DERIVATIZATION OF CANNABINOIDS
TO IMPROVE THE DETECTION OF
THESE WIDELY ABUSED SUBSTANCES
BY LC-MS/MS
Helena Schoon
A Master dissertation for the study programme Master in Drug Development
DERIVATIZATION OF CANNABINOIDS
TO IMPROVE THE DETECTION OF
THESE WIDELY ABUSED SUBSTANCES
BY LC-MS/MS
Helena Schoon
A Master dissertation for the study programme Master in Drug Development
Deze pagina is niet beschikbaar omdat ze persoonsgegevens bevat.
Universiteitsbibliotheek Gent, 2021.
This page is not available because it contains personal information.
Ghent University, Library, 2021.
SUMMARY
Cannabis is a commonly used illegal drug. Therefore, detecting Cannabis consumption requires attention in forensic, driving under the influence of drugs and workplace related cases. In these cases, a biological analysis is mainly performed in venous blood samples, but recently the use of dried blood spot samples has gained growing interest due to the numerous associated advantages. The LC-MS/MS analysis of the cannabinoids in these samples is not straightforward. The lipophilic properties and the low ionization efficiency of the analytes make the analysis and detection of these components challenging.
Recently, a method based on the derivatization of cannabinoids with the derivatization reagent Fast Red RC was introduced in the literature. This Fast Red RC derivatization should, according to the authors, improve the ionization efficiency of the cannabinoids and therefore also the detection sensitivity. In this project, we first examined whether these promising findings are reproducible. Next, this derivatization method was optimized for certain parameters such as the concentration of the Fast Red RC solution. This optimized method was then used to perform a tuning of the LC-MS/MS instrument for the derivatized cannabinoids in order to obtain a more targeted LC-MS/MS analysis of these derivatives. Finally, the optimized method was also used to determine whether the Fast Red RC derivatization approach is also applicable in the presence of the biological matrix blood. This together is an essential step to ultimately achieve one of the initial goals of this project in the future. This primary goal was to test an in-situ reaction for the analysis of cannabinoids on dried blood spot cards, i.e. to answer the question if the derivatization reaction can be performed directly in dried blood spot samples.
When verifying the reproducibility of the literature data, the results showed some similarities and differences. However, it was concluded with certainty that the derivatization of the cannabinoids with the reagent Fast Red RC occurs. It could not be derived directly, even after the optimization of the method that the ionization efficiency and detection sensitivity was improved. By optimizing the derivatization method a better intensity, separation and shape of the peaks was obtained. When the tuning of the LC-MS/MS instrument was finalized, the optimal values of the MRM parameters were determined for each derivatized cannabinoid. In addition, the results after tuning were more in line with the literature data. The Fast Red RC derivatization of the cannabinoids can also be applied in spiked venous blood samples, but further development of the derivatization method is still needed in this context.
After a successful validation of this optimized derivatization method, it can be promising to improve the LC-MS/MS analysis of the cannabinoids in the emerging dried blood spot samples by an in-situ reaction.
SAMENVATTING
Cannabis is een veelgebruikte illegale drug. Het opsporen van Cannabis gebruik vereist daarom aandacht in forensische, rijden onder invloed van drugs en werk gerelateerde zaken. In deze gevallen wordt een biologische analyse voornamelijk uitgevoerd in veneuze bloedstalen, maar sinds kort is de interesse voor gedroogde bloedspots sterk toegenomen vanwege de vele bijhorende voordelen. De LC-MS/MS analyse van de cannabinoïden in deze stalen is niet vanzelfsprekend. De lipofiele eigenschappen en de lage ionisatie-efficiëntie van de analyten bemoeilijken immers de analyse en detectie van deze componenten.
Recent werd een methode gebaseerd op de derivatizatie van cannabinoïden met het derivatizatie reagens Fast Red RC in de literatuur geïntroduceerd. Deze Fast Red RC derivatizatie zou volgens de auteurs de ionisatie-efficiëntie van de cannabinoïden moeten verbeteren en bijgevolg ook de detectiegevoeligheid. In dit project werd eerst onderzocht of deze veelbelovende bevindingen reproduceerbaar zijn. Vervolgens werd deze derivatizatie methode geoptimaliseerd voor enkele parameters, zoals de concentratie van de Fast Red RC oplossing. Deze geoptimaliseerde methode werd vervolgens gebruikt om een tuning uit te voeren van het LC-MS/MS instrument voor de gederivatizeerde cannabinoïden om zo een meer gerichte LC-LC-MS/MS analyse van deze derivaten te bekomen. Tot slot werd de geoptimaliseerde methode ook gebruikt om te bepalen of de Fast Red RC derivatizatie ook toepasbaar is in de aanwezigheid van de biologische matrix bloed. Dit samen is een essentiële stap om in de toekomst één van de initiële doelstellingen van dit project te bereiken. Dit primaire doel was het testen van een in-situ reactie voor de analyse van cannabinoïden op gedroogde bloedspot kaarten, meer bepaald de vraag of de derivatizatie reactie direct in gedroogde bloedspot stalen kan worden uitgevoerd.
Bij het verifiëren van de reproduceerbaarheid van de literatuurdata vertoonden de resultaten zowel enige overeenkomsten als verschillen. Met zekerheid kan er worden geconcludeerd dat de derivatizatie van de cannabinoïden met het reagens Fast Red RC plaatsvindt. Er kon echter niet direct worden afgeleid, zelfs niet na de optimalisatie van de methode, dat de ionisatie-efficiëntie en de detectiegevoeligheid verbeterd waren. Door het optimaliseren van de derivatizatie methode werd een betere intensiteit, scheiding en vorm van de pieken verkregen. Eens de tuning van het LC-MS/MS-instrument was afgerond, werden de optimale waarden van de MRM parameters bepaald voor elk gederivatizeerd cannabinoïd. Bovendien waren de resultaten na de tuning meer in lijn met de literatuurdata. De Fast Red RC derivatizatie van de cannabinoïden kan ook worden toegepast in spiked veneuze bloedstalen, maar verdere ontwikkeling van de derivatizatie methode is in deze context nog nodig. Na een succesvolle validatie van deze geoptimaliseerde derivatizatie methode kan dit veelbelovend zijn om de LC-MS/MS analyse van de cannabinoïden in gedroogde bloedspots te verbeteren door een in-situ reactie.
ACKNOWLEDGMENTS First of all, I would like to sincerely thank my supervisor, Ph.D. student Tim Gelmi, for always assisting me in this learning process of the master thesis. I would like to thank him for his willingness to always answer my questions, for reviewing my work many times and for an unforgettable experience.
My sincere gratitude goes to Professor Christophe Stove for giving me the unique opportunity to write this thesis abroad. I would like to thank him for the feedback conversations and the co-guidance of the thesis.
My gratitude also goes to my promotor Professor Wolfgang Weinmann at the Institute of Forensic Medicine in Bern for passionately exchanging his extensive knowledge and the insightful comments on my work.
I would also like to thank the lab technicians and other employees of the Institute of Forensic Medicine in Bern for always offering help and for the pleasant hospitable environment.
Last but not least, I would like to strongly emphasize my gratitude to my family and friends. I would like to thank my parents, because without them I would never had the opportunity of an Erasmus experience. A loving thank you also goes to Nathan for supporting me in this adventure and for always believing in me.
PREAMBLE: IMPACT OF COVID-19 ON THIS MASTER THESIS
The main objective of this master thesis was to conduct an experimental study regarding the derivatization of cannabinoids by the Fast Red RC reagent by LC-MS/MS within the frame of an Erasmus exchange
at the University of Bern. In the period of February 10th up to March 26th, I was able to work in the lab without
restrictions and about 30 % of the predetermined experimental results for the whole thesis were obtained at
the end of this period. However, on March 27th regulations of the University of Bern and of Ghent obliged me to
stop working in the lab. It was agreed with my Swiss promotor and supervisor that I should return to Belgium. After my return, my supervisor mailed me some more results of the tuning of the LC-MS/MS instrument, which I was able to incorporate in my thesis. I had regular, helpful contact with my supervisor (email, Skype, WhatsApp) about the interpretation/discussion of the results, but this is clearly different and less straightforward than almost daily face-to-face opportunities. However, one of the main objectives was also to experimentally test and optimize the derivatization method on dried blood spots, which I could not execute due to my early return. As agreed with my Swiss promotor and supervisor, this was replaced by a more detailed literature research in the context of dried blood spot sampling and derivatization of the latter.
This preamble was drawn up in agreement between the student and the supervisor and approved by both.
TABLE OF CONTENTS 1. INTRODUCTION ... 1 1.1. CANNABIS ... 1 1.2. CANNABINOIDS ... 1 1.2.1. Natural cannabinoids ... 2 1.2.1.1. (-)-9-tetrahydrocannabinol ... 2 1.2.1.2. Cannabidiol ... 4 1.2.2. Cannabinoid receptors ... 5
1.3. SAMPLING IN THE FORENSIC TOXICOLOGY ...6
1.3.1. Dried blood spot sampling ... 7
1.3.1.1. Automatization of the DBS analysis ...9
1.3.1.2. The extraction procedure ...9
1.3.1.3. DBS cards/papers ...10 1.4. DERIVATIZATION ... 11 1.4.1. Dabsyl chloride ... 12 1.4.2. Fast Red RC ... 14 1.5. DEGLUCURONIDATION ... 16 2. OBJECTIVES ... 17
3. MATERIALS AND METHODS ... 19
3.1. MATERIALS ... 19
3.1.1. Chemicals and reagents ... 19
3.1.2. LC-MS/MS instrument ... 19
3.2. METHODS ... 20
3.2.1. Reproducibility of the literature data ... 20
3.2.1.1. Fast Red RC solution ... 20
3.2.1.2.Sample preparation ... 20
3.2.1.3.LC-MS/MS analysis ... 21
3.2.2. Optimization of the derivatization method... 23
3.2.2.1.Fast Red RC solution ... 23
3.2.2.2.Sample preparation ... 23
3.2.2.3.LC-MS/MS analysis ... 24
3.2.3. Tuning of the LC-MS/MS instrument ... 25
4. RESULTS ... 27
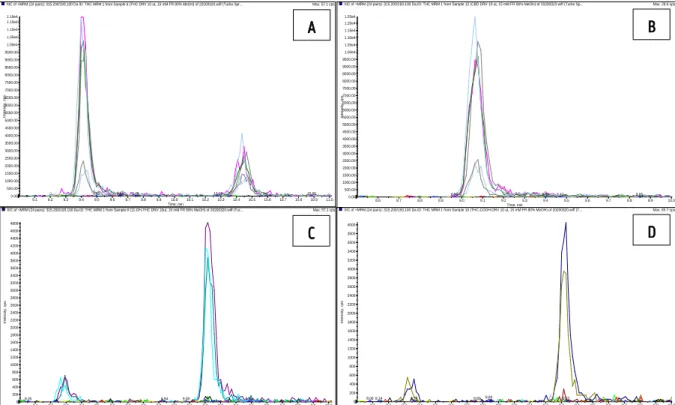
4.1. REPRODUCIBILITY OF THE LITERATURE DATA ... 27
4.2. OPTIMIZATION OF THE DERIVATIZATION METHOD ...30
4.3. TUNING OF THE LC-MS/MS INSTRUMENT... 32
4.4. DERIVATIZATION APPROACH IN WHOLE BLOOD ... 36
5. DISCUSSION... 38
6. CONCLUSION ... 44
7. REFERENCES ... 45
LIST OF ABBREVIATIONS
11-OH–THC: (±)-11-hydroxy-9-tetrahydrocannabinol
CB: Cannabinoid receptors CBD: Cannabidiol
CBDA: Cannabidiolic acid CBGA: Cannabigerolic acid CE: Collision energy
CXP: Collision cell exit potential CYP: Cytochrome P450
DABS-Cl: Dabsyl chloride DBS: Dried blood spots
DMPK: Drug Metabolism and Pharmacokinetics DP: Declustering potential
DRV: Azo-derivative
DUID: Driving under the influence of drugs EMS: Enhanced mass spectrometry scan EPI: Enhanced product ion
FAAH: Fatty acid amide hydrolase FTA: Flinders Technology Associates HCT: Haematocrit
IRM: Institute of Forensic Medicine IS: Ionspray voltage
ISTD: Internal standard
LC-MS/MS: Liquid chromatography tandem mass spectrometry MeOH: Methanol
MRM: Multiple reaction monitoring TEM: Temperature of the ion source
THC: (-)-9-tetrahydrocannabinol
THCA: Tetrahydrocannabinolic acid
THC–COOH: (±)-11-nor-9-carboxylic-9-tetrahydrocannabinol
TR: Retention time
1 1. INTRODUCTION
1.1. CANNABIS
Cannabis is a widely used illegal drug (1,2). This drug is derived from the Cannabis sativa L. plant, a hemp plant native to Central Asia and growing in mild and tropical climates. In the past, the plant was already used for its medicinal and textile characteristics (3,4). The leaves and the flowering tops of the hemp plant yield
cannabinoids like (-)-9-tetrahydrocannabinol (THC), the primary psychoactive substance of Cannabis. Cannabis
is typically smoked as a joint containing the plant material (5).
Frequently, Cannabis is seen as a soft drug because it would lead to poor physical dependence but nothing could be further from the truth. Dependency can certainly occur with drug seeking behaviour and desire for drug use (6). In most cases, Cannabis use can’t cause fatal overdoses because the drug cannot be easily injected (7). However, the acute effects of drug consumption should not be underestimated. Cannabis use has a strong influence on the cognitive and psychomotor abilities, including hallucinations, delayed reflexes and reduced coordination (1,8,9).
Cannabis is often involved in forensic, workplace related and driving under the influence of drugs (DUID) cases but it can also be present in the clinical field (1,2). Cannabinoid medications are being used for a growing number of symptoms (10). At the moment, only one Cannabis extract is licensed for the treatment of moderate to severe spasticity in multiple sclerosis, named Sativex. The formulation of this pharmaceutical product is a buccal spray (11,12). Besides that, Cannabis based medicines are also commonly used for the treatment of nausea, vomiting, loss of appetite and neuropathic pain. The administration of these preparations can be justified for patients whose conventional therapy does not provide sufficient relief of their symptoms. Like all other drugs, these cannabinoid medications show some side effects, e.g. tiredness, dizziness and dry mouth. After a short period of time, there is already development of tolerance to many of these adverse effects. Withdrawal symptoms are rarely occurring in the controlled clinical setting but it may represent a problem in heavy, chronic users of Cannabis after abrupt interruption of the consumption (12).
1.2. CANNABINOIDS
The most well-known natural constituents of the Cannabis plant are the C21 terpenophenolic cannabinoids, also referred to as the phytocannabinoids. THC and cannabidiol (CBD) are the two major examples of the approximately 60 natural cannabinoids. Furthermore, there is also the development of synthetic
2 cannabinoids and the further exploration of endogenous cannabinoids (3).
At the present, synthetic cannabinoids form a group of very diverse compounds which have been quickly incorporated into the illicit market of psychoactive drugs (13–15). On this market, they are easily accessible and sold under several brand names such as Spice and K2. The packaging is usually labelled with “not for human consumption”. The manufacturing of these abusable synthetic variants by clandestine laboratories represents a serious risk for the human society. These synthetic analogues produce comparable psychotropic effects as the natural cannabinoids but with a greater intensity. This can be explained by the fact that these compounds are typically more potent. On top of that, they also exhibit more severe side effects. It is a real challenge for investigators to identify and analyse these structurally modified substances because new ones are continuously being synthesized. Through this constant flow of novel synthetic cannabinoids, the detection of these compounds by routine screening tests can be bypassed which makes them of course interesting for abuse (13–15).
In the human body, the endocannabinoids belong to the group of eicosanoids, with anandamide and 2-arachidonoyl glycerol being the two prominent examples (16,17). It is likely that they act both as neuromodulator and immunomodulator. There is also some evidence that the endocannabinoids are involved in certain pathologies in which they fulfil an autoprotective role. By convention, the endocannabinoids, the cannabinoid receptors and the physiological interaction between them constitute the endocannabinoid system (14,16).
1.2.1. Natural cannabinoids
The natural cannabinoids occurring in the Cannabis plant are divided into two types: the neutral cannabinoids and the cannabinoid acids with the former in much lower quantities. The biosynthesis of the not pharmacologically active cannabinoid acids, tetrahydrocannabinolic acid (THCA) and cannabidiolic acid (CBDA), is mediated through a stereospecific oxidative cyclization of the geranyl group of cannabigerolic acid (CBGA). This biosynthetic reaction is catalysed by respectively THCA- and CBDA synthase. The structural and functional features of these two enzymes are analogous. During storage and smoking of the plant material, the neutral cannabinoids THC and CBD are generated from their corresponding acid precursor via a nonenzymatic decarboxylation (18,19). The biosynthesis of these natural cannabinoids is illustrated in Figure 1.1.
1.2.1.1. (-)-9-tetrahydrocannabinol
THC is an aromatic compound that contains two chiral centers and is optically active (5,9). As a result of the latter, there exist (-) enantiomers which are present in the nature and (+) enantiomers which can only be
3 Figure 1.1: The biosynthesis of the cannabinoid acids and the neutral cannabinoids (19)
obtained synthetically. Moreover, are the (+) enantiomers also pharmacologically inactive. Isomerization of 9
-THC with the formation of 8-THC is also another pathway. However, 8-THC is present in a small amount
resulting in a not significant contribution to the psychoactive effect of Cannabis (5).
The absorption of the cannabinoids is extremely fast when Cannabis is smoked. This rapid absorption causes a fast occurrence of the acute effects. These are the main arguments for so many people to smoke Cannabis as their favourite way of dosing. Nevertheless, the absorption can be quite variable due to the origin of the plant material, the content of the cigarette and the controlled efficiency of smoking by the persons smoking technique. Apart from that, Cannabis can alternatively be taken orally or rectally. The oral absorption is also variable and much slower (5).
The distribution of the cannabinoids starts directly after the absorption. Because THC has a lipophilic character, this compound is largely distributed to the fatty tissues, including the brain. This results in a high volume of distribution. Despite the fact that THC easily passes the blood-brain barrier, less than 1 % of the absorbed amount reaches the brain. The regular use of Cannabis causes that THC will accumulate in the fatty tissues after which it is slowly released from these fatty storage sites into the blood (5).
4 THC is rapidly and extensively metabolized especially in the liver by cytochrome P450 (CYP) isozymes (1,5,12). CYP 2C9 and CYP 2C19 are the principal isozymes that convert THC to certain phase I metabolites (10,12). The initial reaction consists of a hydroxylation at the C11 position of the THC molecule with the formation of the
phase I metabolite (±)-11-hydroxy-9-tetrahydrocannabinol (11-OH–THC). After this reaction, further oxidation
may take place to the acidic phase I metabolite (±)-11-nor-9-carboxylic-9-tetrahydrocannabinol (THC–COOH).
Phase II metabolism catalysed by uridine 5’-diphospho-glucuronosyltransferase can also occur to produce glucuronides of THC, 11-OH-THC and THC-COOH (1,10). The most common THC-COOH glucuronide conjugate is the ester-linked-β-glucuronide where the glucuronic acid is attached to the 11-COOH group. The glucuronic acid molecule may also bind to the phenolic OH group of THC-COOH to form the ether-linked-β-glucuronide. On top of that, it is also an option to have two glucuronic acid groups coupled to THC-COOH (1).
The determination of THC and its two phase I metabolites in biological fluids makes it possible to differentiate between recent or former use. This can be relevant information in certain settings like DUID cases (8). 11-OH-THC appears directly after the use of Cannabis in biological fluids where it has a short detection window. Therefore, the presence of 11-OH-THC is a marker for recent Cannabis use. On the other hand, THC and THC-COOH have a longer detection window (13).
The elimination of THC and the metabolites takes place via the urine and the feces. The metabolites that are found in the urine have mostly an acidic nature and the ones in the feces are as well acid as neutral. The metabolites remain in the feces and the urine for a couple of weeks. Hence, users of Cannabis should be aware of the fact that traces of Cannabis are detectable in the feces and the urine weeks after consumption (5).
1.2.1.2. Cannabidiol
CBD is the main non-psychotropic cannabinoid of the Cannabis plant (12,20). The metabolization of CBD is initiated by a hydroxylation at the C7 position, yielding 7-hydroxy-cannabidiol. Successive further oxidations produce cannabidiol-7-oic acid and several other hydroxylated derivatives of this acid. Glucuronides can also be formed of these oxidized metabolites (20).
There are indications that CBD can supress the THC induced psychosis, anxiety and neurotoxicity (21). The mechanism of action of CBD is not precisely known but it is clear that it does not bind with the cannabinoid receptors, resulting in no activation of these receptors. CBD rather inhibits the hydrolysis of the endocannabinoid anandamide which is normally catalysed by the enzyme fatty acid amide hydrolase (FAAH). In this way, CBD
5 increases the anandamide levels and thus enhances the endogenous signalling of anandamide. The extensively available endocannabinoid can then avoid that THC binds to the cannabinoid receptors resulting in a contribution to the antipsychotic property of CBD. Besides the reduction of the psychotic symptoms of THC by FAAH inhibition, CBD can even interact with other targets such as the serotonin 5-HT1A receptors. This could also explain the antipsychotic and anxiolytic effects of CBD. Finally, due to its influence on the intracellular calcium concentrations, CBD might be able to protect neurons from the potential neurotoxic effects of THC. More studies are needed to clarify which CBD/THC ratio and minimum CBD concentration are necessary to achieve these protective effects (20–22).
1.2.2. Cannabinoid receptors
The human body expresses at least two types of cannabinoid receptors (CB), namely CB1 and CB2. The CB1 receptors were cloned in 1990 and three years later this was also realized for the CB2 receptors. The distinction between the two receptors is based on the amino acid sequence, tissue distribution and signalling pathway. The CB1 receptors are mainly present in the central nervous system on the nerve terminals but they are also observed on peripheral neurons and in certain peripheral tissues like for example the pituitary gland, immune cells, gastrointestinal tissues, heart, lungs, etc. In the brain, the CB1 receptors are not evenly distributed over the different brain regions. One of their functions on the neurons is inhibiting the release of neurotransmitters. The CB2 receptors are predominantly found on immune cells, particularly B-cells and natural killer cells. One of their actions is to regulate the cytokine release. Thus, a common factor of the two cannabinoid receptors seems to be the modulation of the release of chemical messengers. Both receptor types have the structure of a seven transmembrane domain receptor and are coupled to G-proteins. They are negatively linked to adenylate cyclase and positively to mitogen-activated protein kinase. The CB1 receptors are also associated via these G-proteins to several types of calcium and potassium channels. In general, this indicates that the cannabinoid receptors are involved in numerous interactions and physiological actions (12,16,23–25).
THC is a cannabinoid agonist with a comparable affinity for the two cannabinoid receptors. At the molecular level, it interacts as a partial agonist for the CB1 receptors. The endocannabinoids, anandamide and 2-arachidonoyl glycerol are the endogenous ligands for the CB1 and CB2 receptors. Anandamide acts as a partial agonist for both receptors but it has a somewhat higher affinity for the CB1 receptors. The pharmacological properties of 2-arachidonoyl glycerol are not yet fully characterized (13,16,23–25).
6 By activating the receptors, cannabinoid agonists may have a therapeutic potential for a range of indications. It has been reported that they can be used for the treatment of glaucoma, inflammatory and neuropathic pain, cancer and certain multiple sclerosis symptoms (24). This can be further clinically exploited. Long-term administration of CB1 receptor agonists may lead to tolerance for some of their effects. This tolerance is ascribed to the reduction of the receptor’s capacity to trigger the signalling pathways and to internalization of the cell surface-expressed CB1 receptors (16,25).
1.3. SAMPLING IN THE FORENSIC TOXICOLOGY
A part of the forensic toxicology studies the presence or absence of pharmaceutical and illegal drugs in the human body but also of other chemical substances like volatiles, metals, etc. The impact of these analytes on the human performance and behaviour is also assessed. A toxicological analysis is mostly performed by using venous blood samples that are obtained by conventional venipuncture. Blood is the most essential and useful biological matrix in the forensic toxicology but alternative specimens may also be appropriate like for instance urine, breath, oral fluid and hair (13,26). Venous blood is of great importance because a direct relation can be established between the concentration of the analyte and its pharmacological effects. Moreover, not only the concentration of the analyte can be determined but also the concentration of the corresponding metabolites.
If there is any suspicion of a Cannabis intoxication during a roadside testing, this can be evaluated by taking a blood sample but there are a few issues in this case. First, the sampling is invasive, the individual might experience discomfort and the sampling cannot be collected by the police but requires the assistance of medical personnel. As a result, there may be a wide time interval between the initial determination of possible drug abuse and the actual blood collection. In this time interval, the blood concentration of the respective analyte can decrease below a certain limit. Secondly, the blood samples need some pre-treatments such as adding anticoagulants and they need storage precautions (8,27). On top of that the transport of the blood samples is extremely expensive due to the dry ice needed to preserve the samples.
In forensic toxicology, the sample volume is a critical parameter for bioanalyses. By using microsampling, the sample volume can be reduced due to the collection of only a small amount of blood, in particular some tens of microliters. However, it is still important that the obtained sample volume is adequate for the detection and quantification of a variety of drugs, including the cannabinoids (1). Hence, a drawback of microsampling can include sensitivity issues (28). Nevertheless, microsampling is a potential alternative for the classical venipuncture. The sampling procedure can be done by a single skin prick and by using glass or plastic
7 microcapillary tubes of a certain volume for the blood collection. For this reason, it is much less invasive and allows access to populations incapable to offer large sample volumes, for example for children in pediatrics, elderly and severely ill patients. This results in a lower physiological and emotional stress for these populations. The microcapillary tube filled with the blood can then eventually be transferred to a filter paper for making dried microsamples (29,30). Another advantage aside the ease of sampling, is the ability to store and transport the samples at ambient temperature. This benefit provides the opportunity for at home sampling by the persons themselves but where the supervision of correct sampling is lost. In the near future, a broader application of microsampling could be expected. The microsampling techniques have also a positive impact on the ethical problems concerning laboratory animals. It offers the possibility to refine and reduce the number of animals used in experiments (28).
1.3.1. Dried blood spot sampling
Dried blood spot (DBS) sampling is a microsampling technique that was first documented in 1913 by Ivar Bang for the monitoring of glucose. In 1963, Guthrie and Susi introduced the concept of DBS worldwide. They revealed it as a new sampling method for testing neonates on the presence of a congenital metabolic disorder known as phenylketonuria. A few days after the birth of the child, the DBS is obtained via a heel prick that produces a larger sample volume than a finger prick. After the screening test, the remaining DBS matrix has an added value to find out whether the child has been exposed to toxic substances during pregnancy. It should be noted that these outcomes may be biased by post-natal exposure due to breastfeeding. The ability to detect a positive result can make a crucial difference to the child’s quality of life. Since this novel approach, the usage of DBS as an alternative sampling procedure has grown in popularity in the healthcare, pharmaceutical and toxicological field (31,32).
In the context of DUID, DBS are also very promising. Whenever there is a request for more information about a suspected Cannabis consumption, the DBS sampling can be performed directly on-field by the police or not medically trained individuals. Consequently, this has a positive effect on the time span until sampling (13,31– 33). Mercolini et al. developed a new analytical method that provides new advances in the cannabinoid analysis, as their method succeeds in simultaneously identifying and quantifying the analytes THC, 11-OH-THC and THC-COOH in the emerging DBS. This analytical method can be promising because only the simultaneous determination of these analytes ensures the most accurate assessment of the state of Cannabis intoxication (8).
8 The DBS technique shows also other advantages in comparison with conventional blood sampling which makes it an intriguing alternative in toxicological analyses. In the first place, this sampling technique contains all the above-mentioned benefits of the general microsampling technique. Secondly, during the drying of the blood spot, water loss takes place so that enzymatic and/or hydrolytic reactions normally responsible for the decomposition of the analytes are inhibited. As a result, the DBS matrix has a stabilizing effect. This effect also avoids de novo formation of the analytes. Altogether, the DBS increase the reliability of the results, especially if the analysis is carried out a long time after the DBS sampling. Another advantage of the DBS is the improved safety by reducing the biohazard risk. In the context of at home sampling, the DBS card can therefore easily be sent to the appropriate laboratory by post without a high infection risk by different pathogens (13,31–33).
Unfortunately, there are also some drawbacks associated with the DBS sampling. The application of DBS is not suitable for any analyte. Volatile and air-sensitive analytes are less compatible with the DBS sampling. The small sample volume implies also a requirement of high sensitive instruments. Another negative factor to be considered in the DBS sampling is the risk of contamination of the DBS sample. If a person touches a drug powder without taking the drug himself, contamination can happen when taking the blood sample via a fingerprick. This would lead to a false positive result if the DBS is analysed. This negative factor could be reduced by identifying not only the parent molecule, but also the metabolites. In case of external contamination, the latter will not be detected (32,33). It could also be overcome by simply cleaning the finger with an alcohol-free disinfection wipe before the fingerprick.
One of the main concerns in the DBS procedure is the interindividual variability of the haematocrit (HCT). The HCT is the fraction of the whole blood volume that contains the red blood cells and affects the viscosity of the blood. If blood with a high HCT value is spotted onto a filter paper, the fluid will not diffuse widely and will give a small size of the blood spot as opposed to blood with a low HCT value. Analysing a subpunch of this DBS will then result in respectively an over- or underestimation of the analyte concentration. This will obviously lower the quality of the DBS data. The HCT effect can be largely circumvented by analysing a whole, volumetrically applied spot instead of using a partial punch. This also simplifies the validation of a DBS method as the validation parameters punch location, blood volume spotted, blood distribution and HCT have no longer an influence on the assay. Another potential strategy to bypass the HCT problem is to replace the DBS by dried plasma spots. Furthermore, the HCT value of the blood fulfils also a role in the conversion of a DBS concentration to a plasma concentration. Noteworthy is the fact that the similarity or correlation between DBS concentrations and those in venous blood must be evaluated by performing supplementary experiments (32–35).
9 1.3.1.1. Automatization of the DBS analysis
The standard, manual processing of the DBS samples in the laboratory is very labour-intensive and time-consuming (36,37). The classical sample preparation includes punching out the DBS from the filter paper and afterwards subjecting the disk to an extraction. There are several ways to incorporate the internal standard (ISTD) of the analyte in the sample preparation. In the regular methods, it is most of the time added to the extraction solvent but that creates also a problem. This way the ISTD does not come directly in contact with the blood so it cannot take the matrix effects into account. Subsequent, the extract is centrifuged. The supernatant can then be analysed with liquid chromatography tandem mass spectrometry (LC-MS/MS) either directly or after evaporation and reuptake in a reconstitution solvent (36).
Since many applications handle a high throughput of samples, there is a growing demand for automatization of the DBS analysis processes. Nowadays, various instrumental systems are commercially available for partial or fully automation. The CAMAG DBS-MS 500 and the DBS Autosampler are two examples of these systems, which are available for fully automated DBS LC-MS/MS analysis. A review article of Luginbühl et al., discusses the handling and the application of the DBS-MS 500 autosampler from CAMAG (37). A fully automated DBS analysis means that only the DBS card has to be manually inserted in the setup and hereafter the punching, extraction, ISTD addition and analysis by the linked LC-MS/MS system are performed entirely automatic. This creates minimal human interaction, time saving and a remarkable reduction in consumables and costs. However, the exploitation of a fully automated system causes also an increased complexity. There are some challenges such as finding an extraction solvent that is suitable for extracting the analyte, but also fits with the LC-MS/MS starting conditions. This automated procedure in combination with DBS has also proven to be of great interest for the analysis of abused substances (37). Gaugler et al. published an approach for this purpose, including the cannabinoids (38).
1.3.1.2. The extraction procedure
The extraction procedure takes place during the sample preparation of the DBS samples during which the analyte has to be extracted from the punched DBS by using a suitable extraction solvent. This means that the analyte is transferred from the filter paper, a solid form to a liquid phase. The extraction solvent used is chosen based on the solubility and chemical properties of the analyte of interest and with the additional requirement that interfering endogenous components are extracted as few as possible. In addition, this extraction solvent should also provide the highest possible extraction efficiency of the analyte. This extraction procedure is usually done manually, off-line via solvent extraction. In this case, a mixture of aqueous and organic
10 solutions is often used as extraction solvent but for the extraction of large molecules such as proteins, aqueous buffers are rather used (39–42). When the proportion of the organic solution such as methanol (MeOH) and/or acetonitrile increases in the mixture, more analyte is selectively extracted and fewer endogenous, matrix components. This reduces the matrix effect that can interfere with the LC-MS/MS analysis of the analyte. After adding the extraction solvent to the DBS disk in a tube, it is vortexed for a certain time depending on the analyte of interest (41,42). This off-line solvent extraction has some drawbacks like that it demands a lot of time and that a dilution of the concentration of the analyte occurs (39). At the Institute of Forensic Medicine (IRM) in Bern the organic solution acetonitrile is used as extraction solvent in the routine analysis of cannabinoids. During vortexing for about ten minutes, not only the extraction of the cannabinoids occurs but at the same time acetonitrile causes protein precipitation.
Liquid-liquid extraction is another kind of extraction procedure which is done in two steps. First an aqueous solution is added to the DBS sample and after this first extraction, a water immiscible organic solution is added as for example hexane, chloroform or ethyl acetate. After well shaking, the organic layer is separated from the aqueous layer. This organic phase is a more purified extract because of this procedure compared to the above mentioned solvent extraction. However, liquid-liquid extraction has also some drawbacks like high consumption of solutions, labour intensive and low extraction efficiency for hydrophilic, polar analytes (42,43).
Solid-phase extraction is the most universal extraction technique. It provides again a cleaner extract of the DBS samples than solvent extraction and there is a variety of sorbents existing for solid-phase extraction. Therefore, this extraction technique is compatible for almost all types of analytes (42).
1.3.1.3. DBS cards/papers
The DBS cards differ among each other in some parameters such as the composition of the paper, cellulose or non-cellulose matrix, the pore size and the thickness of the paper (40). These last two parameters are of importance because they determine how much of the blood sample can be applied on the paper, i.e. the loading capacity, how fast the blood is sucked up by the paper and the spreading of the blood droplet (33).
Currently, there are a number of commercial, cellulose based DBS cards available, like Whatman 903 cards, Flinders Technology Associates (FTA) Drug Metabolism and Pharmacokinetics (DMPK) cards type A, B, C and FTA Elute cards. They are each used for a different purpose. Whatman 903 cards produced with cotton cellulose linters, are the standard for DBS analysis and are also routinely used for neonatal screening. FTA DMPK cards
11 type A, B, C are mainly utilized in pharmaco- and toxicokinetic studies and the FTA Elute cards are often used for nucleic acids analysis (33,40,44–46).
For all three types of FTA DMPK cards the following two forms are available: regular and indicating cards. The regular cards are colourless white papers, so that an applied spot of a colourless biological fluid like urine is difficult to visualise, especially when this spot is dry. Therefore, indicating cards are more valuable for these colourless biological fluids. These papers contain a blue dye which is displaced when these samples are applied. In this way the spot area is directly visible after both application and drying (40,44). Type A and B FTA DMPK cards are typically chemically coated so whenever biological samples are spotted on these chemically treated papers the cells are lysed, proteins are denatured and endogenous enzymes are inhibited. This provides a stabilizing effect of the analytes and also avoids the growth of microorganisms. Type C FTA DMPK cards are not treated with chemical reagents so that proteins present in the spotted sample are not denatured. These cards are thus suitable for protein based biomolecules analysis. In general, DBS cards can be chemically treated by various coating processes to obtain papers with the preferred properties. This is how the DBS cards/papers for example can be hydrophobized or treated with antioxidant (33,40,44,46).
However, the fact that several chemical card components can interfere with the analytes is an important point of attention. In case of LC-MS/MS analysis, chemicals eluting at the same time as the analyte of interest may supress the ionization of the analyte and alter the chromatographic retention time and peak shape. This makes it necessary to evaluate these potential interactions, especially in the case of chemically coated DBS cards. The LC-MS/MS conditions can be adapted to eliminate these unwanted interferences. This negative aspect will be lower with type C FTA DMPK cards as they are not chemically treated (40,44–46).
1.4. DERIVATIZATION
LC-MS/MS has become an indispensable analytical technique as a result of its intrinsic selectivity and sensitivity. However, for successful LC-MS/MS analyses, the analytes must possess some properties such as chemically stable, ionizable, exhibit retention on the analytical columns and easy to fragment with production of detectable fragment ions. Unfortunately, there are many analytes that are for example difficult to ionize or fragment, which makes LC-MS/MS analyses of these components more complicated. Chemical derivatization of these analytes is a strategy that can be applied to improve the ionization efficiency, chemical stability, chromatographic retention, separation and consequently the detection (47,48).
12 Derivatization techniques have been developed in the 1980s and since then its use has grown in multiple fields such as peptide and protein-, metabolite-, pharmaceutical- and environmental analysis. The derivatization modifies the molecular structure of the analyte and hence its chemical and physical characteristics. Derivatization is a chemical reaction between the reactive functional groups of the analyte and the derivatization reagent. A distinction can be made between the different derivatization reagents based upon the functional group of the analyte with which they react. A selection from the large number of available derivatization reagents is made based on the molecular structure and reactive functional groups of the analyte of interest and the desired purpose of the chemical derivatization in the context of LC-MS/MS analysis (47,48).
Nevertheless, there are some points of attention that need to be taken into account in derivatization-based LC-MS/MS analysis. First of all, a good understanding of the chemical reaction is necessary. The selectivity of the derivatization reagent and the reaction conditions are important parameters. It is therefore favourable to optimize these in such a way that a fast, efficient and specific derivatization reaction occurs with the exclusion of a long reaction time and by-product formation. Secondly, degradation of the derivatives may occur and the excess, unreacted derivatization reagent may interfere with the LC-MS/MS analysis (47,48).
The analytical technique LC-MS/MS with electrospray ionization is often used for the analysis of cannabinoids, but these lipophilic, neutral components have a low ionization efficiency which leads to poor detection sensitivity and limit of quantification. By derivatization of the cannabinoids with the appropriate derivatization reagent, a group can be integrated in the molecular structure, which for example is permanently charged or easily ionizable. This strategy improves the ionization efficiency and detection sensitivity of the cannabinoids (1,47–49). The derivatization reagents dabsyl chloride (DABS-Cl) and Fast Red RC can be utilized for the derivatization of the cannabinoids which will be discussed hereafter.
1.4.1. Dabsyl chloride
The DABS-Cl derivatization of cannabinoids was first discussed and applied in 1983 by Maseda et al (1). The phenolic OH group present in all cannabinoids may react with the sulfonyl group of the derivatization reagent as illustrated in Figure 1.2 for THC. This results in a more lipophilic cannabinoid derivative containing a tertiary amine, a group that is easily ionizable. In this way, the DABS-Cl derivatization can improve the ionization efficiency of the cannabinoids in the electrospray ionization process. This derivatization can also be used for other illegal drugs such as opiates and for substances like amino acids. For any component, the DABS-Cl derivatization should be carried out under alkaline conditions (1,47).
13 Figure 1.2: Dabsyl chloride derivatization of the cannabinoid THC (1)
Lacroix et al. have successfully developed a LC-MS/MS method for the simultaneous quantitative determination of the cannabinoids (THC, CBD, 11-OH -THC and THC-COOH) using small blood sample volumes (50 µL), DABS-Cl derivatization and no time-consuming extraction step (1). The derivatization approach improved the signal intensity and detection sensitivity of the cannabinoids. A limit of quantification was obtained of 0.40 ng/mL on average. Moreover, the developed derivatization-based LC-MS/MS method was successfully validated. The method still needs to be further elaborated to apply it in the context of DBS (1).
The DABS-Cl reagent was prepared in the solvent tetrahydrofuran. For the derivatization of the 50 µL spiked whole blood samples 20 µL of the derivatization reagent and 20 µL of 0.1 M NaOH were used. The samples were also heated to 70 °C for five minutes. The derivatization reaction was stopped by putting the samples in fresh water during one minute so that the temperature decreased. Maseda et al. showed that for the derivatization of THC and CBD a pH of 10.5 in the reaction medium is optimal (1). This alkaline pH can be reached with the use of NaOH. In this study, however, the derivatization reaction was stopped by adding a buffer, which caused the pH to drop below 6. At this low pH no more formation of derivatives was observed. This has the main disadvantage that a dilution of the derivatives occurs. Lacroix et al. also investigated an alternative way to stop the derivatization reaction by keeping the samples at -20 °C for 10 minutes (1).
14 1.4.2. Fast Red RC
Fast Red RC, another derivatization reagent, is a diazonium salt stabilized by resonance (9,50). The salt is complexed with zinc chlorides which makes the compound stable in solid form and allows it to be stored for a long period of time (50). Fast Red RC is a weak electrophile where the electrophilic nitrogen in the molecular structure prefers electron-rich sites in electrophilic aromatic substitution reactions. The Fast Red RC derivatization is typically applied to aromatic compounds such as phenols where the ortho- and para-positions on the aromatic ring are suitable electron-rich places for an electrophilic aromatic substitution reaction. In the derivatization reaction, also called an azo coupling reaction, a hydrogen atom is replaced at these positions by the diazonium molecule. This finally results in an azo-derivative (DRV) of the target compound. The Fast Red RC derivatization of the cannabinoid’s phenolic components produces two regio-isomers for THC DRV, 11-OH-THC DRV and THC-COOH DRV. This implies that the azo coupling reaction may show regioselectivity. Due to the symmetry of the molecular structure of CBD, only one isomer is formed during the derivatization (9,49,50). The Fast Red RC derivatization of the cannabinoid THC is shown in Figure 1.3. The chemical structure of the isomers can be found for all azo-derivatives in Appendix 8.1.
Figure 1.3: The azo coupling derivatization reaction of the cannabinoid THC with the derivatization reagent Fast Red RC, resulting in two regio-isomers of the THC azo-derivative (THC DRV) (49)
The azo-derivatives of the cannabinoids can be readily protonated in the electrospray ionization of the LC-MS/MS instrument, resulting in an improvement of the ionization efficiency of the cannabinoids. In addition, fragments from the diazonium part in the azo-derivative produce a high response in the MS, but as these fragments are formed by all cannabinoid azo-derivatives, they are not specific. The Fast Red RC derivatization cannot be carried out directly in the original sample matrix like blood but the solvents water, methanol, acetonitrile can be used instead. The samples can be analysed by LC-MS/MS directly when the derivatization is complete (9,49).
15 Luo et al. described in two articles the Fast Red RC derivatization approach for cannabinoids (9,49). Both articles discussed a derivatization assay for LC-MS/MS analysis of cannabinoids in human breath samples. In addition, this derivatization assay was also compared with a non-derivative assay in whole blood samples. These whole blood samples were collected from subjects within three hours after smoking Cannabis. On the other hand, drug free whole blood was used to prepare the calibrators and quality controls. The Fast Red RC derivatization method resulted in a limit of quantification ranging from ten pg/mL to sub-ng/mL for the whole blood samples. In general, the authors concluded that the derivatization with the reagent Fast Red RC increases the sensitivity for the LC-MS/MS analysis of cannabinoids in human breath samples. Furthermore, a good correlation was observed between the derivative and non-derivative assay in whole blood samples. They also suggested to widen this approach to other aromatic substances (9,49).
To be more specific, Luo et al. also evaluated several Fast Red RC derivatization reaction conditions in the breath samples, including acidity, fraction of organic solvent and concentration of the derivatization reagent (9,49). For this evaluation, THC was utilized as a reference analyte. To modify the reaction acidity, the derivatization reagent was prepared in different solvents and buffer solutions, each with a specific acidity. The more acidic the reaction medium, the slower the azo coupling reaction was. An increase of the reaction temperature could not significantly increase the reaction rate. Finally, the 5.0 mM ammonium acetate buffer was selected by the authors as optimal solvent for Fast Red RC with the additional benefit that this buffer is well compatible with LC-MS/MS. To evaluate the next condition, i.e. the fraction of organic solvent, different ratios of water/methanol and water/acetonitrile were used in the reaction. A high proportion of organic solvent was preferred and a water/methanol ratio of 8:2 was chosen for further use in the derivatization assay. Finally, for the concentration of the derivatization reagent in the 5.0 mM ammonium acetate buffer, a 1.5 mM Fast Red RC solution was chosen as it showed a stable reaction yield. This derivatization assay with THC as standard was also successfully validated for the parameters precision, accuracy, sensitivity, specificity, matrix effect and carryover (9).
For the LC-MS/MS analysis of a blood sample with a certain concentration of THC, 10 µL of the 1.5 mM Fast Red RC solution was added during the sample preparation to derivatize 100 µL of the sample. Afterwards, the sample was incubated at ambient temperature for half an hour and then analysed. The LC-MS/MS system consisted of a mobile phase A, 5.0 mM ammonium formate in water and a mobile phase B, methanol with both 0.05 % formic acid. A gradient elution was applied. The analytical column used was a Kinetex C18 column (3.0 mm x 50 mm, 2.6 µm particle) at a temperature of 25 °C (9).
16 1.5. DEGLUCURONIDATION
A principal metabolite of THC, THC-COOH, is extensively glucuronidated with the formation of mainly two glucuronide conjugates namely an ester-linked and an ether-linked glucuronide of COOH. The free THC-COOH is mostly found in the blood and the glucuronide conjugates in the urine because the glucuronidation provides easier excretion of the compound (1). When a urine or blood sample is tested for the presence of Cannabis, the total concentration of free THC-COOH is measured. This total concentration is the sum of the already free THC-COOH and the THC-COO-glucuronides that have been converted to the free form via enzymatic and/or alkaline hydrolysis. The ester-linked glucuronide of THC-COOH is simply hydrolysed using the enzyme β-glucuronidase or a base. The ether-linked glucuronide of THC-COOH, on the other hand, is more difficult to hydrolyse with the enzyme β-glucuronidase and is even not sensitive for cleavage under alkaline conditions (1,2). An important aspect is that the enzymatic and/or alkaline hydrolysis may show a variable hydrolysis efficiency which can lead to a bias of the results (2,10).
17 2. OBJECTIVES
Tracing Cannabis is often required in forensic, DUID and workplace related cases. In these cases, a biological analysis is mostly performed using either venous blood by conventional venipuncture or urine as biological matrix. Nevertheless, a new alternative sampling procedure is emerging, namely DBS sampling. This is a microsampling technique whose popularity is growing in the clinical, pharmaceutical and toxicological field due to the numerous associated advantages (ease of sampling, small blood volume, cost-effectiveness, analyte stability and improved safety).
The analysis of the cannabinoids in both venous blood and DBS is not straightforward. The lipophilic properties of the cannabinoids make the extraction procedure challenging and the absence of an ionizable group in the molecular structure makes the ionization of these compounds in the mass spectrometer quite difficult. Given the fact that Cannabis is a widely used drug and is involved in various fields, it is preferable to develop new methods that will significantly improve the analysis and detection of the cannabinoids.
The initial aim of this project is to test two in-situ reactions for the analysis of cannabinoids on DBS cards. The first in-situ reaction concerns the enzymatic and/or alkaline hydrolysis of COO-glucuronide. THC-COOH, one of the main metabolites of THC, is extensively glucuronidated. Generally, venous blood testing for the detection of Cannabis consumption involves measuring the total concentration of THC-COOH. The latter is obtained by the enzymatic and/or alkaline hydrolysis of THC-COO-glucuronide. The question arises whether this reaction can also be performed directly on DBS samples. The second in-situ reaction relates to a derivatization of cannabinoids with the derivatization reagent Fast Red RC or Dabsyl chloride. Here also, the same question arises whether this reaction can be carried out directly on DBS samples. In a subsequent step, these two derivatization reagents will be compared, for example in terms of derivatization efficiency. A last goal of the project is the development and implementation of a validation plan in order to validate a newly designed DBS cartridge. Due to the special Corona circumstances only the derivatization with Fast Red RC could be tested together with a more detailed literature research.
Recently, a novel derivatization method with Fast Red RC has been introduced in the literature. This new method should significantly increase the ionization efficiency of the cannabinoids and consequently the sensitivity and detection. First of all, the reproducibility of this literature data will be investigated. Afterwards, this derivatization method will be optimized for certain parameters, for example the concentration of the Fast Red RC solution, the composition of the reconstitution solvent and the settings of the gradient program for the
18 LC-MS/MS analysis. This optimized derivatization method will then be used for a tuning of the LC-MS/MS instrument for the derivatized cannabinoids. Conditions such as the ionspray voltage (IS), declustering potential (DP) and collision energy (CE) of the LC-MS/MS system will be tuned more towards the LC-MS/MS analysis of the derivatives. Subsequently, it will be examined if the optimized derivatization method is also applicable in spiked venous blood samples. All this together will ultimately provide an essential step to achieve the stated goal of the project in the future.
19 3. MATERIALS AND METHODS
3.1. MATERIALS
3.1.1. Chemicals and reagents
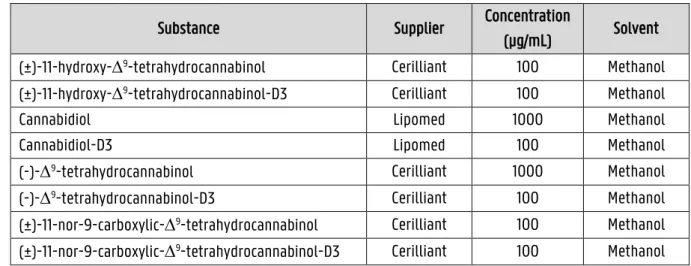
The utilized reference and deuterated ISTD solutions of the cannabinoids are listed in Table 3.1 and were purchased from the suppliers Cerilliant (Round Rock, TX, USA) and Lipomed (Arlesheim, Switzerland).
Table 3.1: The reference and internal standard solutions used in the experiments
Substance Supplier Concentration
(µg/mL) Solvent
(±)-11-hydroxy-9-tetrahydrocannabinol Cerilliant 100 Methanol
(±)-11-hydroxy-9-tetrahydrocannabinol-D3 Cerilliant 100 Methanol
Cannabidiol Lipomed 1000 Methanol
Cannabidiol-D3 Lipomed 100 Methanol
(-)-9-tetrahydrocannabinol Cerilliant 1000 Methanol
(-)-9-tetrahydrocannabinol-D3 Cerilliant 100 Methanol
(±)-11-nor-9-carboxylic-9-tetrahydrocannabinol Cerilliant 100 Methanol
(±)-11-nor-9-carboxylic-9-tetrahydrocannabinol-D3 Cerilliant 100 Methanol
Ultrapure water of Type 1 was produced by a Milli-Q Water System (Millipore Corporation, Billerica, MA, USA). Acetonitrile (99,9 %) was provided by Acros Organics (Geel, Belgium) and methanol was obtained from
Biosolve (Valkenswaard, The Netherlands). FlukaTM/Honeywell (NJ, USA) supplied the ammonium formate powder
and the formic acid solution with a purity of 50 % in water. Ammonium acetate was provided by Merck KGaA (Darmstadt, Germany). The derivatization reagent Fast Red RC was ordered in salt form from Sigma-Aldrich (Buchs, Switzerland) and stored in the refrigerator. To make the blood samples, fresh whole blood was offered by the local blood bank (Bern, Switzerland).
3.1.2. LC-MS/MS instrument
The liquid chromatography system was a 1200 Series of Agilent Technologies (Basel, Switzerland) coupled with a 3200 Q TRAP mass spectrometer of AB Sciex (Brugg, Switzerland). The data acquisition was accomplished by the Analyst® Software version 1.5.1 (AB Sciex, Brugg, Switzerland). The HTS PAL Autosampler was purchased from CTC Analytics (Zwingen, Switzerland). In order to be able to perform a reversed phase chromatography, an analytical column Kinetex® 2.6 µm C18 100 Å (50 x 2.10 mm) from Phenomenex (Torrance, CA, USA) was installed.
20 3.2. METHODS
3.2.1. Reproducibility of the literature data
Luo et al. described in two articles a novel derivatization method with the reagent Fast Red RC to enhance the analysis of cannabinoids (9,49). The following procedure was performed to verify whether this derivatization reaction actually takes place. The procedure was based on the methods described in the two articles and those applied in the routine analyse of cannabinoids at the IRM in Bern.
3.2.1.1. Fast Red RC solution
The derivatization of the cannabinoids required a 1.5 mM Fast Red RC solution in 5.0 mM ammonium acetate. To begin, the 5.0 mM ammonium acetate solution was made by weighing 38.5 mg ammonium acetate on a Mettler Toledo AT200 analytical balance (Greifensee, Switzerland). This quantity was then dissolved in 100 mL ultrapure water. After that, 10.2 mg Fast Red RC salt were weighed and dissolved in 25 mL of the 5.0 mM ammonium acetate solution. The prepared Fast Red RC solution was stored in a refrigerator.
3.2.1.2. Sample preparation
The reference and ISTD solution of each cannabinoid was diluted to a standard solution with a final concentration of 400 ng/mL for both the analyte and its ISTD. Gilson’s Pipetman (Middleton, USA) was always used to pipette the solvents. For the first dilution, 20 µL of the THC reference solution were dissolved in 1980 µL MeOH. In order to obtain the final concentration, 80 µL of this pre-dilution and 8 µL of the THC ISTD solution were added to 1912 µL MeOH. These steps were repeated for the CBD reference and its ISTD solution. The final concentration for 11-OH-THC and its ISTD was achieved by adding 8 µL of the reference solution and 8 µL of the ISTD solution to 1984 µL MeOH. The latter procedure was also followed for THC-COOH. Each prepared solution was always properly mixed by manually vortexing with the Vortex-Genie 2 (Scientific Industries, Bohemia, NY, USA).
Six samples were then prepared for each of the four analytes (THC, CBD, 11-OH-THC, THC-COOH and their corresponding IS) in champagne vials (1.2 mL µ-Vial, Infochroma AG, Goldau, Switzerland). The first sample was a blank containing 200 µL of 100 % MeOH. The second sample contained 100 µL of the appropriate standard solution and 100 µL of the ultrapure water. Into each of the four remaining vials, 100 µL of the standard solution were pipetted and 10, 30, 50 or 70 µL of the 1.5 mM Fast Red RC solution were respectively added to one of these four vials. After capping and vortexing the vials, an incubation period of 30 minutes was set. After the incubation,
21 respectively 90, 70, 50 or 30 µL ultrapure water were added. In this way, a concentration of 200 ng/mL for both the analyte and their associated IS was obtained with a reconstitution solvent of 50 % MeOH and 50 % water. Finally, all the vials were capped, shortly vortexed and put in the autosampler for LC-MS/MS analysis.
Another series of samples was made that contained a mix of the analytes and their corresponding ISTD. To prepare the first sample, 50 µL per standard solution were pipetted into a champagne vial. This was followed by putting the vial in a DB-3D Dri-Block® Heater (Techne, Staffordshire, UK) at 50 °C to evaporate the solvent under a continuous flow of nitrogen. After drying, the cannabinoids were reconstituted in 100 µL MeOH. The vial was put on the vortex and then another 100 µL of ultrapure water were added. The following four samples were prepared in a similar way. In each vial, 25 µL per standard solution were pipetted. Respectively 10, 30, 50 or 70 µL of the 1.5 mM Fast Red RC solution were added. The vials were capped and vortexed. After an incubation time of 30 minutes, the vials were put in the heater block to dry the solvent. Thereafter, 50 µL MeOH were added for re-uptake followed by vortexing. Before capping the vials, 50 µL ultrapure water were added. All samples were then ready to be analysed. This sample preparation resulted for every sample in a final concentration of 100 ng/mL for each analyte and for the equivalent ISTD in a reconstitution solvent of 50 % MeOH and 50 % water.
3.2.1.3. LC-MS/MS analysis
The LC-MS/MS mobile phase A i.e. 5.0 mM ammonium formate with 0.05 % formic acid, was prepared by dissolving 315.28 mg ammonium formate, weighted on the Mettler Toledo analytical balance, in 1 L water. This volume was mixed with 1 mL of the formic acid solution. Mobile phase B i.e. methanol with 0.05 % formic acid, was formulated by mixing 1 mL formic acid with 1 L MeOH. Since there was no degasser installed in the LC-MS/MS system, the mobile phases were put in a Sonorex ultrasonic bath (Bandelin, Berlin, Germany) for 15 minutes to remove the air from the solvents.
In the software program, the following acquisition method was set up. The experiments were operated in the multiple reaction monitoring (MRM) scan type. Electrospray ionization was conducted in the positive ion mode by using a Turbo Spray ion source and the following parameters: IS of 4250 V, temperature of 650 °C and a pressure for ion source gas 1 and 2 of both 40 psi. The pressure of the curtain gas and collision gas were set respectively at 40 and 6 psi. To perform a MRM, the parent ion to fragment ion transitions were imported in the acquisition method. For each analyte, non-derivatized as well as derivatized, two MRM transitions were specified with MRM 1 as the quantifier and MRM 2 as the qualifier. Only one MRM transition was specified for as well the non-derivatized as the derivatized ISTD. Table 3.2 presents an overview of the MRM transitions with the
22 corresponding parameters: dwell time, DP, CE and collision cell exit potential (CXP).
Table 3.2: MRM transitions and related parameters of the non-derivatized and derivatized cannabinoids and internal standards ID Q1 Mass (Da) Q3 Mass (Da) Dwell time (msec) DP (V) CE (eV) CXP (V) 11-OH-THC MRM 1 331.200 313.200 35 87 21 12 11-OH-THC MRM 2 331.200 193.200 35 90 35 7 11-OH-THC-D3 MRM 1 334.200 316.200 20 87 21 12 11-OH-THC DRV MRM 1 499.000 296.000 35 87 21 12 11-OH-THC DRV MRM 2 499.000 324.000 35 90 35 7 11-OH-THC-D3 DRV MRM 1 502.000 299.000 20 87 21 12 CBD MRM 1 315.201 193.101 35 88 32 7 CBD MRM 2 315.201 123.001 35 100 43 8 CBD-D3 MRM 1 318.201 196.301 20 88 32 7 CBD DRV MRM 1 483.001 361.001 35 88 32 7 CBD DRV MRM 2 483.001 142.001 35 100 43 8 CBD-D3 DRV MRM 1 486.001 364.001 20 88 32 7 THC MRM 1 315.200 193.100 35 88 32 7 THC MRM 2 315.200 123.000 35 100 43 8 THC-D3 MRM 1 318.200 196.300 20 88 32 7 THC DRV MRM 1 483.000 361.000 35 88 32 7 THC DRV MRM 2 483.000 142.000 35 100 43 8 THC-D3 DRV MRM 1 486.000 364.000 20 88 32 7 THC-COOH MRM 1 345.200 327.300 35 93 23 12 THC-COOH MRM 2 345.200 299.200 35 102 28 12 THC-COOH-D3 MRM 1 348.200 330.200 20 93 23 12 THC-COOH DRV MRM 1 513.000 338.000 35 93 23 12 THC-COOH DRV MRM 2 513.000 142.000 35 102 28 12 THC-COOH-D3 DRV MRM 1 516.000 341.000 20 93 23 12
The acquisition method also contained a gradient program with the mobile phases A and B. A schematic representation of this gradient program is shown in Table 3.3. A last parameter to be set was the temperature of the oven in which the analytical column was installed. A temperature of 25 °C was selected. Before running the LC-MS/MS analysis, the complete system was equilibrated for 2 minutes. Afterwards, the measurements were
23 started with injecting 20 µL of the sample into the LC by the autosampler.
Table 3.3: The applied gradient program in the LC-MS/MS analysis with mobile phase A, 5.0 mM ammonium formate with 0.05 % formic acid and mobile phase B, methanol with 0.05 % formic acid
Time (min) Flow rate (µL/min) Mobile phase A (%) Mobile phase B (%)
0.00 300 30 70 1.00 300 30 70 4.00 300 0 100 8.00 300 0 100 9.00 300 30 70 11.00 300 30 70
3.2.2. Optimization of the derivatization method 3.2.2.1. Fast Red RC solution
A new Fast Red RC solution with a higher concentration was formulated for the optimization of the derivatization of the cannabinoids. For this purpose, 102.4 mg Fast Red RC salt were dissolved in 25 mL of the already prepared 5.0 mM ammonium acetate solution. The produced 15 mM Fast Red RC solution was stored in a refrigerator.
3.2.2.2. Sample preparation
The following sample was used to optimize the gradient program of the LC-MS/MS system. In a champagne vial, 50 µL per standard solution were pipetted. This vial was then put in the heater block at 50 °C under a continuous flow of nitrogen. When the solvent was evaporated, the compounds were reconstituted in 80 µL MeOH. After putting the vial shortly on the vortex, 20 µL of ultrapure water were added. The vial was capped and put in the autosampler. The final concentration of this sample was 200 ng/mL for each analyte as well as for their relevant ISTD.
The next samples were subjected to the optimized derivatization method. One champagne vial was filled with 25 µL per standard solution. The derivatization was then performed by adding 10 µL of the new 15 mM Fast Red RC solution to the vial and incubating it for 30 minutes after capping and vortexing. When the incubation period was complete, the vial was placed in the heater block. Hereafter, 40 µL MeOH were added for re-uptake followed by vortexing. Finally, another 10 µL of ultrapure water were added. The vial was capped and set up to be tested with the optimized LC-MS/MS analysis. Each analyte and the corresponding ISTD in this sample had
24 both a concentration of 200 ng/mL. A similar procedure was applied to make four samples with now the analytes 11-OH-THC, CBD, THC and THC-COOH and their ISTD each separately. Into each of the four vials, 100 µL of the appropriate standard solution were pipetted. Subsequently, 10 µL of the 15 mM Fast Red RC solution were added to each vial. The vials were capped and shortly placed on the vortex. Then 30 minutes were set as incubation time. Lastly, 15 µL of ultrapure water were added to the four vials. The samples were ready to be analysed. All four samples had a concentration of 320 ng/mL for both the analyte and its ISTD. In this sample preparation, the reconstitution solvent was changed to 80 % MeOH and 20 % water.
3.2.2.3. LC-MS/MS analysis
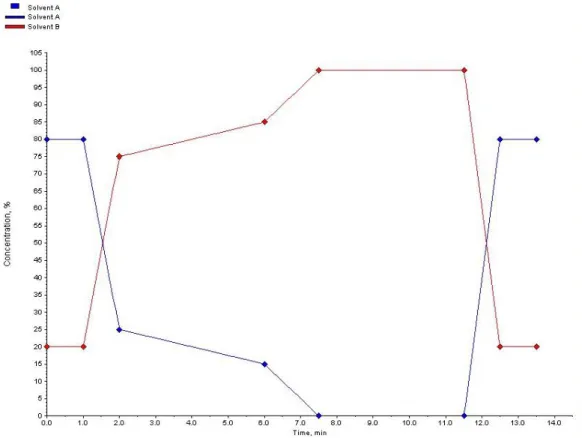
All the instrumental settings were maintained with a few exceptions. The values of the injection volume and the temperature of the oven were adjusted. These were changed to respectively 5 µL and 40 °C. The first sample was injected several times by the autosampler in order to optimize the gradient program of the LC-MS/MS system in multiple steps. The finally obtained gradient program is displayed in Figure 3.1. This resulted in a run time of 13.5 minutes per sample. Every subsequent LC-MS/MS analysis of a sample was performed using these optimized conditions.
Figure 3.1: The optimized gradient program of the LC-MS/MS system with solvent A, 5.0 mM ammonium formate with 0.05 % formic acid and solvent B, methanol with 0.05 % formic acid. The flow rate is 300 µL/min.