Nanomaterials under REACH
Nanosilver as a case study
Report 601780003/2009
RIVM report 601780003/2009
Nanomaterials under REACH
Nanosilver as a case study
M.E.J. Pronk, Centre for Substances and Integrated Risk Assessment, RIVM S.W.P. Wijnhoven, Centre for Substances and Integrated Risk Assessment, RIVM E.A.J. Bleeker, Expertise Centre for Substances, RIVM
E.H.W. Heugens, Expertise Centre for Substances, RIVM
W.J.G.M. Peijnenburg, Laboratory for Ecological Risk Assessment, RIVM R. Luttik, Expertise Centre for Substances, RIVM
B.C. Hakkert, Bureau REACH, Expertise Centre for Risk Assessment, RIVM
Contact: B.C. Hakkert
Bureau REACH, Expertise Centre for Substances, RIVM betty.hakkert@rivm.nl
This investigation has been performed by order and for the account of VROM, within the framework of REACH project number M/601780.
© RIVM 2009
Parts of this publication may be reproduced, provided acknowledgement is given to the 'National Institute for Public Health and the Environment', along with the title and year of publication.
Abstract
Nanomaterials under REACH Nanosilver as a case study
Some adjustments are needed in the European chemicals legislation REACH to assess and control the risks of nanomaterials. The information on substances to be provided under REACH is not sufficient to determine the specific properties of nanomaterials, nor to assess how these properties affect their behaviour and effects in humans and the environment. RIVM concluded this following research into the suitability of REACH for nanomaterials. RIVM therefore proposes an adapted set of minimum information requirements, to be applied to all nanomaterials to be registered under REACH, independent of their volume of production and import. These requirements allow a risk assessment of nanomaterials.
Over the last years the use of nanomaterials has strongly increased. As yet, nanomaterials are defined as substances of which the discrete parts have at least one dimension smaller than one hundred nanometres. Due to their nanosize they have specific properties. Legislation should focus on controlling the potential hazards and risks of these nanomaterials.
By conducting a hypothetical registration of nanosilver it was investigated whether REACH is suitable for assessing the safe use of nanomaterials. From this it appeared that no definition of a nanomaterial is present, and that a relevant measure for expressing harmfulness and exposure is as yet not known. In addition, the standard information requirements are insufficient to assess hazard and exposure. They are also insufficient for a proper characterisation of the nanomaterial. Consequently, it cannot be determined to what extent the nanoform of a substance corresponds to the non-nanoform of the same substance. Furthermore, it is unclear whether current risk reduction measures and extrapolation methods in risk assessment, as established for non-nanomaterials, are applicable to nanomaterials. Key words: REACH, nanomaterials, nanosilver, risk assessment
Rapport in het kort
Nanomaterialen onder REACH Nanozilver als een voorbeeldstudie
Om de risico’s van nanomaterialen te kunnen inschatten en beheersen, zijn enkele aanpassingen nodig in de Europese chemicaliënwetgeving REACH. De gegevens over stoffen waar REACH standaard om vraagt, zijn namelijk onvoldoende om de specifieke eigenschappen van nanomaterialen te bepalen. Hetzelfde geldt voor het bepalen van de invloed van deze eigenschappen op het gedrag en de effecten van nanomaterialen in mens en milieu. Dit blijkt uit onderzoek van RIVM naar de geschiktheid van REACH voor nanomaterialen. Het instituut stelt daarom een aangepaste set minimum informatievereisten voor, voor alle te registreren nanomaterialen onder REACH, ongeacht de omvang van productie en import. Deze vereisten maken het mogelijk de risico’s van nanomaterialen te beoordelen.
Het gebruik van nanomaterialen neemt de laatste jaren sterk toe. Nanomaterialen worden vooralsnog gedefinieerd als stoffen waarvan de deeltjes minstens één dimensie kleiner dan honderd nanometer hebben. Vanwege hun afmeting hebben ze specifieke eigenschappen. Wetgeving moet erop gericht zijn de potentiële gevaren en risico’s van deze nanomaterialen te beheersen.
Aan de hand van een hypothetische registratie van nanozilver is onderzocht of REACH geschikt is om een veilig gebruik van nanomaterialen vast te stellen. Hieruit bleek onder andere dat een definitie van nanomateriaal ontbreekt, en dat de juiste maateenheid om de schadelijkheid en blootstelling in uit te drukken nog niet bekend is. Ook is de verplichte standaardinformatie ontoereikend om de blootstelling en gevaren in te kunnen schatten, en om het nanomateriaal goed te kunnen karakteriseren. Mede door de laatste beperking is niet vast te stellen in hoeverre de nanovorm van een stof overeenkomt met de niet-nanovorm van dezelfde stof. Bovendien is het onduidelijk of de huidige extrapolatiemethoden in de risicobeoordeling en de maatregelen om risico’s te beheersen geschikt zijn voor nanomaterialen. Deze methoden en maatregelen zijn immers vastgesteld voor niet-nanomaterialen.
Preface
This report describes a hypothetical registration of nanosilver under the new EU REACH regulation on chemicals, taking into account the ongoing discussions within the REACH Competent Authorities and its Subgroup on Nanomaterials on how REACH applies to nanomaterials (as described in documents of this subgroup dated December 2008-March 2009). The case study on nanosilver is purely a scientific exercise, with the aim to generate recommendations for future policy guidance on how to deal with first generation nanomaterials under REACH. Given this, it is stressed that this report does not pretend to provide a complete overview of all available toxicity data on (nano)silver, and is as such not to be used for an actual registration under REACH.
Contents
Summary ... 11
1 Introduction... 13
2 REACH and its requirements for registration ... 15
2.1 Gathering and generating information ... 15
2.2 Substance identification ... 16
2.3 Classification and labelling ... 16
2.4 Chemical Safety Assessment and Chemical Safety Report ... 17
2.5 Exposure-related information... 17
3 General risk assessment issues for nanomaterials... 19
3.1 Introduction... 19
3.2 Suitability of current test guidelines for nanomaterials... 19
3.2.1 Physicochemical tests... 19
3.2.2 Human health toxicity tests... 20
3.2.3 Ecotoxicity tests ... 21
3.2.4 Additional OECD results ... 22
3.3 Currently proposed risk assessment approaches for nanomaterials ... 23
4 Nanosilver as a case study for registration under REACH... 25
4.1 Substance identity and physicochemical properties... 26
4.1.1 Information requirements and availability ... 26
4.1.2 Summary of available data for substance identity and physicochemical properties ... 26
4.1.3 Discussion on substance identity and physicochemical properties ... 27
4.2 Environmental hazard and exposure assessment... 29
4.2.1 Information requirements and availability ... 29
4.2.2 Summary of available hazard data for environment... 30
4.2.3 Summary of environmental exposure assessment... 31
4.2.4 Discussion on fate and behaviour and ecotoxicity ... 31
4.2.5 Additional information needs... 33
4.3 Human health hazard and exposure assessment... 34
4.3.1 Information requirements and availability ... 34
4.3.2 Summary of available hazard data ... 35
4.3.3 Summary of consumer exposure assessment ... 36
4.3.4 Discussion human health... 37
5 Lessons learned from the case study for nanomaterials in general ... 43
5.1 General observations... 43
5.2 Substance identification ... 43
5.2.1 Physicochemical properties... 43
5.2.2 Impurities ... 46
5.2.3 Sameness discussion ... 46
5.3 Dosimetry: mass-based or based on another metric? ... 47
5.4.1 Exposure based waiving... 48
5.4.2 Dosimetry... 48
5.4.3 Exposure modelling ... 48
5.5 Hazard assessment ... 50
5.5.1 Dosimetry... 50
5.5.2 Derivation of DNELs and PNECs... 50
5.5.3 Non-testing methods ... 51
5.5.4 In vitro methods ... 51
5.5.5 Data requirements ... 52
5.6 Risk characterisation ... 53
5.7 Lessons learned with respect to existing risk assessment frameworks ... 54
5.8 Conclusions... 55
6 Proposal for a risk assessment framework for first generation nanomaterials under REACH ... 57
6.1 Observations and considerations... 57
6.2 Proposed approach for the risk assessment of first generation nanomaterials under REACH ... 57 Acknowledgement... 61 References ... 63 Appendix 1 ... 65 Appendix 2 ... 69 Appendix 3 ... 71 Appendix 4 ... 81 Appendix 5 ... 83 Appendix 6 ... 85
Summary
Due to their nano-size, nanomaterials (≤ 100 nm) have specific characteristics that may differ from non-nanomaterials. The development of nanomaterials and their increasing use in all sorts of industrial applications and consumer products, challenges the regulating authorities to develop frameworks which can adequately control the potential hazards and risks of these nanomaterials. In Europe such a framework is the new regulation of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). Under REACH, manufacturers, importers and downstream users have to ensure that the substances they manufacture, place on the market or use, do not adversely affect human health or the environment. Since REACH deals with substances, in whatever size, shape or physical state, substances at the nanoscale are also covered by REACH and its provisions apply. This implies that also the safety of nanomaterials to human health and the environment should be ensured under REACH, covering their whole life cycle. The aim of this report is two-fold. First, to investigate the suitability of REACH in ensuring the safety of nanomaterials by conducting a hypothetical registration under REACH of metallic silver, a substance that exists both in nanoform and in non-nanoform (i.e. bulk form). Nanosilver is a first generation nanomaterial, and the case study on nanosilver is used to examine the problems that potential registrants may encounter when trying to register such a substance under REACH. Second, to propose a risk assessment framework for first generation nanomaterials under REACH, based on the information generated in the case study.
In chapter 2 the requirements for registration of a substance under REACH are briefly described. These requirements depend on the tonnage a substance is manufactured or imported in, and may include a chemical safety assessment.
In chapter 3 an overview is presented of some recent analyses on the suitability of existing testing methods in determining the toxicity of nanomaterials. The majority of these methods appears to be suitable for nanomaterials, albeit that in many cases some modifications are needed to address nano-specific issues. These issues have been described for physicochemical, ecotoxicity and toxicity tests. The last part of chapter 3 reviews the (few) risk assessment approaches for nanomaterials that have been described so far. Due to their broad outline and lack of specifics on the kind of testing considered essential for nanomaterials, these frameworks are deemed of limited value.
In chapter 4 the results and conclusions are presented of the hypothetical registration of metallic silver (in bulk and nanoform) under REACH. The complete chemical safety assessment is presented in the Chemical Safety Report in Appendix 6. For this case study some assumptions on the tonnage, characteristics and use of nanosilver were made and only readily accessible public data were used as information source (and should as such not be seen as a complete overview of the toxicity of silver or nanosilver). The available data on silver in bulk form would in principle fulfil the information requirements under REACH for the assumed tonnage and would allow concluding on classification and labelling and a measure of dose-response. For nanosilver, however, several information gaps could be identified. First of all, a proper characterisation of nanosilver was difficult with the standard information requirements for physicochemical properties under REACH (even though some were arbitrarily chosen for the case study), which made it impossible to conclude on the sameness between the nanoform and bulk form of silver. Secondly, without information on the kinetics of dissolution and on partitioning available, it was not possible to predict the fate and behaviour of nanosilver in the environment and the human body. It is thus not clear whether its behaviour is comparable to bulk silver
or not. Consequently, the option of using data on bulk silver to fill the data gaps identified for nanosilver (i.e. read-across) was not considered viable in the absence of a conclusion on the sameness. Chapter 5 presents the extrapolation of the findings from the nanosilver case study to nanomaterials in general. It is concluded that REACH is not sufficiently implementable for nanomaterials. First of all, for the scope of risk assessment of nanomaterials, a proper definition relating to nanomaterials needs to be developed. Also relevant dose metrics need to be established since mass concentration, the metric normally used for non-nanomaterials, may not be the most appropriate for nanomaterials for all endpoints. Further guidance is also needed on proper characterisation of a nanomaterial and its corresponding bulk form, and how to address different shapes, sizes and size distributions of a nanomaterial in substance identification under REACH. This is considered essential for the sameness analysis (between a nanoform and a bulk form of a substance, or between different sizes/shapes of a nanomaterial), and for the propagated use of non-testing methods like read-across under REACH. Also the use of in vitro (screening) methods is propagated. However, for nanomaterials there are at the moment too many knowledge gaps and in vitro assay protocol issues to deem this a viable option, also given the lack of proper databases to translate the in vitro results to the in vivo situation.
With respect to (eco)toxicity testing, kinetic information is currently not a standard requirement under REACH but is considered essential to both human health and environmental hazard assessment of nanomaterials. In addition, for some existing test guidelines extension of parameters to be tested may be necessary, and the influence of physicochemical characteristics of the test material on sample preparation and dosimetry should be considered as well. For a proper exposure assessment, also existing exposure models need to be adapted for use for nanomaterials, by incorporating nano-specific parameters. And finally, in order to demonstrate ‘safe use’, standard default assessment factors used in extrapolation procedures for non-nanomaterials need to be examined for their applicability to nanomaterials, and nano-specific operational conditions and risk management measures need to be developed to control exposure to nanomaterials.
Based on all these observations, in chapter 6 a risk assessment framework is proposed for first generation nanomaterials under REACH. In this proposal the basic requirements of the current REACH legislation still apply, with some nano-specific adaptations. It is proposed that for nanomaterials the tonnage-dependent need for registration and information requirements should be reconsidered. Instead, a base set information requirement is proposed for all nanomaterials to be registered, independent of their production/import volume. This base set covers what is considered to be the primary information needs on nanomaterials. Another recommendation is to request for all nanomaterials to be registered a technical dossier plus, independent of tonnage, a chemical safety assessment documented in a chemical safety report. This approach is proposed to be used at least for the next few years, until further investigations allow certain patterns in the behaviour of nanomaterials to be established, on the basis of which, in time, the data requirements for nanomaterials can be adapted. As discussions on the definition and dose metrics of nanomaterials are ongoing, the proposal recommends for the time being to use the following definition of a nanomaterial: ‘any form of a material that is composed of discrete functional parts, many of which have one or more dimensions of the order of 100 nm or less’, and to use (with caution) mass concentration as the dose metric for nanomaterials.
1
Introduction
The EU Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) suggests a nanomaterial to be defined as any form of a material that is composed of discrete functional parts, many of which have one or more dimensions of the order of 100 nm or less (SCENIHR, 2007a). The nano-size of nanomaterials results in specific physicochemical characteristics that may differ from those of the ‘bulk’ material or particles of larger size. Because of their specific properties, manufactured nanomaterials (of which there are at the moment already first, second and third generations) may bring significant innovation and advances to society and benefits for human health and the environment. At the same time, however, their specific properties may lead to different interactions in the physiology in humans and the environment and to effects that significantly differ from those known of bulk materials without such physicochemical properties. It will thus be necessary to ensure their safety for humans and the environment and to avoid negative impacts on society.
REACH is the new regulation of the European Parliament and of the Council concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals, that is operational in Europe since June 2007 (EU, 2006). Under REACH, manufacturers, importers and downstream users have to ensure that the substances they manufacture, place on the market or use do not adversely affect human health or the environment. It applies to substances on their own, in preparations or in articles. Within the context of REACH, it is of main importance for the development of nanotechnologies and nanomaterials that also their safety to human health and the environment is ensured, covering the whole life cycle. There are no provisions in REACH referring specifically to nanomaterials, nor has a definition relating to nanomaterials been laid down. But since REACH deals with substances, in whatever size, shape or physical state, substances at the nanoscale are also covered by REACH and its provisions apply (European Commission, 2008).
REACH is not the only regulatory framework dealing with the risk assessment of nanomaterials. There are several other types of legislation (like for instance the worker protection legislation) within which the risks of nanomaterials will need to be addressed as well. This report, however, will deal with REACH only, and only with the registration part. The basic principles of risk assessment, i.e. exposure assessment, hazard assessment and a comparison of these two aspects in risk characterisation, are the same in REACH as in any other regulatory framework. Under REACH, however, the focus of the risk assessment is on risk management, i.e. on the demonstration that risks are controlled for both the human target populations (workers, consumers and humans exposed via the environment) and the environmental (aquatic, terrestrial and atmospheric) compartments to be protected.
To date, most presently known nanomaterials are derivatives of existing bulk materials, which are defined as any form of a material with dimensions above 100 nm. Some nanomaterials are only produced in low volumes, whereas others are produced in high volumes, sometimes even higher than the corresponding bulk material. Interestingly, the majority of the bulk materials of nanomaterials currently on the market will have to be registered under REACH before the 1st of December 2010, because they are manufactured or imported in the EU in volumes of 1000 tonnes or more per year (or otherwise fall into other categories of substances that have to be registered by that date, i.e. substances classified as CMR1 category 1 and 2 and manufactured or imported at tonnages of 1 tonne or more per year, and substances classified as very toxic to the aquatic environment and manufactured or imported
at tonnages of 100 tonnes or more per year). A ‘sameness’ analysis should indicate whether the substance at nanoscale can be considered as a specific physical form of the bulk substance (i.e. both are covered by the same EINECS2 entry), or is a different substance than the bulk substance (i.e. separate EINECS entries, or no EINECS entry for the nanoscale substance). The outcome of this substance identification is critical, as it forms the basis for data sharing obligations and for the joint submission of a registration dossier under REACH (the one substance – one registration principle). In case a nanomaterial is identified as a nanoform of a bulk substance, data sharing obligations with the related bulk substance apply within one common SIEF3. In case a nanomaterial is identified as a different substance, its registration needs to be addressed in a different SIEF, with data sharing obligations within that SIEF. At the moment, however, the issue of substance identification and what kind of information plays a role in deciding on the sameness of a substance are still under discussion within the REACH Competent Authorities and its Subgroup on Nanomaterials. Also the substance definition in REACH does not provide clues for differentiating substance identities on the basis of physicochemical characteristics. Furthermore, the science of nanotechnology might not yet be advanced enough to allow in all cases an easy distinction between nanoforms and other forms of a substance. Moreover, the shape, size and subsequently the specific properties of nanomaterials may change during the cascade of production, processing, use, and emission. All this seriously complicates the ‘sameness’ analysis, which under REACH is to be carried out by potential registrants. Therefore, further work and guidance on the ‘sameness’ analysis is urgently needed, especially for nanomaterials that can be considered a specific form of a bulk material that already has to be registered before the 1st of December 2010. In that case, the registration date for the bulk material also applies to the nanomaterial. In addition, the registration dossier should include any relevant nano-specific information next to information on the bulk substance. Further work and guidance is therefore also urgently needed on (specific) information requirements for nanomaterials.
This report deals with a case study on nanosilver, a first generation nanomaterial, conducted by order of the Netherlands Ministry of Housing, Spatial Planning and the Environment (VROM). In order to examine the problems that potential registrants may encounter when trying to register a nanomaterial under REACH, a hypothetical registration of silver was conducted. Silver exists both in bulk form and in nanoform, and assumptions were made on its tonnage and on the use and size and shape of nanosilver. The objective of performing this case study was to generate information that could be helpful in formulating a risk assessment framework for first generation nanomaterials under REACH. Following some general information on nanomaterials and REACH (chapter 2) and on (approaches for) risk assessment of nanomaterials (chapter 3), in chapter 4 the results and conclusions are presented of the hypothetical registration of nanosilver under REACH, where possible extrapolated to nanomaterials in general (chapter 5). Finally, in chapter 6 a proposal is made for an approach for risk assessment of first generation nanomaterials. This report is intended to be of help to the REACH Competent Authorities and its Subgroup on Nanomaterials in developing (guidance for) a risk assessment framework for nanomaterials under REACH, from which subsequently industry and society can benefit.
2 EINECS: European Inventory of Existing Commercial Chemical Substances. REACH distinguishes between phase-in substances (= substance with an EINECS entry) and non-phase-in substances (= substance without an EINECS entry).
2
REACH and its requirements for registration
Substances, including substances at the nanoscale, manufactured or imported in volumes of 1 tonne or more per year have to be registered under REACH. For the registration of a nanomaterial it is first of all important to establish whether the nanomaterial meets the criteria of a phase-in substance (REACH article 3(20)) or whether it is a non-phase-in substance4: only phase-in substances can benefit from extended registration deadlines, provided they have been pre-registered. Further, when a substance not only exists in the nanoform but also in the bulk form (which is true for most presently known nanomaterials), a ‘sameness’ analysis should make clear as to whether the substance at nanoscale can be considered as a specific physical form of the bulk substance, or is a different substance than the bulk substance. In case the nanomaterial is identified as a nanoform of a bulk substance, obligations for data sharing and joint submission of a registration dossier apply. In case a nanomaterial is identified as a different substance, data cannot be shared easily and the registration should be dealt with separately. The requirements for registration under REACH are described in the following paragraphs (based on EU, 2006; European Commission, 2008). It is to be noted that the registration of a substance existing in the nanoform as well as in the bulk form can be complex, because not only the information of the substance in the bulk form should be included in the registration dossier, but also any information regarding intrinsic properties where the properties of a substance in the nanoform differs from the bulk form, any different classification and labelling, any different chemicals safety assessment as well as all identified uses and relevant exposure scenarios for the nanoform of the substance. With the separate registration of a nanomaterial, the production/import volume is of extreme importance, since data requirements for registration are tonnage-dependent. For nanomaterials produced/imported in quantities below 1 tonne per year, no safety testing is required under REACH. However, when produced/imported in quantities of 1000 tonnes or more per year, a full (eco)toxicological data set is required (see Appendix 1).
2.1
Gathering and generating information
All available and relevant information (Annex VI of REACH) on the substance, regardless of whether testing for a given endpoint is required or not at the specific tonnage level, should be gathered and considered for the registration. In addition, information on exposure, use and risk management measures should be provided. Based on the information required for registration (Annexes VI and VII– XI), further testing may be needed. A registrant may decide that he needs to generate further information beyond the information required through Annexes VII–X of REACH (see Appendix 1) in order to be able to demonstrate and document that the risks of the substance (in all its forms) are controlled. This also applies to nanomaterials which have specific properties that may not in all cases be covered by the endpoints currently included in the REACH annexes.
The information requirements increase with the tonnage manufactured or imported. The tonnage triggers for registration apply to the total volume of a substance manufactured or imported by a
4 A phase-in substance is a substance which has been listed in EINECS in the past and was considered an existing substance before the entry into force of REACH. A non-phase-in substance is a substance which has no EINECS entry and was considered a new substance before the entry into force of REACH.
registrant. Thus, for substances which exist both in a conventional form and in a nanoform, and will be covered in one registration, the total volume determines the information requirements.
2.2
Substance identification
Any substance needs to be identified by a combination of the appropriate identification parameters (Annex VI of REACH): name or other identifier of the substance, information related to molecular and structural formula and composition of the substance. Under REACH, a substance is defined as ‘a chemical element and its compounds in the natural state or obtained by any manufacturing process, including any additive5 necessary to preserve its stability and any impurity6 deriving from the process used, but excluding any solvent which may be separated without affecting the stability of the substance or changing its composition’. Based on the chemical composition, the registrant has to decide whether the substance to be registered is either 1) a mono-constituent substance7, 2) a multi-constituent substance8, 3) a mono- or multi-constituent substance needing some additional physical parameters for proper characterisation, or 4) a UVCB substance9. The substance identification information should allow a ‘sameness’ analysis, at least for bulk substances. Nanomaterials, for which the discussion on the definition is still ongoing, probably belong to category 3) and thus may need further parameters/descriptors for proper characterisation, such as for example those mentioned by OECD10 in their Sponsorship Programme (see section 3.1, and Appendix 2 under ‘Nanomaterial information/ identification’ and ‘Physical-chemical properties and material characterization’), although these partly go beyond the current REACH requirements. Additional considerations such as different physicochemical, toxicological and ecotoxicological properties may in certain cases play a role in deciding on the sameness of the substance.
2.3
Classification and labelling
A substance with different sizes or forms can have different classifications, as for example is the case for nickel powder (particle diameter < 1 mm) and nickel. So, when it comes to evaluating the available information for the purposes of classification, one shall consider the forms or physical states in which the substance or mixture is placed on the market and in which it can reasonably expected to be used.
5 An additive is defined as a ‘substance that has been intentionally added to stabilise the substance’.
6 An impurity is defined as ‘an unintended constituent present in a substance as produced. It may originate from the starting materials or be the result of secondary or incomplete reactions during the production process. While it is present in the final substance it was not intentionally added’. Normally, impurities present in a concentration ≥ 1 % should be specified. However, impurities that are relevant for the classification and/or for PBT assessment shall always be specified.
7 A mono-constituent substance is a substance in which one main constituent (not being an additive or impurity) is present to at least 80 % (w/w).
8 A multi-constituent substance is a substance in which more than one main constituent (not being an additive or impurity) is present in a concentration ≥ 10 % (w/w) and < 80 % (w/w).
9 Substance of Unknown or Variable composition, Complex reaction products or Biological materials. 10 Organisation for Economic Co-operation and Development.
2.4
Chemical Safety Assessment and Chemical Safety Report
In accordance with Annex I of REACH, at production/import volumes of 10 or more tonnes per year, a Chemical Safety Assessment (CSA) documented in a Chemical Safety Report (CSR) is required. This includes a hazard assessment and, if the substance meets the criteria for classification and labelling or is assessed to be a PBT/vPvB11 substance, an exposure assessment including the generation of exposure scenarios and finally a risk characterisation. A registrant may decide to develop exposure scenarios even if the substance does not meet the above mentioned criteria, in order to describe and implement how he controls the nanomaterial at his own site and recommend downstream users to control exposures to human health and the environment.
The behaviour and effects of nanomaterials are dependent on several characteristics, including size, number concentration, surface area, charge and overall surface reactivity. The risk assessment related to both human health and the environment has to take into account these characteristics. In order to address the specific hazards associated with nanomaterials, additional testing or information may be required. To determine specific hazards associated with nanomaterials, current test guidelines may need to be modified. Until revised and specific test guidelines for nanomaterials exist, toxicity testing will have to be carried out according to already existing guidelines and/or by corresponding test methods, as much as possible supplemented with nano-specific additions because these can provide very useful and relevant information (see section 3.2).
2.5
Exposure-related information
Aside from data to characterize the hazard of a substance, REACH also requires information on exposure, use and risk management measures to be provided upon registration. For substances manufactured or imported in quantities below 10 tonnes/year, however, the data requirements are very limited. For these substances the following information should be provided (Annex VI):
− Information on the tonnage; a brief description of the technological process(es) used in manufacture or production of articles; information on the form (substance, preparation or article) and/or physical state under which the substance is made available to downstream users; a brief general description of the identified use(s).
− Guidance on safe use, consistent with information in the safety data sheet on e.g. safe handling and exposure control/personal protection.
− Information on exposure for substances registered in quantities between 1 and 10 tonnes/year: the main use category (industrial, professional and/or consumer); whether use is in closed system, non-dispersive, dispersive and/or results in inclusion into or onto matrix; significant route(s) of exposure for humans and the environment; pattern of exposure (accidental/infrequent, occasional and/or continuous/frequent).
It is important to note that these limited requirements also hold for substances manufactured or imported in volumes over 10 tonnes/year when they are not classified as dangerous or not PBT/vPvB. The resulting information is more of a qualitative than quantitative nature, and will not likely provide details on the various sizes and shapes/forms of the nanomaterials to which humans and the environment are exposed in different exposure scenarios.
Only for substances manufactured or imported in volumes over 10 tonnes/year that are classified as dangerous or PBT/vPvB, a more detailed exposure assessment is required, considering all life-cycle stages resulting from the manufacture and identified use(s). This assessment includes development of exposure scenarios (including description of operational conditions and risk management measures) and exposure estimation (Annex I). Question is, however, if monitoring data are available for nanomaterials or if existing exposure models like EUSES12, ConsExpo13 and EASE14, are suitable for providing exposure estimates for nanomaterials.
12 European Union System for the Evaluation of Substances; a quantitative risk assessment tool for chemicals. 13 Software model to calculate consumer exposure.
3
General risk assessment issues for nanomaterials
3.1
Introduction
In the past years, several analyses on the suitability of current risk assessment approaches for nanomaterials have been performed. These are carried out by various international scientific organizations and committees such as the EU Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR, 2006, 2007b, 2009), the Royal Society and Royal Academy of Engineering (2004), VDI Technologiezentrum GMbH (2004), Nanotechnology Research Co-ordination group (NRCG, 2006, 2007), Environmental Defense – Du Pont (2007), Nanotechnology Industries Association (Arnold, 2007), US Environmental Protection Agency (US EPA; 2008) and Defra (Crane and Handy, 2007).
Recently, an extensive summary of these analyses has been reported by Defra (Rocks et al., 2008). All the information available until May 2008 is clearly and comprehensively reviewed in this report. Furthermore, it also contains an evaluation of the suitability of the current OECD test guidelines for the testing of nanomaterials. A similar evaluation has been made by the OECD Working Party on Manufactured Nanomaterials (WPMN) (OECD, 2008a).
It is also to be noted that the OECD-WPMN has set up a Sponsorship Programme to provide guidance on the methods used to assess safety, and to derive valuable and relevant information on the safety of manufactured nanomaterials. This with the intention to improve the understanding of nanomaterials and, if possible, to understand what information may be generalized across different nanomaterials or classes of nanomaterials. In order to derive that information a list of ‘endpoints for testing nanomaterials’ has been formulated (see Appendix 2).
3.2
Suitability of current test guidelines for nanomaterials
For chemical risk assessment, a range of critical toxicity endpoints and associated test guidelines have been established by organizations and regulatory bodies worldwide (including OECD, US EPA, EU). In Appendix 3 a comprehensive overview of these test methods for physicochemical properties and human toxicity is presented together with their suitability to determine the toxicity of nanomaterials (copied from Rocks et al., 2008). A similar analysis of test guidelines was performed by the OECD- WPMN (OECD, 2008a). The testing methods can be split in three parts: physicochemical properties, human health effects, and environmental effects. A more detailed description of all tests can be found in the review report (Rocks et al., 2008). Here only the relevant issues with respect to the testing of nanomaterials, as identified in the review report by Rocks et al. (2008), are summarized. However, some of the issues mentioned are not specific for nanomaterials, but are also relevant for bulk substances.
3.2.1
Physicochemical tests
The physicochemical properties of a substance will, to some extent, determine the exposure route to be used in further toxicity studies. Within general risk assessment frameworks for non-nano (bulk)
materials, there is a suggested tiered approach to determine the physicochemical properties of a substance in order to eliminate unnecessary tests (reviewed by Rocks et al., 2008). In this testing scheme the particle size distribution, if appropriate, is determined at a later stage. For nanomaterials, however, it is suggested that the particle size distribution is already to be determined in an early stage, because it is thought to have a major impact on toxicity testing.
Other relevant issues identified in physicochemical tests (based on Rocks et al., 2008):
− Observation of material – Manufactured nanomaterials are generally assumed to be solid particles with at least one dimension less than 100 nm. The materials will not be observable by the human eye, and testers need a microscope to be able to observe the particles. With a number of the physicochemical tests, the endpoint is assumed to be observable by eye using the apparatus listed in the Testing Methods in Appendix 3.
− Amount of material – A number of methods require large amounts of material to be used in the test, which may pose a problem with nanomaterials in development.
− Use of appropriate controls – Any testing methods should be standardized using ‘known’ nanomaterials to ensure reproducibility.
− Appropriate tests – A number of tests are not appropriate for solid material (which most of the manufactured nanomaterials are). Also some properties can not be determined for certain nanomaterials such as melting and boiling point for metal oxides.
− Methods of analysis – There is uncertainty whether analytical techniques such as HPLC et cetera are suitable for nanomaterials with variable surface chemistry, solubility and reactivity. New techniques have to be developed and validated for each new variation, a long and laborious process. Electron microscopy (EM) can be used for determining the presence of nanomaterials; the composition can be analyzed by using Energy Dispersive X-ray (EDX) and X-ray photoelectron spectroscopy (XPS). For larger amounts, techniques such as X-ray Diffraction (XRD) and electron paramagnetic resonance spectroscopy (EPR) can be used to identify crystal structure.
− Characterisation of the specific surface area (SSA) – This can be done for a non-porous material by mathematical derivation from measurements obtained from electron micrographs. Porous nanomaterials will have an increased surface area which is more difficult to determine by electron microscopy, however the BET theory measuring the physical adsorption of gas molecules onto a solid surface may be suitable to determine the surface area of porous nanomaterials (Brunauer et al., 1938).
3.2.2
Human health toxicity tests
In general, toxicity testing methods as established for non-nanomaterials are considered suitable for the determination of the effects of nanomaterials on human health. However, there are a couple of concerns (based on Rocks et al., 2008):
− Mass concentration – Mass concentration (in mg/kg or mg/mL) may not be an appropriate metric for dosage of nanomaterials.
− Appropriate route of exposure – For initial in vivo toxicity testing methods normally the oral exposure route is used. However, for testing of nanomaterials, this may not be sufficient and administration via dermal or inhalation routes is likely to be more applicable. Furthermore, the effect of oral administration of nanomaterials on gut flora may show toxic effects which are not investigated and identified during routine toxicity testing (which also counts for bulk materials). − Duration of tests – Sub-chronic or chronic studies are likely to be the most appropriate to study the
toxic effects of nanomaterials since the duration of human exposure to small amounts of nanomaterials will be over a longer period of time. Single or short-term exposures are likely to
occur with high concentrations of nanomaterials as a result of accidental release. This point also holds for non-nanomaterials.
− Detection of nanomaterials – Whereas the potential toxic effects of nanomaterials will be detectable by using light microscopy, their presence, as single particles or in small aggregates, will not be. Therefore, to show the presence of nanomaterials within a histological sample it will be necessary to use EM, which may be very laborious and time consuming.
− Distinction and identification of nanomaterials – As the normal analytical detection methods may not be suitable to detect the presence of nanomaterials within a sample (see above), and EM techniques only show their presence, not their chemical structure, additional techniques such as EDX and XPS should be applied to elucidate the structure. This is essential for the identification of nanomaterials (both manufactured and naturally occurring).
− Systemic effects of toxicity – The most probable scenario is that a nanomaterial, after entering the body, will relocate in the organism and exert a systemic effect at a target site. This cannot be determined by single cell in vitro studies and therefore the need for animal experimentation remains until more developed screening tests are available or the relationship between the physicochemical properties of a nanomaterial and its toxic effect can be determined. Again this concern also holds for bulk materials.
− Effect of particulate number – Given the small particle sizes of nanomaterials and the normal dosimetrics in toxicity studies (mass concentration in mg/kg), there is a distinct possibility that due to the large amount of nanomaterial to be administered (which may no longer be representative for the actual exposure situation), toxic effects induced are a consequence of an overload phenomenon, rather than a consequence of exposure to the nanomaterial itself (or a combination of both).
− Solution or suspension of (nano)material – the distinction between a solution or suspension of a material, whether in nanoform or in bulk form, for use in sample preparation must be considered. However, it is likely that this will only be a problem with long term administration of the test substance as the suspension may precipitate out over time (sediment).
− Use of appropriate solvent – whilst the test nanomaterial may be soluble and stable in an organic solvent, the effects of the solvent on the test system must also be considered. Conversely, the potential of the nanomaterial to interact with the surrounding media (e.g. plastic of syringe, cell culture media) must also be considered in the administration of the nanomaterial. This concern also holds for bulk materials.
3.2.3
Ecotoxicity tests
The ecological information required under REACH of manufactured nanomaterials was considered in depth by Crane and Handy (2007).
The areas where the current ecotoxicological testing methodology was identified as not fit for purpose were:
− Relating macroscale to nanoscale – Current chemicals regulations (including REACH) do not distinguish between the nanoscale and macroscale forms of substances, so ecotoxicity tests performed on the macroscale form may, from a legal point of view, need to be accepted for both macroscale and nanoscale forms by regulatory authorities. This needs to change so that, at the very least, an evidence-based case is presented by manufacturers to show that there is no difference in the hazards of nanoscale and macroscale forms of the same substance. At present macroscale material toxicity cannot be related to nanoscale material toxicity, so currently evidence can only come from (rapid) testing;
− Exposure in test systems – Organisms in ecotoxicity tests should be exposed to nanomaterials in a way that is environmentally relevant. The homogenous dispersion currently recommended in
ecotoxicological testing may not reflect this. In the environment nanomaterials may react to their surroundings by agglomeration and aggregation after which precipitation is likely to occur, or they may react with other (naturally occurring) substances that may attach themselves to the surface of nanomaterials;
− Acute to chronic extrapolation – In most environmental risk assessment frameworks chronic toxicity is predicted from acute toxicity data by applying (large) assessment factors. For nanomaterials there is currently not enough empirical data (including data on bioaccumulation potential) to derive such assessment factors;
− Mass concentration is commonly used as a determinant of dose, but other metrics like for instance (combinations of) specific surface area, particle size, zeta potential, and shape might be better suited to quantify adverse effects across nanomaterials.
− Partition coefficient – There are some concerns about whether or not the partition coefficient test works for nanomaterials. This has implications for risk assessment strategies that use the partition coefficient as a trigger for requiring either sediment toxicity tests or bioaccumulation studies.
3.2.4
Additional OECD results
In the progress report of the OECD-WPMN (OECD, 2008a), all the OECD test guidelines were reviewed for applicability for nanomaterials. Only for physicochemical properties the tests were divided in 3 categories (I: applicable for nanomaterials, II: applicable for some nanomaterials or under some circumstances and III: not applicable for nanomaterials). The outcome of the evaluation (shortly summarized below) was generally similar to the one conducted in the review report by Rocks et al. (2008), with some slight deviations (e.g. on boiling point).
With respect to health effects, the general conclusion is that the OECD guidelines are appropriate for investigating the effects of nanomaterials with the important proviso that additional consideration needs to be given to the physicochemical characteristics of the materials tested, including such characteristics in the actual dosing solution (see section 3.2.2). However, in some cases there is need for further modification of the OECD guideline. This applies in particular to studies using the inhalation route and to toxicokinetic (ADME15) studies. Finally, it is important to build upon current knowledge and practical solutions in relation to in vitro test approaches.
More specifically:
1. The current test guideline on toxicokinetics is old (1984) and is currently being revised. This test guideline as well as the draft revised version only give very general guidance. The question is whether general modifications of this guideline for nanomaterials are sufficient, possibly specific studies are needed on a case-by-case basis. Absorption through cellular membranes is crucial. It is unknown which characteristics determine the absorption via the different exposure routes. Distribution, metabolism and excretion studies are also very important, especially the passage of nanoparticles through barriers like the blood-brain barrier and the placenta.
2. Current test guidelines for oral exposure are appropriate but the test endpoints may need to be extended, e.g. with cardiovascular effects. Test guidelines for inhalation exposure have recently been updated. One important aspect that has been changed in these revised versions is the inclusion of examination of the entire respiratory tract, which makes the tests more suited for assessment of nanomaterials. However, specific attention to the translocation of nanoparticles from the lung to blood and brain, as well as the dose metric relevant for nanomaterials are as yet not included in
these revised guidelines. For all repeated dose studies, including those via the respiratory tract, more information on cardiovascular effects, immunological effects and inflammation is desirable. Furthermore, also endpoints like reproductive toxicity/embryotoxicity as well as genotoxicity/carcinogenicity are worthwhile to investigate.
3. It is yet unknown whether the currently used assays for determination of genotoxicity will be valid for nanomaterials. This is in particular the case for bacterial assays since mechanisms of genotoxicity of nanomaterials may differ from that of other chemicals.
With respect to environmental effects, the validity and appropriateness of existing testing methods for nanomaterials is often questioned. According to the OECD (2008a), there is a lack of standardized protocols for testing ecotoxicity; guidance on preparation, delivery measurement and metrology in the existing test guidelines is currently insufficient for testing nanomaterials. The interactions of nanomaterials with the environmental matrices need to be assessed (like aggregation, shielding of the surface of nanomaterials with (dissolved) humic acids in water, complexation with organic carbon in soil as well as with other soil constituents like clay minerals). Exposure and dose-effect models need to be adapted. There is a general need for guidance for sample preparation and dosimetry. On this latter issue, a draft document has recently been written by the OECD-WPMN, in which guidance for nanomaterials has been proposed (OECD, 2009).
3.3
Currently proposed risk assessment approaches for nanomaterials
Of the currently proposed risk assessment approaches for nanomaterials, the one proposed by SCENIHR (2007b) is the most extensive one. The starting point in their approach, which was developed for assessing the potential risks from engineered nanomaterials, is the adequately characterized nanomaterial. Both human and environmental risks from exposure to this nanomaterial are then to be identified in a process involving 4 different stages (see also Appendix 4):
Stage 1: to identify whether the manufacture, use and/or end of use disposal or recycling could result in exposure of humans or environmental species and ecosystems;
Stage 2: to characterize the nature, level and duration of any exposure;
Stage 3: to identify the hazardous properties of any form of the nanomaterial to which significant exposure is likely;
Stage 4: to assess the risk.
In steps 1 and 2 the focus is on exposure to the nanomaterial, and when no human or environmental exposure is expected or this exposure is expected to be very low, the process stops or the nanomaterial is considered a low priority for hazard assessment. When, however, there is a potential for exposure, then the assessment proceeds to stage 3, the hazard assessment stage. In step 3, the focus is first on carefully selected in silico and/or in vitro testing and then, when effects are observed, either limited in
vivo testing when effects are very similar to those of the bulk chemical, or further more specialised in vitro testing followed by in vivo testing when effects are not very similar to those of the bulk chemical
or this is unknown. SCENIHR, however, does not provide details on the kind of in vitro or in vivo testing to be performed.
In 2009, SCENIHR concluded that ‘this framework remains appropriate although a few further details can be added in the light of recent publications’. These details include relevant physicochemical properties (size and size distribution, specific surface area, stability in relevant media, surface
adsorption properties and water solubility, if necessary extended with considerations about photoactivation and potential to generate active oxygen) and possibilities for read-across. This latter focuses on broad properties (e.g. fibres, rods and tubes versus particles) that might help identifying a testing strategy, although it is recognized that there is insufficient information to identify opportunities for read-across based on the general chemical composition of nanomaterials (SCENIHR, 2009).
On the basis of information on production volume, release and exposure and on toxicological screening information, a hazard trigger algorithm as a potential prioritization tool for regulators has been proposed by Howard and De Jong (2004), reviewed in VDI Technologiezentrum GMbH (2004) (see Appendix 5). In this algorithm a first step is to focus on the larger production volume materials (> 1 tonne/year) as well as those for which aerosol release and/or direct exposure (consumers, workers and/or environment) can be expected. If neither of these conditions is met, or if the material is rapidly soluble in water, it is considered as a low priority material for risk assessment.
For all other materials the next step is to distinguish between fibres and particles. Fibres < 5 µm in length and particles > 100 nm in diameter are considered as intermediate priority materials for risk assessment. In the final step toxicity and ecotoxicity is screened for the larger fibres (length > 5 µm) and smaller particles (diameter < 100 nm). If no (eco)toxicity is expected they are considered as intermediate priority materials, but if either human or environmental toxicity (or both) is expected or unknown, the material is considered as a high priority material for risk assessment. In this final step, toxicological screening is on lung toxicity, systemic effects, oxidative stress, endocrine disruption and sensitising potential, and ecotoxicological screening is on persistence, long range transport and biomagnification. However, no details are provided on the kind of tests (in vitro and/or in vivo) that would provide such screening information, nor is specified what kind of studies should be performed to complete the risk assessment (VDI Technologiezentrum GMbH, 2004).
Another approach which is currently explored within the RIVM and which was originally proposed with EU partners as part of a 7th EU Framework Programme proposal (but did not make it), is the NAPIRA Framework. This proposal is based on tiered testing, using (standard) in vitro and in vivo testing methods with, if necessary, nano-specific modification. In the proposal, tier 1 testing consists of local toxicity tests as well as in vitro kinetics studies. Depending on the outcome of tier 1 the next tier comprises four options for further testing, based on the presence or absence of local effects and systemic availability.
Despite a lot of attention the last few years for nanomaterials, it can be concluded that at the moment there are only a limited number of risk assessment approaches described. And those that have been described, just broadly outline the framework, with first a focus on exposure and in later stages/steps, only when a potential for exposure has been identified, a focus on hazard, however without specifying exactly what kind of in vitro and/or in vivo testing is to be performed in order to provide the necessary (screening) information for nanomaterials.
4
Nanosilver as a case study for registration under
REACH
The case study concerns a hypothetical registration of a substance (metallic silver) that exists in the nanoform (nanosilver, a first generation nanomaterial) as well as in the bulk form, with a total tonnage within the 10 – 100 tonnage band.
The nanoform, characterized by a spherical particle (no aggregates or agglomerates) with a size of 15 ± 5 nm, is used in a bathroom cleaning product, i.e. a trigger spray containing 1 % nanosilver particles.
Emission to the environment may occur during all life cycle steps. For consumers the use of the product may result in both dermal and inhalation exposure to nanosilver. Workers may also be exposed (during the production of nanosilver particles and the formulation of the cleaning product), but are not dealt with in this report.
As working hypothesis, it can be assumed that nanosilver forms dissolved free silver ions in aqueous solutions by dissolution and subsequent oxidation. Dissolution is a process that basically differs from the process of dissolving of chemicals. Chemicals that dissolve will become hydrated and will yield molecules that are surrounded by water molecules without losing their chemical integrity. Salts that dissolve in water generally yield hydrated cations and anions, i.e. charged ions surrounded by water molecules. Metal solids (i.e. metal not in salts) do not dissolve as such, independent of the metal solids being present in bulk form or in nanoform. Instead, oxidation of the metal will take place at the surface of the solids. This will yield oxidized metal ions (like Ag+) that are released in the water compartment surrounding the (bulk or nano) metal solids. Similar to the case of metal salts, the metal ions thus formed will become hydrated and surrounded by water molecules. Metal solids are not distributed in the environment according to equilibrium-based processes and instead will deposit to either sediment or soil instantaneously. Deposited salts will distribute according to the fundamentals of equilibrium partitioning. As the chemical form (silver metal) of nanosilver initially is similar to bulk silver, the nano-specific physicochemical properties of nanosilver are to be compared to the physicochemical properties of silver metal. As dissolution of nanosilver yields dissolved silver ions, the environmental distribution of nanosilver is best compared to the distribution of silver salts. Influence of nano-specific characteristics on nanosilver toxicity are the sum of the contribution of the nanoparticles sec and the contribution of the silver ions released. As bulk silver solids are non-toxic, the kinetics of dissolution are of importance in this respect, resulting in the silver cation (as measure for dissolved silver species) being the determining factor for (systemic) toxicity. This means that in principle toxicity data on any silver compound, when expressed as the silver cation, can be used to determine the (systemic) toxicity of silver (cf. former Technical Guidance Document for environmental risk assessment for metals and metal compounds), with data on soluble silver salts probably more worst case for (nano)silver than data on less soluble/insoluble silver salts, provided that the anion does not significantly contribute to the toxicity of the silver salt.
In conformity with the requirements under REACH and based on the above-mentioned assumptions for the nanoform and on the dissolution to the ionic species, a CSR has been completed on the hazard assessment and exposure assessment parts (as far as possible and considered relevant for the purpose of this case study). This CSR can be found in Appendix 6. In this chapter, the information requirements as well as the conclusions on the available silver data are summarized.
Disclaimer: The data presented in the CSR on bulk silver should not be seen as a complete overview of the toxicity of this compound. No in depth literature search was performed, only readily accessible data
were used. The primary information source in this case was an assessment report on silver thiosulphate, drafted within the pesticide framework (CTGB, 2004). For nanosilver the primary information source was a review by Wijnhoven et al. (2009). This was supplemented with relevant information found on nanosilver upon searching the literature for studies that became available since submission of the review.
4.1
Substance identity and physicochemical properties
4.1.1
Information requirements and availability
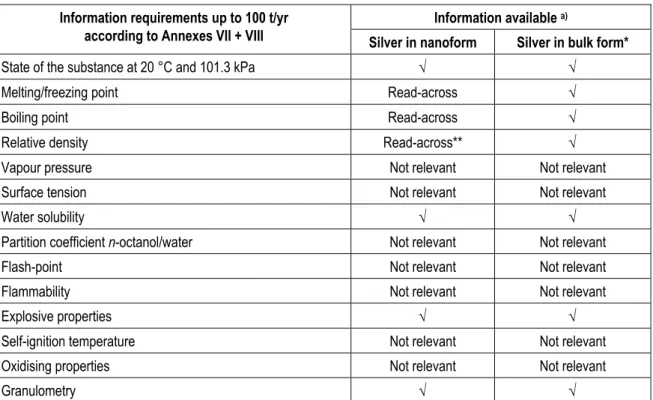
For substance identity and physicochemical properties the data requirements under REACH and available information are given in Table 1.
Table 1. Information required under REACH and information available.
Information available a)
Information requirements up to 100 t/yr
according to Annexes VII + VIII Silver in nanoform Silver in bulk form*
State of the substance at 20 °C and 101.3 kPa √ √
Melting/freezing point Read-across √
Boiling point Read-across √
Relative density Read-across** √
Vapour pressure Not relevant Not relevant
Surface tension Not relevant Not relevant
Water solubility √ √
Partition coefficient n-octanol/water Not relevant Not relevant
Flash-point Not relevant Not relevant
Flammability Not relevant Not relevant
Explosive properties √ √
Self-ignition temperature Not relevant Not relevant
Oxidising properties Not relevant Not relevant
Granulometry √ √
a) * information on metallic silver; ** caution is necessary (see section 4.1.2.2); √ information is available; Read-across:
for nanosilver this information is assumed to be the same as for silver in bulk form; Not relevant: the endpoint given is by definition not of relevance for the risk assessment of either the nanoform or the bulk form of (metallic) silver.
4.1.2
Summary of available data for substance identity and physicochemical properties
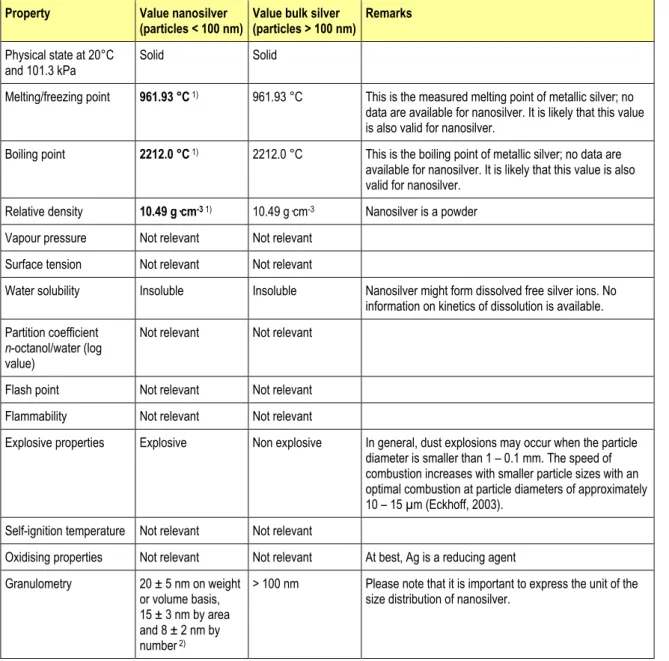
4.1.2.1 Substance identityFor silver and nanosilver, the names and other identifiers (CAS number, IUPAC name, et cetera) are the same. The typical purity of nanosilver is > 99.99 %.
4.1.2.2 Physicochemical properties
Nanosilver is a solid under standard conditions. Specific data for melting/freezing point, boiling point and relative density are not available for nanosilver, but it can be assumed that these are similar to those of metallic bulk silver. Interpretation of relative density of nanosilver, however, needs caution. Very small nanosilver particles will have a density > 1, but will not precipitate due to upwards pressures in the water that might very well counteract gravitational forces. This may complicate the prediction of the exchange of nanosilver between water and sediment/soil and may ask for an additional parameter for risk assessment of nanoparticles, especially since this may also influence fate and behaviour in aquatic toxicity tests.
Although no studies on the explosive properties of nanosilver are present, in general dust explosions may occur when the particle diameter is smaller than 1 – 0.1 mm. The combustion rate increases with smaller particle sizes with an optimal combustion at particle diameters of approximately 10 – 15 µm (Eckhoff, 2003). With respect to granulometry it is obvious that nanosilver particles are much smaller than bulk silver particles. Other properties are not relevant for silver.
Metallic bulk silver is a solid, for which the relevant properties are available. Bulk silver is not explosive.
4.1.3
Discussion on substance identity and physicochemical properties
4.1.3.1 Substance identityChemical identity
Distinct Chemical Abstract Service (CAS) numbers for silver and nanosilver are not available. CAS numbers are assigned for unique chemical species, with a molecular formula and a unique structure but not for ‘materials’ per se. When different CAS numbers are to be assigned to nanoforms of a bulk substance, it needs to be decided what makes a nanoform different from the bulk, and one nanoform different from the other.
Composition of the substance
The nanosilver particles in the case study are produced in a narrow particle size range, 15 ± 5 nm. Effectively, this means that the size distribution is assumed to be more or less monodisperse. In reality, however, the particle size distribution may be more multidisperse than monodisperse, i.e. the mean particle size may still be 15 nm but the distribution is much wider. Since physical, chemical and toxicological properties of nanosilver particles may depend on particle size, it is important to know how to deal with particles outside the intended range of 10 – 20 nm. Are they to be considered as impurities, or as part(s) of the substance, making the substance possibly a multi-constituent substance? As a further complication, metallic bulk silver is not specifically defined/characterized, other than that it consists of particles > 100 nm. This may give difficulties in comparing measured properties of metallic silver with those of nanosilver. So, for a proper read-across between the two, both forms in principle need to be properly characterized.
4.1.3.2 Physicochemical properties
Overall, most basic physicochemical properties of nanosilver are similar or assumed to be similar to those of bulk silver. Exceptions are explosivity, granulometry, and water solubility. Nanosilver may have explosive properties which are solely due to the small particle size. Although obvious that
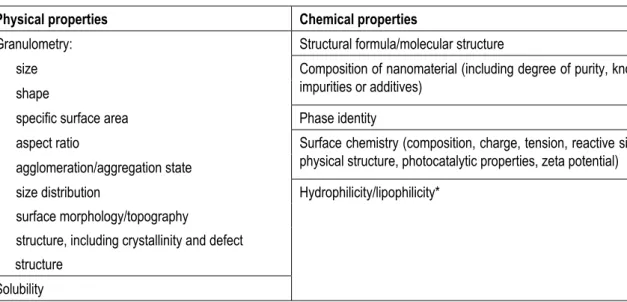
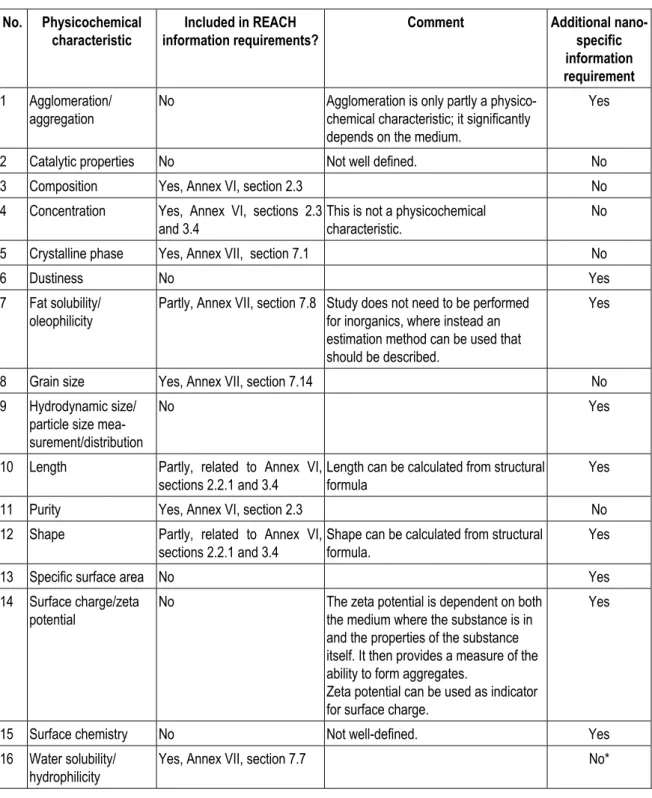
nanosilver particles are smaller than bulk silver particles, for a proper risk assessment other information on granulometry than solely particle size distribution may be essential. Parameters such as specific surface area, surface charge and shape of the particles (e.g. rods versus ball-shape particles) are important as well. This is in agreement with the opinion of SCENIHR (2009, with reference to the work of OECD and ISO), who identified the main physical and chemical properties with respect to nanomaterial safety (see Table 2). At the moment, it is not clear which properties are the key parameters to predict hazard and exposure, and therefore information on all properties should be provided. In the future when more information on nanomaterials becomes available it may be possible to make a selection of the most relevant parameters.
Various methods are available for measuring the parameters given in Table 2, like dynamic light scattering, electron microscopy, dynamic imaging (Nanosight), thermal optical transmission, et cetera. Silver is insoluble, but nanosilver might form dissolved free silver ions in aqueous solutions by dissolution and subsequent oxidation, or it may be dispersed in water following agitation. There is no information available on the kinetics of dissolution in dependence of nanosilver particle properties (such as (time-dependent shifts in) size distribution, shape) and properties of the medium (like pH, dissolved organic carbon, silver-complexing ions). This information is important, as also stated by SCENIHR (2009, see Table 2), especially for nanosilver because dissolution will yield highly toxic free silver ions. Free silver ions in water can relatively easily be measured by means of a silver selective electrode. Recently, a new approach is introduced to differentiate the effect of silver ions from silver nanoparticles by scavenging the silver ions with glycine (Navarro et al., 2008). Also, OECD guidance (OECD, 2001) is available for the determination of the rate and extent to which metals and sparingly soluble metal compounds can produce soluble available ionic and other metal-bearing species in aqueous media under a set of standard laboratory conditions representative of those generally occurring in the environment. In human toxicology studies distinguishing between nanosilver and silver ions may be much more difficult, because dissolution may take place within the organism, which complicates measuring this process (see section 4.3.4.3).
Table 2. Main physical and chemical properties with respect to nanomaterial safety as defined by SCENIHR (2009).
Physical properties Chemical properties
Granulometry: Structural formula/molecular structure - size
- shape
Composition of nanomaterial (including degree of purity, known impurities or additives)
- specific surface area Phase identity - aspect ratio
- agglomeration/aggregation state
Surface chemistry (composition, charge, tension, reactive sites, physical structure, photocatalytic properties, zeta potential) - size distribution Hydrophilicity/lipophilicity*
- surface morphology/topography - structure, including crystallinity and defect structure
Solubility * Not relevant for nanosilver.
In all cases, the fate and effect properties of the nanosilver particles basically need to be compared to those of bulk silver and the properties of silver salts like silver nitrate. This is the only way to assess the
nano-specific properties of nanosilver as compared to properties of the bulk material and dissolved silver ions. In essence, one wants to compare the risk of nanoparticles to the risk posed by bulk silver (i.e. of size in all dimensions > 100 nm). As the ultimate fate of nanosilver is dissolution to yield silver ions, the risks of silver salts need to be included too. For a proper comparison, however, additional data are needed, such as information on rate and extent of dissolution (also in comparison to metallic bulk silver) and on stability of the nanoparticle.
On basis of the evaluation of substance identity and physicochemical properties, there are some essential information gaps for the substance to be registered (i.e. (nano)silver). As indicated in the introduction of this chapter, it is assumed for the present case that some of these data is available/given. For instance, nanosilver is characterized by a spherical particle (no aggregates or agglomerates) with a size of 15 ± 5 nm.
These general assumptions will be used in the following paragraphs to assess the relevance of the toxicity data and to assess the exposure of the various groups.
4.1.3.3 Additional data needed
As indicated in Table 2, several specific properties for nanomaterials are needed to properly assess the risk. For the presented case of nanosilver (spherical particles of 15 ± 5 nm) some of these properties are known (e.g. size, shape, agglomeration/aggregation state), but there are also still some uncertainties. For the REACH requirements, however, only two main issues of uncertainty remain, which require additional information.
1. Nanosilver is assumed to be insoluble, but it might form dissolved free silver ions. As no information on the kinetics of dissolution is available, additional data on this kinetics is needed. 2. For fate estimations partition coefficients (i.e. Kp values) are needed, especially for the partitioning
between sediment and water and for soil and water. At present specific values for nanosilver are not available.
4.2
Environmental hazard and exposure assessment
4.2.1
Information requirements and availability
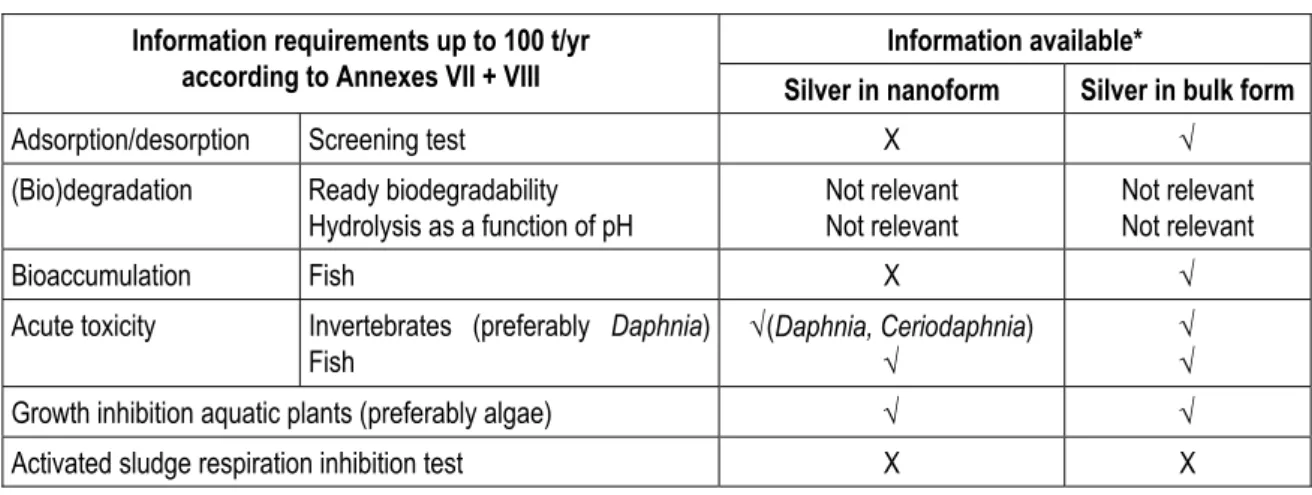
For the environment the data requirements under REACH and information available are given in Table 3.