µL/mL was added. ZAN is used as an internal standard to calculate the response for the components of AF and FUM. The standard mixture was added to the 5 spikes for calibration and the control spike, the exact amounts can be found in Table 3.1. At last, all the samples were put in the dark for 15 minutes.
Table 3.1. Preparation of samples and calibration curve
Position Name Spike Standard mixture (3.2.2)(µL)
Internal Standard ZAN of 2.5 µL/mL (µL) 1 Blank / 0 60 2 Spike 1 1/4 X 15 60 3 Spike 2 1/2 X 30 60 4 Spike 3 1 X 60 60 5 Control spike 1 X 60 60 6 Spike 4 2 X 120 60 7 Spike 5 4 X 240 60 8 Unknown samples / 0 60
3.2.2 Mycotoxin standard mixture
Preparation of stock solutions
Mycotoxin standards (FB₁, FB₂, FB₃, AFB₁, AFB₂, AFG₁ and AFG₂) were delivered as a powder. For each of these standards, a stock solution of 1 mg/mL was made by dissolving the powder in 1000 µL of acetonitrile per mg. Only FB₃ standard was dissolved in a mixture of acetonitrile and water (50/50, v/v). The dissolved powder was transferred into a Sovirel tube, wrapped in aluminium foil, and stored in the freezer.
Preparation of work solutions
Work solutions were dilutions of stock solutions. These solutions were made when the volume to be pipetted from the initial stock solution was too small to be accurate (< 10 µL). The internal standard ZAN work solution of 2.5 ng/µL was made by diluting the stock solution of 1 mg/mL 400 times, this dilution was done in two steps. First, 1000 µL of acetonitrile were transferred into a Sovirel tube with a micropipette and 100 µL of this acetonitrile was removed from this tube. Into this tube, 100 µL of the stock solution were pipetted as a first diluted concentration of 100 ng/µL. Next, 2000 µL of acetonitrile were transferred into a Sovirel tube with the help of a micropipette and 50 µL of acetonitrile (100 ng/µL) was removed and pipetted into this Sovirel tube. The tube was wrapped in aluminium foil, vortexed, and stored in the freezer.
The AFs work solutions of 100 ng/µL were made by diluting the stock solution of 1 mg/mL ten times. A volume of 1000 µL acetonitrile was transferred using a micropipette into a Sovirel tube and 100 µL of this acetonitrile removed. Thereafter, 100 µL of the stock solution of 1 mg/mL were pipetted into this tube and vortexed. Only AFG₂
was additionally diluted 10 times to obtain a stock solution of 10 ng/µL. 1000 µL were transferred into a Sovirel tube and 100 µL of this acetonitrile removed from this tube. Then, 100 µL of the obtained work solution of 100 ng/µL were transferred into this tube. The tube was wrapped in aluminium foil and stored in the freezer.
The working solution of FB₁ was made by diluting the stock solution of 1 mg/mL (10x). Analogue to the AFs working solutions, 1000 µL of acetonitrile were transferred using a micropipette into a Sovirel tube and 100 µL removed. Thereafter, 100 µL of the stock solution of 1 mg/mL were pipetted in this tube and vortexed. The tube was wrapped in aluminium foil and stored in the freezer.
Preparation of the standard mixture
The standard mixture was made by adding different mycotoxin standards and contained FB₁, FB₂, FB₃, AFB₁, AFB₂, AFG₁, AFG₂. The concentration in the middle point of the calibration curve (Spike 3 = control spike) is used as a cut-off value. Based on this concentration, the mass needed for each standard in the 3 g blank can be calculated, which is the same value that needed to be present in 60 µL standard mix. A volume of 1000 µL standard mix was made ( Table 3.2).
Table 3.2. Calculations for standard mix
Mycotoxin Concentration in control spike (60 µL) (ng/g) Mass in 3g blank (ng) Mass in 60 µL standard mix (ng) Mass in 1000 µL standard mix (ng) FB1 100 300 300 5000 FB2 200 600 600 10000 FB3 200 600 600 10000 AFB1 40 120 120 2000 AFB2 20 60 60 1000 AFG1 20 60 60 1000 AFG2 10 30 30 500
The volume of each standard that needed to be taken out of the stock or work solution was calculated based on the required concentrations in the standard mix with the help of the formula C₁ x V₁ = C₂ x V₂ ( Table 3.3).The mix was evaporated until dryness at 40°C under a nitrogen flow and again dissolved in 1 mL of acetonitrile. This solution was vortexed and transferred into a Sovirel tube and wrapped in aluminium foil. The mix was stored in the
Table 3.3. Volumes to prepare the mycotoxin standard mix Mycotoxin Concentration stock or work solution (ng/µL) Volume stock or work solution (µL) Concentration in standard mix (ng/µL) Concentration in control spike (60 µL) (ng/g) FB1 100 50 5 100 FB2 1000 10 10 200 FB3 1000 10 10 200 AFB1 100 20 2 40 AFB2 100 10 1 20 AFG1 100 10 1 20 AFG2 10 50 0.5 10 3.2.3 Extraction
An extraction solvent of methanol/ethyl acetate/water (70/20/10, v/v/v) was made. A volume of 500 mL of methanol was added in a volumetric flask of 1000 mL together with 200 mL of ethyl acetate and 100 mL of Ultra-pure water. Methanol was added up to the grade mark of the volumetric flask and shaken vigorously. The extraction solvent was labelled and kept at room temperature. To all samples (blank sample, spiked blank samples for the calibration curve, control sample and unknown samples), 20 mL of the extraction solvent were added. The extraction tubes were put in a rack and wrapped in aluminium foil. All the tubes were shaken for 40 minutes using an end-over-end shaker. Next, the samples were centrifuged for 10 minutes at 4000 g using a centrifuge. Thereafter, the supernatant of each sample was transferred in a new extraction tube of 50 ml using a plastic Pasteur pipette.
3.2.4 Defatting
Ten mL of n-hexane were added to each sample extract. The extraction tubes were shaken using an end-over-end shaker. Thereafter, the samples were centrifuged for 15 minutes at 4000 g. The upper layer (hexane) was removed and discarded using a Pasteur pipette.
For further purification, the sample extract was split into two parts. One part of the defatted extract underwent liquid-liquid extraction and/or precipitation (Section 3.2.5), while the other part was subjected to solid phase extraction (SPE) purification using an amino SPE column as described subsequently in Section 3.2.6.
3.2.5 Liquid-liquid extraction/precipitation
An extraction solvent consisting of dichloromethane/formic acid (99/5, v/v) was made. A volume of 500 mL of dichloromethane was added into a 1000 mL volumetric flask containing 50 mL of formic acid. The mixture was diluted with dichloromethane up to the grade mark and agitated. The extraction solvent was labelled and kept at room temperature. Five mL of each defatted sample extract were transferred into a clean test tube of 50 mL. From the 5 mL of the defatted extract, 2.5 mL was pipetted into a clean 50 mL extraction tube. To all samples, 10 mL of the extraction solvent were added. Next, samples were vortexed for 1 minute and then centrifuged for 10 minutes at 4000 g. The remaining 2.5 ml of extract were discarded.
3.2.6 Purification on an amino SPE-column
For each sample, an amino SPE-column was placed on a vacuum elution manifold. The columns were conditioned twice using 6 mL of the extraction solvent consisting of methanol/ethyl acetate/water (70/20/10, v/v/v). The eluate accumulated in the vacuum manifold was later discarded after which time, the vacuum manifold was rinsed with Ultra-pure water. Below the amino SPE-columns, a tube holder was placed with the test tubes. The remaining fraction of the defatted extract (Section 3.2.4) was passed through the column and the eluate was immediately collected in the test tubes below.
The supernatants obtained from the liquid-liquid extraction (Section 3.2.5) were transferred in new extraction tubes together with the corresponding eluate. Finally, all combined fractions were evaporated at 60°C under a gentle nitrogen flow.
3.2.7 Evaporation and reconstitution
An injection solvent was made consisting of a mixture of mobile phase A and mobile phase B. Mobile phase A (water/methanol/acetic acid (94/5/1, v/v/v) + 5 mM ammonium acetate) was made by first transferring 250 mL of ultra-pure water in a 500 mL volumetric flask. Next, 192.5 mg of ammonium acetate were dissolved in a small
amount of Ultra-pure water and added quantitativelyto the volumetric flask. Afterwards, 5 mL of acetic acid and
25 mL of methanol were added. The mixture was diluted with ultra-pure water up to the grade mark of the volumetric flask, the flask was tightly sealed and vigorously shaken. The solution was filtered through a Durapore® membrane filter and transferred into a brown labelled Duran bottle, and stored at room temperature.
filled in a 50 mL graduated cylinder. Next, 385 mg of ammonium acetate was transferred quantitatively to the volumetric flask with the residual Ultra-pure water. Thereafter, the mixture was diluted with methanol up to the grade mark of the volumetric flask and shaken vigorously. The solution was filtered with a Durapore® membrane filter and transferred into a labelled brown Duran bottle, and stored under room temperature conditions.
The injection solvent was made by transferring 50 mL of mobile phase A and 50 mL of mobile phase B in a Duran flask of 100 mL. The flask was tightly sealed and vigorously shaken. The injection solvent was labelled and kept at room temperature.
The residue obtained in Section 3.2.6 was redissolved in 300 µL mobile phase and vortexed very well using a vortex mixer. After adding 200 µL n-hexane, it was vortexed again for 2 minutes. This mixture was transferred into a centrifuge filter and was ultra-centrifuged for 10 minutes using a centrifuge (program setting: slow start and slow stop). Using a micropipette, 100 µL of the defatted filtrate (lower phase) were pipetted into a vial containing an insert together with 100 µL of the mobile phase. Thereafter, the vial was closed using a cap foreseen of a septum and vortexed to homogenize. Finally, possible formed air bubbles at the bottom of the insert were removed by gently tapping the vial.
3.3
LC-MS/MS
For the determination of mycotoxins in maize an HPLC system (Waters Acquity) was coupled to a Quattro PREMIER mass spectrometer. The HPLC column used was a Symmetry C₁₈, 5 µm 150 x 2.1 µm, Waters (WAT 056975). A mixture of two mobile phases was used in gradient mode. The composition of the mobile phase changed over time as illustrated in Table 3.4.
The injection volume applied on the HPLC was 20 µL with a flow rate of 0.3 mL/min and a runtime of 28 minutes for each sample. The injection solvent consisted of 50% mobile phase A and 50% mobile phase B. The sequence of injection is illustrated in Table 3.6. Nitrogen was used as a nebulizer and desolvation gas. The ESI+ mode was used as an operating mode in the mass spectrometer. The capillary voltage was set at 3.50 kV, the extractor voltage at 3 V and the RF lens voltage at 0.20 V. The source block and desolvation temperatures were set at 130°C and 350°C, respectively. Table 3.5 illustrates the parameters of Quattro PREMIER used for the analysis. The precursor ions and
the selected fragment ions for each component of AF and FUM are given in Table 3.7 together with their ionisation
mode, cone voltage and collision energy. The data processing was done using QuanLynx software. The tune file method is called ‘Rapidmultimyco-T’ and the MS file ‘Rapidmultimyco-MS’.
Table 3.4. Gradient composition used on the Quattro Premier LC-MS/MS
Time (min) Mobile Phase A Mobile Phase B Flow (mL/min)
0.00 95 5 0.3 7.00 35 65 0.3 11.00 25 75 0.3 13.00 0 100 0.3 14.00 0 100 0.3 14.10 95 5 0.3 17.60 35 65 0.3 18.60 25 75 0.3 19.80 0 100 0.3 19.90 95 5 0.3 22.40 35 65 0.3 23.40 25 75 0.3 25.00 0 100 0.3 26.00 95 5 0.3 28.00 95 5 0.3
Table 3.5. Parameters used on the Quattro PREMIER
Parameters Value LM res 1 14.0 LM res 2 14.0 HM res 1 14.0 HM res 2 14.0 Ion energy 1 14.0 Ion energy 2 2.00 Multiplier 750 V
Table 3.6. Order of injection (Quattro PREMIER)
Sequence of injection Name 1 Standard mix 2 Mobile phase 3 Blank 4 Spike 1-5 calibration 5 Mobile phase 6 Unknown samples
Table 3.7. Selected ions aflatoxin and fumonisin LC-MS/MS (Quattro PREMIER)
Component Precursor ion
Fragment ions (m/z) Collision Energy
Cone voltage (V) Predicted AFB1 313 285.27/241.42 21.00/34.00 47.00 7.87 AFB2 315 287.30/259.40 25.00/29.00 50.00 7.60 AFG1 328.85 311.17/243.36 25.00/20.00 43.00 7.21 AFG2 331 313.15/245.30 24.00/30.00 46.00 6.99 FB1 722.5 334.36/352.26 37.00/36.00 51.00 9.35 FB2 706,4 336.26/318.00 35.00/38.00 51.00 11.94 FB3 706,4 336.30/354.00 35.00/29.00 51.00 10.53
3.3.1 Data interpretation
Confirmation of the presence of a component is only possible if the four following identification criteria are simultaneously fulfilled.
1. The presence of at least 3 identification points. One signal or transition of one fragment ion is good for 1,5
identification points. From each component, at least 2 signals and thus 2 fragment ions must have been found.
2. The signal to noise ratio (S/N-ratio) of each ion must be more than 3. In that case, the peak height and area
have a sufficient size.
3. The relative retention time of the analyte in the unknown sample (relative to the internal standard) cannot
deviate more than 2.5% from the relative retention time of the spike.
4. The relative intensity of the selected ions in the unknown sample should not deviate more than a certain
percentage form the relative intensity of the selected ions in the closest spike. Table 3.8. illustrates the acceptable limits of deviation in LC-MS/MS based on relative intensity.
Table 3.8: The acceptable limits of deviation in the LC-MS/MS based on the relative intensity (identification criteria 3)
Relative intensity (% of mean peak) Accepted limits LC-MS/MS > 50 % ± 20 %
20 % - 50 % ± 25 % 10 % - 20 % ± 30 %
≤ 10 % ± 50 %
The data received from the Quattro PREMIER were processed and analyzed using Massalynx (coupled to the calculation program Qualynx). To meet the four identification criteria, an investigation with the help of the obtained datasheet was performed. Internal quality control was equally done. Every sample was checked if it contained the internal standard ZAN. The concentration found in the spiked samples, forming the calibration curve, had to be comparable with the theoretical spiked concentrations. The R or R² of the calibration curve had to be higher than 0.95. The recovery of the control spike needed to fall between the range of 80 – 120 %. The concentration of mycotoxins in the blank sample was also determined. If the blank sample turned out not to be totally ‘blank’, the results were corrected by taking the response factor into account and led to a shift of the calibration curve.