A
Randomized
Controlled
Trial
comparing prone and supine position
in breast irradiation: 5-year results
Marie Vergotte
Student number: 01510627Promotor: Prof. Dr. Liv Veldeman
Co-promotor: Dr. Vincent Vakaet
A dissertation submitted to Ghent University in partial fulfilment of the requirements for the degree of Master of Medicine in Medicine
A
Randomized
Controlled
Trial
comparing prone and supine position
in breast irradiation: 5-year results
Marie Vergotte
Student number: 01510627Promotor: Prof. Dr. Liv Veldeman
Co-promotor: Dr. Vincent Vakaet
A dissertation submitted to Ghent University in partial fulfilment of the requirements for the degree of Master of Medicine in Medicine
Deze pagina is niet beschikbaar omdat ze persoonsgegevens bevat.
Universiteitsbibliotheek Gent, 2021.
This page is not available because it contains personal information.
Ghent University, Library, 2021.
ACKNOWLEDGEMENTS
First and foremost, I would like to thank my promotor Prof. Dr. Liv Veldeman for giving me the opportunity to write this master thesis and for guiding me through these two master years with quick and comprehensive feedback at all times. Without her availability and thorough answers, I could never have achieved this result.
Second, I want to thank my co-promotor Dr. Vincent Vakaet for the extensive support with the statistical analysis and the writing of this master thesis; and above all for helping me to become more at home in the world of radiotherapy with clear explanations regarding the principles of radiotherapy.
Next, I am thankful for Dr. Pieter Deseyne for providing me with the photographs to perform the objective analysis on and Mr. Hans Van Hulle for the accurate and meaningful remarks. Last but not least, I want to thank my sister Justine for the help with the design of this master thesis, my friends for the endless support and my boyfriend for always believing in me. I can honestly say that I enjoyed working on this master thesis and that it helped me grow as a person and as a future doctor.
Table of Contents:
Abstract (English): --- 1
Abstract (Dutch): --- 2
Introduction --- 3
Breast cancer and epidemiology --- 3
Standard treatment --- 4
Side effects of radiotherapy --- 7
Radiotherapy after BCS --- 8
Prone position and breath hold --- 9
Aim of the thesis --- 10
Materials and methods --- 12
Patient selection --- 12
Treatment schedule and technique --- 12
Assessment of late toxicity and cosmesis --- 13
Statistics --- 15
Results --- 17
Patient selection --- 17
Patient, tumor and treatment characteristics --- 18
Late toxicity at 5 years --- 19
Evolution of late toxicity: Clinical Endpoints --- 24
Oncological outcome --- 25
Discussion --- 27
Patient, tumor and treatment characteristics --- 27
Late toxicity: Clinical Endpoints--- 28
Late toxicity: Objective scoring using the photographs --- 31
Oncological outcome --- 32
Conclusion --- 33
ABSTRACT (ENGLISH):
Introduction:
Breast cancer is the most common cancer in women in Belgium. Due to the better treatment techniques, an excellent 5-year survival is achieved and as a result the long-term side effects and cosmesis have become more important. Radiotherapy plays a major role in this long-term outcome, in addition to the surgery. A reduction in this toxicity is already seen thanks to better irradiation techniques of which intensity-modulated radiation therapy (IMRT) is responsible for a major improvement. Prone position irradiation delivers promising results but little long-term evidence is already available. The purpose of this master thesis is to compare the 5-year cosmetic and oncological outcome between prone and supine irradiation in women with large breasts.Materials and methods:
Between 2010 and 2012, 100 women with large breasts (cup size C or more) were included in a non-blinded randomized controlled trial comparing the prone and the supine treatment position. All patients received hypofractionated IMRT as adjuvant whole breast radiation therapy after breast-conserving surgery. The 5-year toxicity was scored in two different ways: subjective by using clinical endpoints scored by the radiation oncologist and objective by using the BCCT.core software on photographs.Results:
In the supine cohort, 17,1% more patients showed pigmentation change 5 years after radiation therapy (RT) than in the prone cohort (P-value = .01). For retraction, edema, fibrosis as well as for patients who endured any toxicity after RT, a consistently higher percentage was found in the supine treated patients than in the prone treated patients; though no significant difference was calculated. In the supine group, more patients showed a deterioration of the BCCT.core classification (e.g. excellent -> good) and in addition a more serious deterioration (e.g. excellent -> fair) was seen on the photographs 5 years after RT in the supine group than in the prone group. The objective scoring using the pBRA value showed no differences between prone and supine irradiation. No local nor regional recurrence was reported in the study population and two patients developed distant metastases (1 in the prone cohort and 1 in the supine cohort).Discussion:
Breast irradiation in prone position after breast-conserving surgery is a promising new treatment option because of its dosimetric features and better dose homogeneity, especially for patients with large breasts. The acute toxicity was already described to be decreased when using the prone position technique (1). We reported less long-term toxicity in the prone group compared with the supine group, in particular when using the clinical endpoints. For the objective scoring, smaller differences were observed but the trend in favor of the prone position was also described. The oncological outcome in prone breast irradiation appears to be equal to the outcome in supine breast irradiation and no additional recurrences were seen in the prone treated patients. The limitations of the study are: the limited study population and the single-institutional non-blinded nature of the trial. Despite the limitations, this is the first randomized controlled trial comparing the cosmetic and oncological outcome 5 years after IMRT between prone and supine breast irradiation. In conclusion, prone breast irradiation seems to be at least as favorable as supine breast irradiation when considering the oncological outcome and in addition, less toxicity is seen in the prone position which results in a better cosmetic outcome.ABSTRACT (DUTCH):
Inleiding:
Borstkanker is de meest voorkomende kanker bij vrouwen in België. Tegenwoordig wordt er een uitstekende 5-jaars overleving bekomen dankzij de nieuwe therapie technieken en bijgevolg hebben de lange termijn bijwerkingen en esthetiek aan belang gewonnen. Radiotherapie speelt naast de chirurgie een belangrijke rol in deze lange termijn uitkomst. Dankzij betere radiotherapie technieken is er een belangrijke reductie gezien in deze toxiciteit, waarbij de introductie van intensity-modulated radiation therapy (IMRT) gezorgd heeft voor een grote verbetering. Veelbelovende resultaten werden al beschreven over bestraling in buiklig maar slechts weinig lange termijn resultaten zijn bekend. Het doel van deze master thesis is om de cosmetische en oncologische uitkomst 5 jaar na bestraling te vergelijken tussen ruglig en buiklig bestraling bij vrouwen met volumineuze borsten.Methodologie:
Tussen 2010 en 2012 werden 100 vrouwen met volumineuze borsten (cup maat C of meer) geïncludeerd in een niet-geblindeerde, gerandomiseerde gecontroleerde studie die ruglig en buiklig bestraling vergelijkt. De patiënten kregen allen een volledige borst bestraling met gehypofractioneerde IMRT na borstsparende heelkunde. De 5-jaars toxiciteit werd gescoord op twee verschillende manieren: subjectief aan de hand van klinische eindpunten die gescoord werden door de radiotherapeut-oncoloog en objectief door middel van de BCCT.core software op foto’s.Resultaten:
In de ruglig cohorte vertoonden 17,1% meer patiënten een pigmentatie verandering 5 jaar na radiotherapie dan in de buiklig cohorte (P-waarde = .01). Voor zowel retractie, oedeem, fibrose en voor alle patiënten die enige vorm van toxiciteit vertoonden, werd een consistent hoger percentage gevonden in de ruglig groep in vergelijking met buiklig; dit leverde echter geen significant resultaat op. Meer patiënten vertoonden een slechtere BCCT.core classificatie (vb. excellent -> goed) 5 jaar na radiotherapie in de ruglig cohorte dan in de buiklig cohorte en bovendien werd er een meer uitgesproken verslechtering gezien op de foto’s in de ruglig groep. De objectieve scoring met behulp van de pBRA waarde toonde geen verschillen tussen buiklig en ruglig bestraling. Er werd noch lokaal noch regionaal herval gerapporteerd in de studie populatie, daarentegen ontwikkelden wel 2 patiënten metastasen op afstand (in beide cohortes telkens 1 patiënt).Discussie:
Borstbestraling in buiklig na borstsparende heelkunde is een interessante nieuwe behandeloptie dankzij zijn dosimetrische eigenschappen, vooral voor patiënten met volumineuze borsten. Een vermindering van acute toxiciteit werd al beschreven bij het gebruik van buiklig bestraling (1). Wij rapporteerden minder lange termijn toxiciteit in de buiklig cohorte in vergelijking met de ruglig cohorte, in het bijzonder wanneer we de klinische eindpunten beschouwen. Voor de objectieve score waren de geobserveerde verschillen kleiner maar de trend in het voordeel van buiklig werd ook hier beschreven. De oncologische uitkomst in buiklig bestraling lijkt gelijkaardig als die in ruglig bestraling en geen bijkomend herval werd gezien in de buiklig cohorte. De beperkingen van onze studie zijn de volgende: de beperkte studie populatie en het monocentrische, niet-geblindeerde karakter van de studie. Ondanks deze beperkingen is deze studie de eerste gerandomiseerde, gecontroleerde studie die het verschil beschrijft tussen ruglig en buiklig bestraling betreffende de esthetische en oncologische uitkomst 5 jaar na IMRT. Concluderend lijkt buiklig bestraling minstens even gunstig te zijn als ruglig bestraling voor wat betreft de oncologische uitkomst en bovendien wordt er minder toxiciteit gezien in buiklig bestraling wat resulteert in een betere cosmetische uitkomst 5 jaar na borstbestraling.INTRODUCTION
Breast cancer and epidemiology
Breast cancer is by far the most common cancer in women in Belgium, with the exception of skin cancer and hematological cancer that are usually not included in these solid organ cancer statistics. An average of 10 500 new cases of breast cancer were reported per year during the period of 2011-2016. Roughly 1 in 3 women with cancer have breast cancer. The average age at diagnosis is 63 years old and there is a clear increase in incidence starting at the age of 35 with a peak incidence in the age group of 65 to 70 years old. It has a relatively good prognosis with a 5-year relative survival of 90%; on the other hand, it still is the most important cause of cancer deaths in females. There clearly is a lower 5-year relative survival rate in the older age groups: 84,1% in the age ‘70+ years’ versus 93,0% in the age ‘50-69 years’ and 93,3% in the age ‘15-49 years’ (2). Breast cancer is the most common cause of death in women aged between 40 and 55 years old. 1 in 10 women will also be diagnosed with breast cancer in their lifetime (3).
The most frequent types of breast cancer are the ductal carcinoma (80%) and the lobular carcinoma (10%). Breast cancer can be considered as a hormone-dependent malignant disease in the majority of the cases. Early menarche, late menopause, nulliparity and first parturition at a higher age are risk factors to develop breast cancer. Women who take hormonal contraceptives since puberty will have a slightly increased risk, but this effect will disappear 10 years after stopping this hormonal treatment. A full-term pregnancy has a protective effect against breast cancer. Some other risk factors to develop breast cancer are an older age, major inheritance susceptibility (the presence of a BRCA-1 or BRCA-2 mutation), obesity, family health history, alcohol intake, breast tissue density and radiation exposure to the breast. Using mammography as a screening method decreases the breast cancer mortality (3, 4). The sensation of a lump in the breast is often the first clinical symptom of breast cancer but recently more and more women are diagnosed by having an abnormal screening mammography. Others complain about skin or nipple retraction, edema of the skin or fluid loss from the nipple. Breast cancer can be diagnosed based on three different aspects. The diagnosis of malignancy can be strongly suspected with a correct clinical examination. However, this has to be confirmed by imaging (mammography or ultrasound breast and ultrasound axilla) and (cyto)histopathology. Staging – according to the TNM classification - is obtained by performing a few additional imaging examinations such as a chest x-ray, a bone scan and an ultrasound of the liver. The T indicates the size of the tumor (categories range from 1 to 4), N indicates
the lymph node invasion (ranges from 0 to 3) and M indicates the presence of metastases (0 = no metastases, 1 = metastases elsewhere in the body) (3).
Standard treatment
The treatment of breast cancer is situated on three different levels: local (surgery to remove the tumor in the breast or radiotherapy (RT) to the breast and/or thoracic wall), regional (axillary dissection or radiotherapy to the lymph node regions) and systemic (endocrine therapy, chemotherapy, and/or targeted therapy are used to reduce the risk of developing metastases). The decision of the exact therapy will be made individually and depends on the type of breast cancer and the staging. Much progress has been made recently in this study area. Unfortunately, there are still many side effects and complications (both early and late) that can occur in any part of the treatment (3, 5).
The centerpiece of breast cancer treatment remains surgery. This consists of radical mastectomy or breast-conserving surgery (BCS: lumpectomy, partial mastectomy, segmental mastectomy or quadrantectomy); in some cases combined with axillary dissection. When performing a mastectomy the whole breast is removed, in contrast to BCS where only the tumor and a certain margin are removed. To avoid the complications of an axillary dissection (e.g. arm lymphedema), a sentinel lymph node (SLN) procedure is generally performed in clinically lymph node negative patients. This SLN is the first lymph node that will receive drainage from the primary breast cancer and is also the first lymph node where the cancer is likely to spread to from the primary tumor. By injecting a radioactive substance or a blue dye in the proximity of the tumor this SLN can be found and removed. It will then be histologically examined by the pathologist. If the lymph node is clear, an axillary dissection may not be required. If cancer cells are found in this lymph node, an axillary dissection is considered, but in some patients radiotherapy can be an alternative for axillary surgery as shown in recent publications (6, 7). The most common side effects of surgery are wound infection, fatigue, pain and an impaired self-image especially after radical mastectomy. When axillary dissection is performed, some other side effects may also occur such as lymphedema, numbness, loss of strength and reduction of arm movement amplitude (5). BCS is carried out more and more often because it has a number of advantages: it is less invasive and thereby the recovery time is shorter and a better aesthetic outcome is to be expected. No significant difference is found in the overall survival between the two different surgical procedures (breast-conserving surgery followed by radiotherapy vs. mastectomy) (8, 9).
Both chemotherapy and endocrine therapy (ET) result in a longer disease-free period and overall survival in patients up to the age of 70. If the tumor is hormone receptor positive (ER+ and/or PR+), an adjuvant hormonal therapy is always indicated. Some patients will need both chemotherapy and hormonal therapy (after the chemotherapy) (3, 10). Tamoxifen is a selective estrogen receptor modulator, combining both estrogenic and anti-estrogenic functions: it inhibits hormone receptor positive breast cancer cells but contrariwise it stimulates the endometrium (higher risk of endometrial hyperplasia and carcinoma), the bone (protective effect against osteoporosis), the lipid metabolism (protective effect) and blood clotting (increased risk of thromboembolism) (3, 11, 12). Tamoxifen reduces the 15-year absolute risk of death and also reduces the relative risk of recurrence by 50%. This results in a reduced risk of 5-year recurrence (3, 13). Together with tamoxifen, aromatase inhibitors (AI) are the most commonly used ET in breast cancer. AI are inhibitors of the aromatase enzyme, which is responsible for the conversion of androgens to estrogens. As a result, the oestradiol concentrations are strongly reduced causing the stimulation of oestradiol on the hormone-sensitive breast cancer cells to be lost (3). The patient’s menopausal status is the most important factor that determines the agent that will be used (11). In postmenopausal women, AI are often preferred. In premenopausal women, AI can only be used in combination with suppression of the ovarian production of estrogens by performing a bilateral ovariectomy or by giving a long-acting GnRH analogue (3). Alternatively, tamoxifen is used in premenopausal women. AI combined with ovarian ablation has a benefit over tamoxifen in younger women (under 45) (14, 15). ET has some important advantages over chemotherapy: higher efficiency in hormone receptor-positive tumors, easy medical administration, better tolerance and quality of life and the reversibility of the treatment. Nevertheless, some important side effects such as hot flashes, vaginal dryness, sleep disorders, fatigue, weight gain, arthralgia, osteoporosis and endometrial hyperplasia/carcinoma can occur (3, 5). Due to the increased risk of osteoporosis with an AI, a bone densitometry is recommended before starting treatment (3).
In order to reduce the risk of recurrence and death after breast cancer surgery, adjuvant chemotherapy is used to destroy the micrometastases. It stops the cell division of rapidly dividing cells. These micrometastases are most prevalent in lymph node positive breast cancer. Patients with hormone negative tumors will usually receive chemotherapy but chemotherapy is also considered in other patients. An individual decision is made based on the prognostic factors of the tumor and the risk factors for relapse, but the side effects of the chemotherapy, age of the woman, cardiac risk factors and other comorbidities of the patient are also taken into account. Chemotherapy is started 4 to 6 weeks after the surgery when the wound is healed. The most frequently used chemotherapy at this moment in breast cancer is a sequence of an anthracycline and a taxane. Short-term side effects such as bone marrow
depression (leucopenia, thrombopenia and anemia), nausea, fatigue, mucositis, alopecia and long-term side effects such as induction of menopause and infertility, heart insufficiency and myelodysplasia or leukemia can occur. Currently, a number of supporting measures are used to avoid or reduce these side effects as much as possible. In some cases, chemotherapy is given before surgery (neoadjuvant) to reduce the tumor, for example in large or HER2 positive or triple negative (ER, PR and Her2/Neu negative) tumors (3, 6, 11, 16).
Targeted therapy focuses on a specific characteristic of the cancer cells and therefore it has less adverse effects on normal cells. In tumors with HER2/neu overexpression, the monoclonal antibodies trastuzumab and pertuzumab are used (3, 5, 11). Unfortunately, only one in three patients will benefit from this treatment. In combination with chemotherapy, it reduces the recurrence risk by half, compared with chemotherapy alone in these patients. On the other hand, it is important to do a baseline cardiac function test and a periodic monitoring of cardiac function during the treatment due to the cardiotoxicity of trastuzumab. This is certainly necessary when combined with anthracycline-containing chemotherapy because they can both lead to cardiomyopathy (3, 11, 17).
Radiation therapy destroys cancer cells or keeps them from growing by using high-energy x-rays or other types of radiation. In breast cancer, external radiation therapy is the most frequently used technique (6). In this technique, the linear accelerator directs the radiation beam to the affected breast. For partial breast irradiation different techniques can be used like brachytherapy where tubes or needles are placed in the affected breast wherein radioactive wires are introduced. (3, 6). Postoperative radiotherapy is standard after breast-conserving surgery (3, 11, 18). After modified radical mastectomy, radiation therapy is only indicated in selected cases, e.g. if the tumor was large, if the lymph nodes are affected or if the patient is young (4, 6). Adjuvant radiotherapy after BCS and radical mastectomy for early-stage breast cancer significantly reduces the risk of locoregional recurrence, in BCS it even halves. In women with a high risk of locoregional recurrence, the absolute benefit of irradiation is bigger. Furthermore, it also improves the cancer-specific survival in patients undergoing systemic therapy, resulting in a breast cancer death rate reduced by a sixth (4, 18-21). For every four recurrences avoided, one breast cancer death can be avoided within the next 15 years. Radiation therapy can be delayed till after the completion of the chemotherapy (two to seven months after surgery) without having any effect on the rate of local recurrence nor on the overall outcome. Radiation therapy is therefore usually given after chemotherapy. Radiation therapy delivered concomitantly with trastuzumab appears to be safe. (4)
Side effects of radiotherapy
Irradiation also has an effect on the normal tissue surrounding the target volume and therefore various side-effects can be observed (4, 5). Short-term side effects of radiation therapy on the skin are erythema (over 50% of the women), dry or wet desquamation (6-50%), edema of the subcutaneous fat. These side effects are often represented by the term radiation dermatitis. Erythema is mostly transient and usually has a minor effect on overall cosmesis; this reaction starts generally after 2-3 weeks of treatment and peaks at the end of or in the first week after the radiation therapy schedule (3, 22). Some other possible side effects are fatigue (more than 1 in 2 patients), pain, anxiety, depression and decreased quality of life (5, 22). Long-term cosmetic changes are also observed after breast radiotherapy: color change, edema, retraction, telangiectasia and subcutaneous fibrosis (23). Lymphedema of the arm is an adverse effect seen after lymph node irradiation (6-10% of women, increasing to up to 40% in combination with axillary dissection) (4, 24).
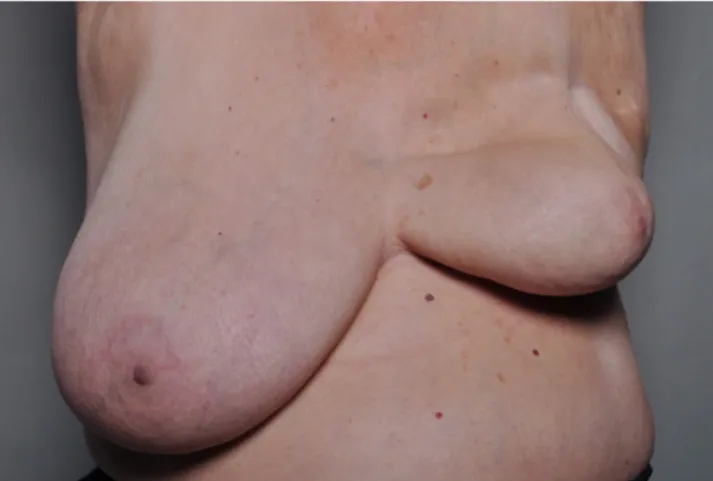
Figure 1. Acute radiation dermatitis (25) Figure 2. Late toxicity: severe retraction
The two major factors affecting the overall cosmetic outcome are tumor size and patient age. One study showed a higher frequency of breast induration and changes in normal breast color in younger patients (22). A study about the additional boost dose showed no significant difference in the grade of fibrosis between patients who received a boost dose of 16 Gy and those who did not (26). Some studies registered that undergoing a boost as well as having a large excision volume or simultaneous administration of chemotherapy has a negative effect on cosmesis (27, 28). In large-breasted women an increased RT-induced skin toxicity is seen (29).
Other possible side effects with a more serious long-term impact are pulmonary disorders like radiofibrosis, pneumonitis or lung cancer (particularly smokers) and heart problems such as ischemic heart disease, pericarditis and arrhythmias (3-6, 9, 30, 31). An increase in the
incidence of contralateral breast cancer was also observed, especially in patients younger than 45 years. In older trials, these side effects limited the beneficial effect of radiotherapy on 15-year breast cancer mortality (6, 21, 22). Multiple studies showed an increase of non-breast-cancer deaths in the follow-up after radiotherapy due to radiation-induced ischemic heart disease and secondary lung cancer (30, 32). The risk of ischemic heart disease increases with a higher mean dose to the heart, and women with other cardiac risk factors such as smoking show a greater absolute risk (9, 30). Epidemiological studies registered a higher cardiac mortality and incidence of lung cancer in women with left-sided breast tumors than in patients with right-sided tumors because of the increased cardiac and lung irradiation dose (9, 32). Important is to note that these data on heart and lung toxicity are based on studies that took place years ago and that these late effects are now less likely thanks to new techniques in radiation therapy that decrease the cardiac and lung dose (4, 9, 30, 31). The current mean doses of irradiation to the heart are typically less than 2 Gy when only the breast (and not the lymph nodes) is irradiated with contemporary techniques (9, 30, 33).
Radiotherapy after BCS
After BCS, a whole breast radiation therapy (WBRT) is the standard, although recent studies show promising results with partial breast irradiation (PBI) in selected patients with a low risk of relapse (26, 34). An extra focal radiation dose to the surgical cavity (boost) is indicated in certain cases (4, 21, 35). This additional boost on the tumor bed gives a further reduction of the risk of local recurrence, especially in patients younger than 50 years of age (3, 4, 11, 36) and can be delivered either by external-beam radiation (with photons or electrons) or by using brachytherapy. Nodal irradiation is considered for patients with involved lymph nodes and includes one or a combination of the draining regions of the breast: the axilla, the supraclavicular region and the internal mammary nodes (3, 11, 37, 38).
Conventionally, adjuvant WBRT was administered in 25 to 28 daily fractions of 1.8 Gy to 2 Gy over about 5 to 6 weeks with a total dose of 45 Gy to 50 Gy. Shorter fractionation schedules of 15 or 16 fractions over 3 to 4 weeks (hypofractionation) have shown equivalent results and have now become standard for WBRT without nodal irradiation. The results on local recurrence and survival, side-effects or overall aesthetic outcome were not inferior to the standard schedule of 25 fractions (39, 40). Some studies even found additional benefits of the hypofractionated scheme such as less normal tissue effects (breast shrinkage, breast edema
and telangiectasia) and a better overall survival compared with patients on the normal schedule (22, 40, 41).
In the last decade, intensity-modulated radiation therapy (IMRT) has become the standard for WBRT. Large beams, encompassing the whole breast, are divided in additional sub-beams of different intensity to allow intensity to vary depending on the individual anatomy of the breast. Randomized trials have shown that this results in a better dosage, less skin toxicity and a better cosmesis (29, 42).
Prone position and breath hold
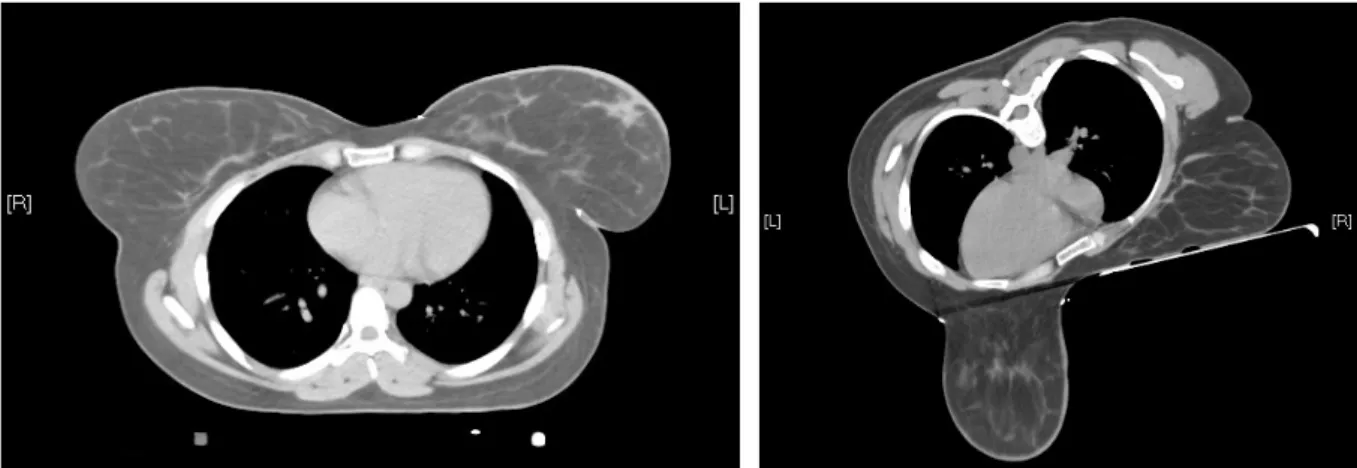
WBRT is according to the international standard performed in supine position (43). Prone positioning provides a displacement of the breast anteriorly away from heart, lungs and chest wall (44). Figures 3 and 4 show the distinct anatomy of the breast and neighboring organs in supine and prone position in the same patient. The unique anatomy and shape of the breast in prone position with unfolding of the skin leads to a better distribution of the target dose, a better dose homogeneity and less hotspots (44, 45).
Figure 3. CT-scan in supine position Figure 4. CT-scan in prone position
Radiation therapy in prone position is shown to deliver a lower mean dose to the heart, left-anterior-descending-coronary-artery (LAD) and above all to the lung in comparison with supine position, especially in patients with large breasts (31, 43, 45-48). The effect of the prone positioning upon the heart and LAD dose is variable between patients but in most patients a reduction of doses is found, mainly in the women with larger breasts (45, 46, 49). In prone position the heart tissue - especially the lateral and superior aspect of the heart - will move towards the chest wall and therefore a higher heart dose is observed in some patients with left-sided breast cancer, particularly in patients with small breasts (because the smaller breasts
are not pulled anteriorly under gravity) and in target tissues that include the chest wall or deep breast (44-46). In women with right-sided breast cancer a reduction in dose was seen regardless of the breast volume therefore these women are all likely to benefit from prone positioning (46).
A study from 2015 demonstrated better cardiac sparing with the supine deep-inspiratory breath-hold technique (DIBH) than with prone position radiotherapy with free breathing in the majority of the patients (33). In this breath-hold technique, the patient inhales to a specific threshold and this level of inspiration needs to be maintained during each irradiation wave delivered. With deep inhalation, the heart shifts caudally to a more favorable anatomic position further away from the treated breast. By increasing the distance between heart and breast tissue, cardiac dose can be reduced significantly. Combination of prone position and DIBH seems to be the best technique for heart sparing. Left-sided WBRT in prone position using DIBH reduces the heart and LAD doses compared to supine position using DIBH (50).
Nevertheless, the set-up in prone position is more complex than in the standard supine position and therefore the position is less reproducible (1, 50, 51). In general, prone position treatment is found less comfortable than supine position radiotherapy (33).Difficulties with positioning in prone position are shoulder positioning, underarm discomfort and shoulder tension and some women reported problems of neck and inframammary discomfort as well (33, 43). Continuous improvements in prone breast board technology are made and are likely to increase the patient comfort and the reproducibility (33).
Overall, irradiation in prone position is found to be feasible and well-tolerated by patients and prone deep-inspiratory breath-hold (DIBH) has the ability to acquire optimal heart and lung sparing for left-sided breast irradiation (43, 50, 52). However, the long-term effects of prone WBRT on cosmesis, late toxicity, local control and overall survival are not well studied and randomized controlled trials on prone WBRT are very scarce.
Aim of the thesis
The purpose of this thesis is to report the 5-year cosmetic and oncological outcome of a randomized trial comparing prone and supine WBRT in large-breasted patients. Already two studies have been published about the acute toxicity (2013) and the 2-year cosmetic outcome (2016) in this specific study population of breast cancer patients with large breasts treated at
edema and pain were significantly reduced in the prone positioning group compared to the supine patient cohort. No significant difference was found between the two groups for pruritus. The prone positioning also had a higher dose coverage index, dose homogeneity and less over-dosage. A lower average heart dose, LAD dose and especially lung dose were found in the prone-treated patients, although for heart dose this was not significant. The second study on this population showed that edema and a worsening of color change appeared explicitly more frequently in the supine cohort 2 years after WBRT and this color change was also found outside of the areola in contrast to the prone group where the color change was located solely at the areola. Scores for retraction and fibrosis were generally better in the prone group, though no significant differences were found between the two position cohorts. Overall, a better cosmetic outcome in the 2-year follow-up was obtained in the prone-treated patients than in the supine-treated patients which indicates a reduction in late skin effects in prone position irradiation after two years (23). In this thesis, the 5-year results are presented since cosmesis might further decline with increasing time and differences between the two groups might become more prominent (23).
MATERIALS AND METHODS
This study was designed as a phase III, non-blinded, mono-centric, randomized controlled trial comparing prone and supine setup in women who received IMRT as adjuvant WBRT after BCS. The primary endpoint was acute moist desquamation but other acute toxicity besides moist desquamation, late toxicity, Quality Of Life (QOL) and dose-volume parameters on organs at risk (OAR) were also compared between the two study arms. This master thesis evaluates the five-year results (both oncologically and cosmetically) comparing both radiotherapy positions (prone vs. supine), after radiotherapy following BCS. The study was originally approved by the Ethics Committee of Ghent University Hospital on 10/06/2010 and an additional approval was obtained on 15/11/2018 to write this master thesis. The study was registered on clinicaltrials.gov (reference number: NCT00887523).
Patient selection
Between December 2010 and October 2012, 100 female patients aged 18 and up with an European cup size C or more who needed WBRT were included in this randomized trial in Ghent University Hospital. The exclusion criteria included: undergoing a mastectomy, having metastases, the need for axillary irradiation, bilateral breast irradiation and previous irradiation at the same site. Patients who were unlikely to complete the trial (e.g. uncooperative attitude) or who had an inability to come back for follow-up appointments, were excluded as well. The patients had no mental conditions that would make them unable to understand the nature, extent and possible consequences of the trial. Because of the randomization, each patient had a possibility of 50% to be assigned to either the prone or the supine group. The randomization was performed independently of any features of the patients, the tumor or the treatment. An informed consent was obtained, signed and dated by all participants before the start of the study. Patients with less than five years of follow-up were excluded from this analysis.
Treatment schedule and technique
The patients underwent CT-simulation, planning and treatment in prone or supine position. All patients received hypofractionated WBRT and this was administered in 15 fractions of 2.67 Gy over a period of three weeks with a total dose of 40.05 Gy. When it was indicated, patients received an additional boost of 10 Gy in 4 fractions to the tumor bed (75 patients: 39 supine,
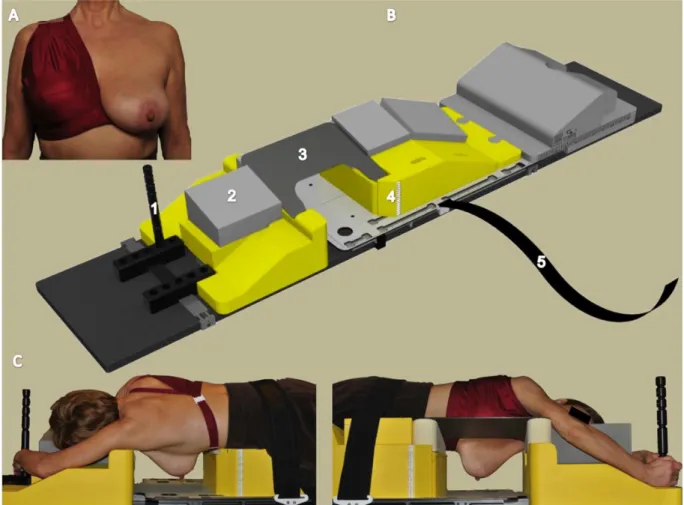
technique and a 2-beam tangential field (TF) IMRT was used in the prone position (1, 23). For boost irradiation, a multi-beam (MB)-IMRT was used in most patients. Only two patients received an electron boost, they were part of the supine group. For the prone setup a unilateral breast holder (U-BH) (Van de Velde; Schellebelle, Belgium) and a prone breast board (Orfit Industries; Wijnegem, Belgium) were used.
Figure 5. Setup in prone position with the unilateral breast holder (U-BH) (Van de Velde; Schellebelle, Belgium) and the prone breast board (Orfit Industries; Wijnegem, Belgium). (1)
Assessment of late toxicity and cosmesis
Two methods were used to evaluate the toxicity and cosmesis: subjective assessment by the radiation oncologist in the follow-up consultation and objective analysis by a software program on photographs. Clinical endpoints were scored before, 2 years and 5 years after radiation therapy by the treating radiation oncologist (who was conscious of the treatment position) using the Late Effect of Normal Tissue-Subjective, Objective, Medical Management and Analytical evaluation (LENT-SOMA) scale through observation and clinical examination.
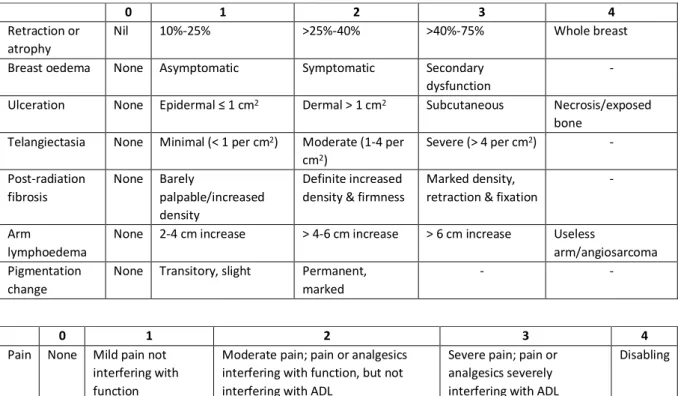
Table 1. LENT-SOMA scale, adapted from the doctoral thesis of L. Veldeman (53)
In a separate room, four digital photographs were taken in standard conditions with the patient in front of a gray background, an equal distance between lens and nipple and with the same presets of the camera.
Figure 6. Illustration of the setup in the photo studio, inserted from the doctoral thesis of L. Veldeman (53)
0 1 2 3 4
Retraction or atrophy
Nil 10%-25% >25%-40% >40%-75% Whole breast Breast oedema None Asymptomatic Symptomatic Secondary
dysfunction
- Ulceration None Epidermal ≤ 1 cm2 Dermal > 1 cm2 Subcutaneous Necrosis/exposed
bone Telangiectasia None Minimal (< 1 per cm2) Moderate (1-4 per
cm2) Severe (> 4 per cm2) - Post-radiation fibrosis None Barely palpable/increased density Definite increased density & firmness
Marked density, retraction & fixation
-
Arm
lymphoedema
None 2-4 cm increase > 4-6 cm increase > 6 cm increase Useless
arm/angiosarcoma Pigmentation
change
None Transitory, slight Permanent, marked
- -
0 1 2 3 4
Pain None Mild pain not interfering with function
Moderate pain; pain or analgesics interfering with function, but not interfering with ADL
Severe pain; pain or analgesics severely interfering with ADL
Two photos were taken: one with the arms alongside the patient and one with the arms elevated 180°, each with markers on the nipples, the suprasternal notch and the xyphoid. One additional photo was taken to identify the patient and as a reference for the color settings of the subsequent photographs. For this thesis, only the first two (anonymized) photos were used for quantitative scoring of cosmesis by using the Breast Cancer Conservation Treatment. cosmetic results (BCCT.core) software which provides an aesthetic classification ranging from poor to excellent and calculates the BRA and relative BRA (pBRA) as well. (54-56). This software can perform a semi-automatic analysis on two-dimensional anteroposterior photographs. By manually placing red markers on the existing markers in the photographs (nipples, suprasternal notch, xiphoid) and additionally on the both breast-axilla margins, the software can calculate the breast contour (white markers) which can be manually adjusted if necessary. Cosmetic outcome is defined by color differences (compared with the other breast), asymmetry and the appearance of the surgical scar. The BRA measures the rate of retraction of the treated breast with the contralateral breast and the BRA is calculated as following: 𝐵𝑅𝐴 = &(𝒳2 − 𝒳1)!+ (𝒴2 − 𝒴1)! with 1 = treated breast and 2 = contralateral breast. The pBRA is the dimensionless equivalent of the BRA and is calculated as following: pBRA = (BRA/ reference length) X 100 with 𝑟𝑒𝑓𝑒𝑟𝑒𝑛𝑐𝑒 𝑙𝑒𝑛𝑔𝑡ℎ = &𝒳1!+ 𝒴1! (54-57). Placing the right markers on the (anonymized) photographs for evaluation by the BCCT.core software was also part of the assignment for this master thesis, just like the statistical analysis of the study data.
Statistics
The difference between the scores before (baseline) and after radiotherapy (at 5 years of follow-up), both for the quantitative cosmetic outcome and the clinical endpoints, were included in the statistical analysis. The difference was used so that the pure effect of radiotherapy on cosmesis and late toxicity could be evaluated without any influence from the surgery. For the quantitative analysis (BCCT.core software), the outcome difference was described as following: 0 (no change or better), 1 (one category worse after treatment, e.g. from good to fair), 2 (two categories worse after treatment, e.g. from excellent to fair). As the null-hypothesis, the two treatment positions were assumed to cause a similar extent of late toxicity. These outcome differences were statistically analyzed using the Fisher’s Exact Test with a rejection of the null-hypothesis if the significance was < 0.05. The difference between the scores before and after radiotherapy (at 2 years of follow-up) were already evaluated in 2016 (23). In addition, new variables were created representing the difference between baseline and 5 years of follow-up, both for the quantitative cosmetic outcome and the clinical endpoints.
The dataset was cleaned and analyzed using the software program SPSS Statistics 25. The characteristics of the two cohorts were evaluated using the Mann-Whitney U-test for the continuous variables and the Fisher’s Exact Test for the categorical variables. The two cohorts were considered homogeneous if the statistical test (with a significance level of 0.05) was not significant.
RESULTS
Patient selection
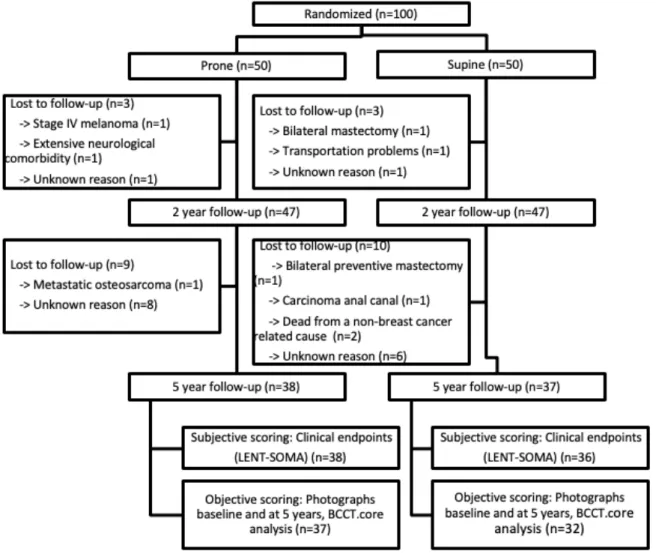
From the 100 patients included between 2010-2012, the 5-year up data (median follow-up of 61 months) was available for 75 patients (38 in the prone cohort and 37 in the sfollow-upine cohort). The clinical endpoints were present for 74 patients (38 prone vs. 36 supine) and the photographs for 69 patients (37 prone vs. 32 supine). In the timeframe between 2 years of follow-up and 5 years of follow-up 19 patients (9 prone vs. 10 supine) were reported ‘lost to follow-up’ (LTFU) due to various causes. Figure 7 shows the CONSORT diagram for the study population with the different reasons for the lost to follow-up.
Figure 7. CONSORT Diagram. Abbreviations: LENT-SOMA = Late Effect of Normal Tissue-Subjective, Objective, Medical Management and Analytical evaluation; BCCT.core = Breast Cancer Conservation Treatment.cosmetic results.
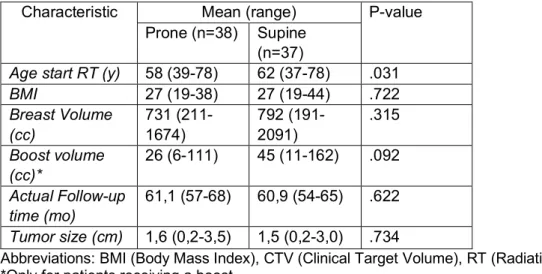
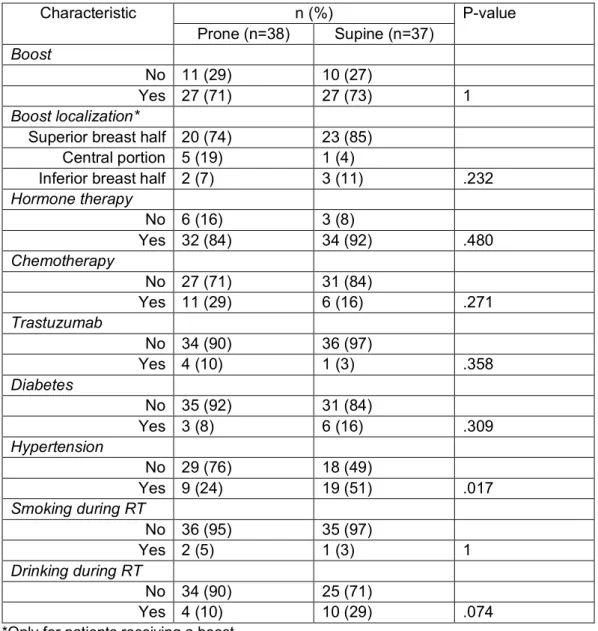
Patient, tumor and treatment characteristics
Table 2 and 3 show patient, tumor and treatment characteristics of the study population with 5-year follow-up data. The BMI, Breast volume (cc), Boost volume (cc), Actual follow-up time (mo) and tumor size (cm) showed no significant differences between the two groups. As for the age, a mean difference of 4 years was found between the two groups with supine treated patients being older than prone treated patients (P = 0.031). The difference in range for age is nearly non-existent with a range of 39-78 for the prone cohort and 37-78 for the supine cohort. Considering the categorical variables, no significant differences were found between the two cohorts. In the supine group, 27% more patients had hypertension with a P value of 0.017. Table 2. Baseline characteristics (5-year follow-up patients): patient, tumor and treatment; continuous variables
Characteristic Mean (range) P-value Prone (n=38) Supine
(n=37)
Age start RT (y) 58 (39-78) 62 (37-78) .031
BMI 27 (19-38) 27 (19-44) .722 Breast Volume (cc) 731 (211-1674) 792 (191-2091) .315 Boost volume (cc)* 26 (6-111) 45 (11-162) .092 Actual Follow-up time (mo) 61,1 (57-68) 60,9 (54-65) .622 Tumor size (cm) 1,6 (0,2-3,5) 1,5 (0,2-3,0) .734
Abbreviations: BMI (Body Mass Index), CTV (Clinical Target Volume), RT (Radiation Therapy). *Only for patients receiving a boost.
Table 3. Baseline characteristics (5-year follow-up patients): patients, tumor and treatment; categorical variables Characteristic n (%) P-value Prone (n=38) Supine (n=37) Boost No 11 (29) 10 (27) Yes 27 (71) 27 (73) 1 Boost localization*
Superior breast half 20 (74) 23 (85) Central portion 5 (19) 1 (4)
Inferior breast half 2 (7) 3 (11) .232 Hormone therapy No 6 (16) 3 (8) Yes 32 (84) 34 (92) .480 Chemotherapy No 27 (71) 31 (84) Yes 11 (29) 6 (16) .271 Trastuzumab No 34 (90) 36 (97) Yes 4 (10) 1 (3) .358 Diabetes No 35 (92) 31 (84) Yes 3 (8) 6 (16) .309 Hypertension No 29 (76) 18 (49) Yes 9 (24) 19 (51) .017 Smoking during RT No 36 (95) 35 (97) Yes 2 (5) 1 (3) 1 Drinking during RT No 34 (90) 25 (71) Yes 4 (10) 10 (29) .074
*Only for patients receiving a boost.
Late toxicity at 5 years
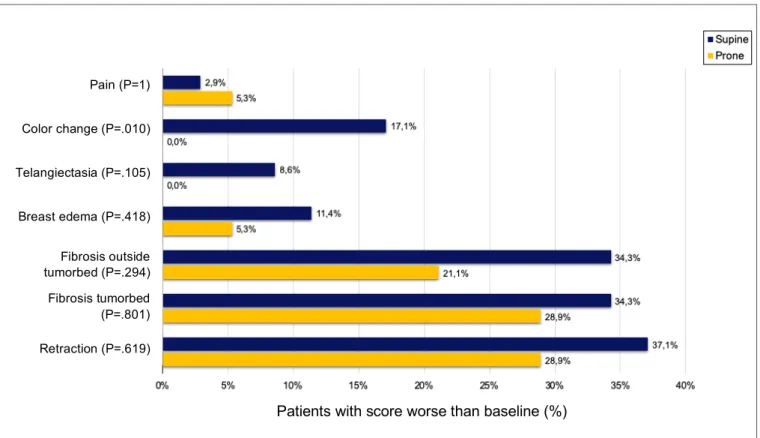
A) Subjective scoring: Clinical Endpoints
As shown in figure 8, some differences were seen between the prone and the supine cohorts as to the extent of late toxicity when looking at the clinical endpoints. None of the patients showed ulceration after RT, not in the prone group nor in the supine group. No aggravation of telangiectasia or color change was seen in the prone cohort. As for the supine group, a worse score was respectively present in 3 and 6 patients. For color change this leads to a significant difference between the prone and the supine cohorts with a P value of .01. Worsening of the pain was seen in 2 patients in the prone group versus 1 patient in the supine group (P = 1). For retraction, edema and both fibrosis outside the tumorbed and fibrosis in the tumorbed a
worse score was consistently found in more cases in the supine cohort than in the prone cohort; though none of them showed a significant difference in the statistical analysis.
Figure 8. Late toxicity: Patients with score worse than baseline for the Clinical Endpoints (5 years after RT).
Figure 9 shows the percentage of patients that endured any kind of toxicity 5 years after RT that wasn’t present before RT. This data was available for 35 supine treated patients and 38 patients of the prone cohort. All previously mentioned clinical endpoints were included in this equation. In the supine cohort, 8,5% more patients endured any sort of late toxicity after RT than in the prone cohort. A P value of .456 was calculated and thus considered not significant.
Pain (P=1) Color change (P=.010) Telangiectasia (P=.105) Breast edema (P=.418) Fibrosis outside tumo Fibrosis tumorbed (P=.801) Retraction (P=.619)
Patients with score worse than baseline (%) Fibrosis outside
tumorbed (P=.294) Fibrosis tumorbed (P=.801)
Figure 9. Any kind of toxicity after RT (at 5 years of follow-up).
B) Objective scoring: Photographs
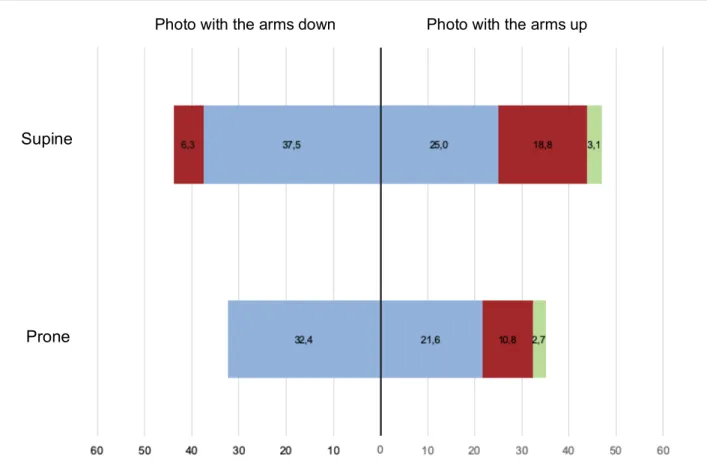
è Categorical BCCT.core scoreAs shown in table 4, a worsening of the BCCT.core classification was seen in one additional patient both for the prone and the supine group on the photograph with the arms elevated in comparison with the photograph with the arms alongside the patient. In the supine treated cohort, more patients endured an aggravation of BCCT.core classification (e.g. excellent -> good) compared with the prone cohort, although the difference was small and not statistically significant. The difference was described in both the photographs with the arms elevated and the photographs with the arms alongside the patient.
Table 4. Patients with a score equal or better than baseline according to the BCCT.core software on the photographs.
Prone Supine P-value
Count Percentage Count Percentage
Photo with arms down 25 67,6% 18 56,3% .46
Photo with arms up 24 64,9% 17 53,1% .34
On the photo with the arms down, a worsening of two categories (e.g. excellent -> fair) was described in two patients in the supine group versus none in the prone group (prone = 0% vs. supine = 6,3%, P = .45). For both groups, more deterioration of BCCT.core classification was seen on the photo with the arms elevated than on the photo with the arms alongside the patient.
Five patients endured a worsening of two categories or more (e.g. excellent -> fair; excellent -> poor) in the prone group against seven patients in the supine group on the photo with the arms elevated (prone 13,5% vs. supine 21,9%, P = .71). The differences were not statistically significant (Figure 10).
Figure 10. Patients with a score worse than baseline according to the BCCT.core software at 5 years of follow-up (Photo with the arms elevated and photo with the arms alongside the patient). Blue = 1 category worse, red = 2 categories worse, green = 3 categories worse.
Table 5. Patients with good/excellent cosmesis according to the BCCT.core software on the photographs at baseline and 5 years after RT.
Before RT Prone Supine P-value
Count Percentage Count Percentage Photo with the arms
down
35 94,6% 28 87,5% .382
Photo with the arms up 32 86,5% 28 87,5% .229
5 years after RT
Photo with the arms down
34 91,9% 24 75% .213
Photo with the arms up 29 78,4% 19 59,4% .151
At baseline, the number of patients presenting with a good or excellent cosmesis were comparable in the supine and prone cohort (> 85%). However, in the supine cohort, 12,5%
Supine
Prone
patients evolved to a fair or poor cosmesis at 5 years whereas in the prone cohort only 2,7% and 8,1% of patients evolved to a fair or poor cosmesis. These findings were not significant.
è Continuous with the pBRA value:
When comparing the evolution of the pBRA value on the photographs, a slight, but not significant, difference can be seen between the prone and the supine cohort. In the supine cohort, this data was available in only 27 patients whereas in the prone cohort the pBRA value difference was present in 35 patients. The pBRA value should be present in every patient of whom photographs were taken but some data was probably lost during the processing of the photos. In the supine group, more patients showed a worsening of the pBRA value on the photo with the arms down than on the photo with the arms up (21 vs. 18). Table 6 shows that this difference between the two photos is smaller for the prone group (photo 4 = 22 vs. photo 5 = 23). No clear differences can be seen between the prone and the supine group for the evolution of the pBRA value and this for both photographs. When the difference calculated between baseline and the 5-year follow-up is greater than 0, the pBRA value has worsened after RT. A percentage of 77,8% of the supine patients showed a worsening of their pBRA value on the photo with the arms alongside the patient, in comparison with 62,9% in the prone treated patients. None of these findings were significant.
In the prone group, a mean pBRA value of 0,107802 was seen at baseline and a mean pBRA value of 0,137277 at the 5-year follow-up. For the supine treated patients, a mean pBRA value of 0,106635 was found at baseline and 0,134879 at 5 years of follow-up. A mean difference between the prone and the supine group of 0,0087 was found for the photo with the arms elevated and 0,0067 for the photo with the arms alongside the patient, favoring the supine cohort. These findings show that only little difference can be seen between the prone and the supine cohorts for the evolution of the pBRA value.
Table 6: Patients with score worse than baseline for the pBRA value at 5 years of follow-up.
Prone Supine P-value
Count Percentage Count Percentage Photo 4: Arms down 22 62,9% 21 77,8% .27 Photo 5: Arms up 23 65,7% 18 66,7% 1
Figure 11. Evolution of pBRA value (mean of both photographs) between baseline and 5 years after RT. The outliers were excluded from this graph.
Evolution of late toxicity: Clinical Endpoints
Figure 12 shows the evolution of toxicity from baseline to 5 years for the prone and the supine group. At baseline, no large differences in toxicity were seen between both cohorts except for retraction or atrophy. In the supine group, a large percentage of patients was seen with retraction already at baseline whereas in the prone group this percentage was about half of it (supine = 62,9% vs. prone = 34,2%). Both retraction and fibrosis of the tumorbed were seen in more than 30% of the patients before RT whereas only a maximum of 10% of the patients was affected for the other clinical endpoints and this for the two groups. For all of the clinical endpoints except for pain, a higher percentage of patients was affected in the supine cohort than in the prone cohort at the 5-year follow-up. The curves are not for all endpoints similar for the prone and supine treated patients. The number of patients experiencing retraction remained constant in the supine group, while it clearly augmented in the prone group, especially between 2 and 5 years of follow-up. The difference between the both groups was reduced from 32,7% to 11,3% from baseline to 5-year follow-up. For breast edema, a fairly large difference was seen between the prone and the supine patients. At the 2-year follow-up, three times as much patients showed breast edema in the supine group than in the prone group while they showed the same amount at baseline (prone = 4 (10,5%) vs. supine = 12 (33,3%)).
Figure 12. Evolution of Clinical Endpoints: prone versus supine position. Continuous line = Prone, dashed line = Supine. Dark Blue = Retraction/Atrophy, yellow = Fibrosis tumorbed, grey = Pigmentation change, light blue = Breast edema, red = Fibrosis outside tumorbed, purple = Pain.
Two different trends can be seen in the evolution of the clinical endpoints: the types of defects that show their peak percentage at the 2-year follow-up and the ones that keep increasing until the 5-year follow-up. Breast edema, color change and pain fit into this first group and are all present in less than 1 in 5 patients at the 5-year follow-up. Retraction and both fibrosis outside tumorbed and fibrosis of the tumorbed show a further increase of patients after 2 years of follow-up. Figure 12 shows that a higher percentage of patients in the supine group experience pain at baseline, while at the 5-year follow-up more prone treated patients show pain. For breast edema, a clear difference is seen between the prone and the supine group. The number of patients with breast edema remains similar in the prone cohort, while in the supine cohort an increase is seen between baseline and 2 years of follow-up followed by a decrease between the 2-year and 5-year follow-up.
Oncological outcome
Neither local recurrence nor regional recurrence was seen in any of the patients included in our study. No recent data could be obtained for one early LTFU patient and therefore no information about possible recurrence was available for this patient. For three additional patients who became LTFU after the 2-year follow-up, no evidence of recurrence was found but recent data was absent. Distant metastases occurred in two patients, one in the prone
Timeframe: Baseline, 2-year follow-up, 5-year follow-up Patients
with toxicity (%)
treated group and one in the supine treated group. The first of these patients showed bone metastases 56 months after RT and the other one was diagnosed with pleural metastases 78 months after RT. Two patients underwent a preventive bilateral mastectomy after receiving the diagnosis of a BRCA mutation and one patient died from primary stomach cancer. One patient was LTFU due to a metastatic osteosarcoma, another because of a carcinoma of the anal canal, and a third one because of metastatic melanoma. One patient got LTFU after 5 years of follow-up due to fast evolutionary CML.
DISCUSSION
Breast irradiation in prone position after BCS is considered to be a feasible new treatment option for WBRT after BCS. In prone position, a decreased mean heart and LAD dose and above all a lower irradiation dose to the lung is seen, especially in women with large breasts. The anatomical features of the breast in prone position result in a safer administration of the radiation dose with less irradiation of healthy tissue surrounding the breast. Another benefit of using the prone technique in patients with large breasts is the reduced total tissue thickness and fewer volumes of over-dosage generated in the skin and subsequently a potential reduction of the fibrosis. Because of these favorable anatomical and dosimetric features, interest in it is rising and a lot of research is being performed to make it more of a standard in certain types of patients (1, 45, 46, 58, 59). The biggest issue is that only little long-term evidence is already available on this subject. The purpose of this thesis is to evaluate the cosmetic and oncological differences between the standard supine position and the more recently developed prone position for WBRT 5 years after RT. The acute skin toxicity was already observed to be reduced in the prone position compared to the supine position (1). In addition, a better cosmetic outcome was seen in the prone treated patients in comparison with the supine cohort two years after RT (23). Because of the much-improved life expectancy of breast cancer patients, it is important to also consider the aesthetic outcome as a major endpoint. Multiple studies showed that a better aesthetic outcome is associated with an improved psychological recovery and better QoL (Quality of Life) (60, 61). We evaluated the cosmetic outcome 5 years after WBRT in two different ways: subjective analysis through the clinical endpoints scored by the treating radiation oncologist and objective assessment with the BCCT.core software on the photographs. This study is the first randomized study that evaluates the difference in oncological and cosmetic outcome between prone and supine breast irradiation 5 years after WBRT.
Patient, tumor and treatment characteristics
While there were no significant differences in age between the 2 treatment arms in the originally randomized population, at 5 years differences were seen due to patients being lost to follow-up. The mean age difference between the prone and the supine cohorts at 5 years is significant but fairly small with only 4 years between them (prone = 58 vs. supine = 62 years). Age has been reported to have an influence on late toxicity, but age differences are probably the most relevant between pre- and post-menopausal women and this 4 year difference can probably be considered as not clinically relevant (62).
Some other patient-related factors such as hypertension, smoking history and medication use might affect the tolerance to radiation therapy and therefore the development of toxicity after RT (63, 64). Chon et al. reported 9 studies where the hypertension patients endured more late toxicity after RT than the control groups (65). Other studies reported a rather minimal effect of hypertension on the development of toxicity (66). Mbah et al. described no significant impact of hypertension on the toxicity endpoints (64). In our study population with 5 years of follow-up, 24% of the prone treated patients and 51% of the supine treated patients had hypertension (significant P value of .017). It is possible that this difference biased our results, but due to the conflicting results in the literature, no statement about a possible effect of hypertension on our study findings can be made.
Late toxicity: Clinical Endpoints
The clinical endpoints were scored in three consultations: before RT, 2 and 5 years after RT. The difference between this first and last one was calculated to find the net effect of the RT independently from the effect of the surgery. This because a poor surgical cosmesis before RT will lead to a poor overall cosmesis years after RT (67). All patients received hypofractionated WBRT after BCS, which is proven to have a similar efficacy and toxicity as the standard radiation schemes (39). Various studies showed less acute and late toxicity when using the IMRT technique in comparison with more conventional techniques. When using IMRT, a more homogenous dose distribution is obtained and less hotspots can be found. This can be translated in less skin telangiectasia and even a superior overall cosmesis (29, 67, 68). The fact that none of the patients in the study population endured major toxicity can partly be attributed to this innovative radiation technique. The study population consisted of woman with a cup size C or more and it is already described that women with large breasts often have an inferior cosmetic outcome than smaller-sized patients after BCS because of the marked dose inhomogeneity in these patients (63, 69). A first step towards a better dose homogeneity in these patients is done with the implementation of the IMRT technique (61, 70). The development of prone position RT is a next big step towards a better dose homogeneity, less hotspots and eventually a better cosmetic outcome, especially in patients with big breasts.
As expected because of the rather small differences between the prone and the supine group at the 2-year follow-up, the differences now are also not very pronounced. This study was not powered for late toxicity and due to patients being lost to follow-up even a smaller study population (n=75) was left at 5 years of follow-up. Only for color change a significant difference
endpoints the number of patients presenting with a toxicity score worse than baseline were consistently lower in prone than in supine position. The lower incidence of late toxicity in prone position is not unexpected.
In prone position, the shape of the breast resembles a cylinder whereas in the supine position the breast falls flat against the chest wall and describes an irregular shape. Because of these anatomical features, a more homogeneous dose is obtained in prone position IMRT which results in even fewer hotspots (regions of overdosage) than in supine IMRT.
The biggest and only significant difference between prone and supine position was found for color change (prone = 0% vs. supine = 17,1%). Color change is an adverse effect that usually has its peak percentage around 1-2 years after RT and afterwards a reduction is seen (71). As shown in figure 12, our findings confirm this theory. The percentage of color change in the supine group is similar to the numbers found in the literature, namely 15,7% and 20% (67, 70). As for the results of the prone group, a higher percentage is consistently found in the literature. In a study from Osa et al., 404 patients were included in a non-randomized trial where 92% of the patients were treated in prone position. They reported a percentage of 19,6% (at 5 years of follow-up) and Bergom et al. described a percentage of 22,9% (at 3,5 years of follow-up) (72, 73). A third study from Takahashi et al. described a percentage of 35% of the prone treated patients with color change at 4 years of follow-up (70).
Fibrosis is considered to be the hallmark of chronic RT damage and affects the function, shape and aesthetic outcome of the skin (74). A difference of 5,4% is seen between prone and supine for fibrosis inside the tumorbed and as expected because of the anatomical features of prone positioning, an even bigger difference was found when considering fibrosis outside this area (13,2%), favoring prone position. Osa et al. reported a similar percentage of 24,3% patients with fibrosis at 5 years of follow-up (in a population where 92% received RT in prone position), in comparison with 21,1% outside the tumorbed and 28,9% in the tumorbed in our findings (72). Bergom et al. on the other hand, described a much higher percentage of 53,3% prone treated patients with fibrosis with a mean follow-up of 3,5 years (73).
Ulceration is a severe adverse effect that was not seen in our patient cohort. Pain was present in less than 15% of the patients in the study population (5 patients) at 5 years of follow-up. No telangiectasia were seen in the prone group whereas in the supine group 8,6% of the patients showed this long-term adverse effect after RT. Osa et al. described a percentage of 21,3% for telangiectasia after RT in a study population of 404 patients where 92% received hypofractionated prone IMRT in two prospective trials with 5 years of follow-up (72). Another
study from Bergom et al. reported 7,6% patients with telangiectasia. This percentage was obtained in a study population of 109 WBRT prone treated patients with large breasts and a significantly shorter mean follow-up time of 45,9 months (versus 61,0 months in our population) (73). Barnett et al. reported a percentage of 14,9% for telangiectasia in the Cambridge Breast IMRT trial in 329 patients that received breast IMRT in the standard supine position (67). Both the prone and the supine group in this study scored better for telangiectasia than most of the already published studies. The use of two innovative and recent radiation techniques in this study can be an explanation for this finding.
Breast edema is an early adverse effect frequently seen after RT and in a small portion of patients an evolution towards chronic breast edema is seen. Barnett et al. reported a higher percentage of patients with breast edema 2 years after IMRT in supine treated patients than we found (42,5% vs. 33,3%) (67). This shows we already have advantageous results for the supine group. In the prone group moreover, 22,8% less patients showed breast edema in comparison with the supine cohort 2 years after RT. As expected, after this 2-year follow-up a big reduction is seen in the percentage of patients with breast edema. Breast edema was present in 11,1% of the supine treated patients and in 7,9% of the prone patients at the 5-year follow-up. Osa et al. reported an even lower percentage of 2,7% patients with breast edema 5 years after IMRT for mainly prone treated patients (72). When looking at breast edema that solely occurred after RT, a more similar percentage of 5,3% of the prone treated patients is found.
Most of the retraction we see in breast cancer patients after BCS can be attributed to the BCS and not to the RT, our findings displayed in figure 8 support this. In the supine cohort, 37,1% showed retraction because of RT as for the prone cohort a percentage of 28,9% was found. In the literature, Osa et al. reported a percentage of 20,8% in a group where 92% was treated in prone position but four times as many patients were included in this prospective trial than in our trial (72).
We reported any toxicity in 65,8% of the prone treated patients and in 74,3% of the supine treated patients 5 years after WBRT whereas Bergom et al. described any toxicity in 70,5% of the prone treated patients (73).
Late toxicity: Objective scoring using the photographs
Quite a few methods have already been described to evaluate the cosmetic outcome in an objective way that is reproducible and allows comparison between different studies. Cardoso et al. introduced the BCCT.core software which focusses on the asymmetry, color differences and scar appearance of the breasts and is demonstrated to be a useful tool to evaluate the cosmetic outcome (75). In our evaluation we considered both the categorical (category with range excellent to poor) and the continuous (pBRA value) results calculated by the BCCT.core software. Overall, no big differences were found between the prone and the supine cohort in the objective scoring. Some possible explanations have already been described earlier when discussing the clinical endpoints but for the objective scoring even fewer differences were calculated between the prone and the supine patients. This data was available for 37 of the prone treated patients and 32 of the supine treated patients and thus in less patients than the clinical endpoints. Some possible explanations for this smaller number are: the possibility of someone forgetting to take the photographs, the patient’s refusal or inability to wait for the photographs to be taken and the possible loss of photographs through these years. The differences between prone and supine were similar on both photographs with a higher percentage of patients in the supine cohort with a score worse than baseline (photo with the arms down = diff. of 11,4% and photo with the arms up = diff. of 11,8%). As seen in figure 10, more patients endured a deterioration of 2 categories or more in the supine group than in the prone group. Five years after RT, 12,5 % (photo with the arms down) and 28,1% (photo with the arms up) more patients showed a fair or poor cosmetic outcome than at baseline in the supine group. In the prone group, these percentages were noticeably lower (2,7% and 8,1%, respectively). We can conclude that more patients showed deterioration of BCCT.core classification in the supine group and in addition, a more serious deterioration was seen in the supine treated patients, but results were not significantly different, possibly due to the low sample size.
At 5 years of follow-up, we reported 91,9% and 78,4% (mean = 85,2%) of the prone patients and 75% and 59,4% (mean = 67,2%) of the supine patients with a good or excellent cosmetic outcome (mean difference of 18%). Various studies reported higher percentages for the part of patients with an excellent or good cosmetic overall outcome after RT as we did (prone = 89% - 94%, supine = 90% - 100%) (70, 71, 73). However, the overall cosmetic outcome also depends on surgery. Therefore, in contrast with other studies, we looked at the net effect of