CHARACTERISATION OF AN IN VITRO
INTESTINAL MODEL OF THE MUCOSAL
INTERFACE IN THE CONTEXT OF
CROHN’S DISEASE
Laure Maes
Student number: 01504117
Promoter: Prof. dr. ir. Tom Van de Wiele
Tutor: ir. Annelore Beterams
Master’s Dissertation submitted to Ghent University in partial fulfilment of the requirements for the degree of Master of Science in Bioscience Engineering: Cell and Gene Biotechnology
Deze pagina is niet beschikbaar omdat ze persoonsgegevens bevat.
Universiteitsbibliotheek Gent, 2021.
This page is not available because it contains personal information.
Ghent University, Library, 2021.
ACKNOWLEDGMENTS
Finalising this master’s dissertation feels like the icing on the cake of my education as a bioscience engineer. I started my thesis with full enthusiasm in August, not exactly knowing what to expect. Not only did I quickly encounter a strong curiosity for the topic, I also experienced a great satisfaction for performing research and laboratory work. During this year, I have obtained tons of hands-on lab experience and knowledge on the subject which I primarily owe to the people guiding me through this endeavour.
First of all, I would like to extensively thank my promoter, professor Van de Wiele, for the great opportunity to execute this master’s dissertation at CMET. Thank you for sharing your expertise on the topic with me and for all the insightful contributions to the design of the experiments and to the interpretation of the results. In addition, I would like to express my deepest gratitude to my tutor Annelore. Thank you for the vast amount of time you spent on teaching me all the necessary lab techniques and how to analyse results. Your frequent and constructive feedback has lifted this thesis to a higher level. Combining the laboratory work for this dissertation with the courses that I still had to follow was not an easy task. However, thanks to your support and patience, I was able to bring many experiments to a good end.
Of course I would like to extend my gratitude to my parents and brothers. Thank you, mama and papa, for giving me the opportunity to study bioscience engineering, but also for your consolation when times were difficult. Your encouragement and optimism were very much appreciated. Thank you, brothers, Marijke, Helena and Charlotte, for sharing your experience in writing a thesis and giving me constructive feedback after proofreading this dissertation.
Last but absolutely not least, special thanks go out to Simon, who has supported me unconditionally in the last few months. Due to corona, these months felt like an emotional rollercoaster, but your love and support made these strange times a lot more bearable.
PREAMBULE
Due to the corona measures, laboratory work was terminated on the 16th of March before all the planned
lab work for this master’s dissertation was finished. A running co-culture experiment was aborted and a differential gene expression analysis with microarrays, planned at the end of March, could no longer be executed. However, enough experimental data were obtained during the first semester and the month February to complete the dissertation without additional difficulties. This preamble was drawn up after consultation between the student and the supervisor and is approved by both.
I
TABLE OF CONTENTS
LITERATURE REVIEW ... 1
D
ISCOVERY AND DEFINITION OF INFLAMMATORY BOWEL DISEASE... 1
F
ACTORS INFLUENCING THE AETIOLOGY OF INFLAMMATORY BOWEL DISEASE... 2
2.1 Impact of the host’s genetic background ... 2
2.2 Link with the environment ... 3
2.3 Importance of the gut microbiota ... 4
A
NATOMY AND PHYSIOLOGY OF THE INTESTINAL WALL... 5
T
HE GASTROINTESTINAL TRACT MICROBIAL COMMUNITY... 6
4.1 Composition of the gut microbiota ... 6
4.2 Beneficial functions of the gut microbiota ... 7
P
ATHOGENESIS OFC
ROHN’
S DISEASE... 10
5.1 Intestinal dysbiosis ... 10
5.2 The leaky gut hypothesis ... 11
5.3 Disrupted bacterial clearance ... 12
C
URRENT THERAPEUTIC TREATMENTS OFC
ROHN’
S DISEASE... 12
I
N VITRO MODELS TO STUDY HOST-
MICROBE INTERACTIONS... 15
OBJECTIVES... 17
MATERIALS AND METHODS ... 18
H
UMAN CELL CULTURES... 18
B
ACTERIA... 18
I
N VITRO MULTICELLULAR CO-
CULTURE MODEL... 18
O
PTIMISATION EXPERIMENTS... 19
4.1 Adhesion capacity of LGG ... 19
4.2 Effect Triton X-100 on viability LGG ... 20
4.3 LGG growth quantification after 16, 24 and 48 h ... 20
V
ALIDATION OF THE IN VITRO MULTICELLULAR CO-
CULTURE MODEL... 21
5.1 Experimental set-up in vitro multicellular co-culture model ... 21
5.2 Analyses in vitro multicellular co-culture model ... 22
II
6.1 Fixation, permeabilization and blocking of human cells ... 25
6.2 MUC2 staining ... 25
6.3 DAPI and phalloidin-rhodamine staining ... 26
S
TATISTICS... 26
RESULTS ... 27
O
PTIMISATION EXPERIMENTS... 27
V
ALIDATION OF THE IN VITRO MULTICELLULAR CO-
CULTURE MODEL... 29
2.1 Inflammation induced with TNFα ... 29
2.2 Inflammation induced with SHIME bacteria ... 33
S
TAINING AND IMAGING... 37
3.1 Visualisation of nucleus and actin cytoskeleton ... 37
3.2 Visualisation of mucus layer ... 38
DISCUSSION ... 39
R
ATIONALE OF THE CONDUCTED STUDY... 39
O
PTIMISATION EXPERIMENTS... 39
V
ALIDATION OF THE IN VITRO MULTICELLULAR CO-
CULTURE MODEL... 42
CONCLUSION ... 50
FUTURE PERSPECTIVES ... 51
BIBLIOGRAPHY ... 53
APPENDIX ... 72
A
PPENDIXA:
LDH
ASSAY RESULTS... 72
III
ABBREVIATIONS
AIEC Adherent-invasive Escherichia coli
AMP Antimicrobial peptide
ASC Antigen-sampling cell
BCA Bicinchoninic acid
BSA Bovine serum albumin
AP-1 Activator protein 1
CD Crohn’s disease
CFU Colony forming unit
CLS Cell Lines Service
DAPI 4’, 6-diamino-2-phenylindole
DC Dendritic cell
DMEM/F-12 Dulbecco’s modified Eagle medium/nutrient mixture F-12
DMSO Dimethyl sulfoxide
(D)-PBS (Dulbecco’s) phosphate buffered saline
ECACC European Collection of Authenticated Cell Cultures
ECM Extracellular matrix
ELISA Enzyme-linked immunosorbent assay
FBS Foetal bovine serum
FCM Flow cytometry
GIT Gastrointestinal tract
HMI Host-microbiota interaction module
HuMiX Host-microbial crosstalk platform
IBD Inflammatory bowel disease
IEC Intestinal epithelial cell
IESC Intestinal epithelial stem cell
IFN-γ Interferon gamma
IL Interleukin
LDH Lactate dehydrogenase
LGG Lactobacillus rhamnosus GG
MAMP Microbe-associated molecular pattern
M cell Microfold cell
MEM Minimum essential medium
MRS de Man, Rogosa and Sharpe
MUC2 Mucin type II
NF-κB Nuclear factor kappa B
IV
PC Polycarbonate
PE Polyester
PMA Phorbol 12-myristate 13-acetate
PRR Pattern recognition receptor
RH Relative humidity
RPMI Roswell Park Memorial Institute
SCFA Short-chain fatty acids
SGPI SYBR Green propidium iodide
SHIME Simulator of the human intestinal microbial ecosystem
(s)IgA (secreted) immunoglobulin A
TH T helper cell
TLR Toll-like receptor
TNFα Tumour necrosis factor alpha
Treg Regulatory T cell
TRITC Tetramethylrhodamine B isothiocyanate
V
ABSTRACT
Crohn’s disease (CD) belongs to a group of disorders known as inflammatory bowel disease (IBD) and is characterised by a chronic and relapsing inflammation, primarily affecting the colon, although it can also spread throughout the whole digestive tract. In fact, the disease has been often associated to extraintestinal manifestations and autoimmune diseases. Clinical symptoms include bloody diarrhoea, abdominal pains, fever, vomiting, fatigue and weight loss. Since no cure has been found yet for CD, individuals are condemned to a life-long burden on their physical, emotional and economic well-being. Worldwide, the number of new cases of IBD is increasing every day, turning the disorder into a major global health problem. The exact cause of IBD is not yet understood, but scientific research points into the direction of a complex interplay of genetic susceptibility, environmental factors and the gut microbiota leading to a disturbed mucosal innate and adaptive immune response. In the last 20 years, the contribution of the gut microbiota to the pathogenesis of IBD has gained interest as accumulating scientific evidence has correlated IBD susceptibility genes to host-microbial interactions. Moreover, a disbalance in the gut microbial composition was demonstrated in many IBD patients.
Most scientific evidence of the host-microbial crosstalk is based on associations derived from genomic and metagenomic analyses, while little is known about the underlying molecular pathways. Currently, there is a lack of representative intestinal in vitro models to perform mechanistic studies. In this master’s dissertation, the main objective was to optimise and validate a multicellular co-culture model of the colonic mucosal interface to investigate the host-microbial interplay during inflammation. The co-culture model was based on an indirect interaction model between bacterial cells and a combination of T84 epithelial, LS174T mucus-producing and THP-1 macrophage-like cells. Bacteria were added on top of an agar-mucin layer in the apical compartment and grown as a biofilm, while the human cells were seeded in the basolateral compartment. Direct contact between the bacterial compartment and the human cells was prevented through the use of an agar-mucin layer on top of removable filter inserts with a semipermeable membrane.
During the optimisation experiments, the goal was to restrict the bacterial growth to representative numbers to mimic the in vivo situation as closely as possible. A small adhesion assay was performed to determine the adhesion capacity of the probiotic strain, Lactobacillus rhamnosus GG (LGG), on the agar-mucin layers. Approximately 20 % of LGG was able to adhere to the agar-agar-mucin layers after 2 h of incubation when a washing step was applied. The washing step was included in the experimental protocol to replace the planktonic bacterial cells by anaerobic PBS so that the adhered cells could form a biofilm during co-culture and the growth of planktonic cells was limited. The survival of LGG after Triton X-100 treatment was assessed in order to evaluate the possibility of using flow cytometry for viable bacterial enumeration instead of the viable plate count technique. Triton X-100 did not significantly affect the viability of the LGG bacteria and was therefore used to detach the biofilm cells from the agar-mucin layers and dissolve them as single cells for flow cytometry measurements. Multiple incubation end points
VI
were tested to evaluate the survival and growth of the LGG bacteria and the complex microbial community on the agar-mucin layers under aerobic conditions. In the absence of human cells, both LGG and the complex microbial community were able to grow on the filters, but bacterial concentrations higher than the in vivo situation were already reached after 16 h of incubation. The incubation end point of 16 h was thus chosen for the co-culture experiments to diminish the possible risk of cytotoxicity. During the co-culture experiments, the growth of the LGG bacteria and the complex microbial community was controlled by the presence of human cells. The LGG bacteria reached a concentration similar to the in vivo situation. The complex microbial community on the other hand, still attained a too high bacterial concentration after 16 h of co-incubation, but no cytotoxic effects on the human cells were observed.
The model was challenged with the pro-inflammatory cytokine TNFα and with a complex microbial community to imitate biochemically and microbiologically induced inflammation. In addition, the effect of LGG, with its reported immunomodulatory effects, was explored on the introduced inflammation. The viability and cytotoxicity of the human cells was determined with an lactate dehydrogenase (LDH) assay and a resazurin test. No reduction in viability was observed after administering bacteria or inflammatory stimuli to the multicellular co-culture model. Moreover, the three different cell types could be distinguished from each other on the microscopic images, they were able to survive on the collagen coating and they did not negatively affect each other’s viability. The inflammatory response following the introduction of the bacteria and inflammatory stimuli was evaluated by measuring the cytokine release with enzyme-linked immunosorbent assays (ELISA) after 16 h of co-incubation and the results were compared to a T84 monocellular model. The multicellular model responded more representatively to the inflammatory stimuli and bacteria in comparison to the monocellular model. A decreasing trend in IL-8 response was noticed when LGG was added to the multicellular model, while the IL-1β release appeared to be increased. This is in accordance to what is reported in literature. At last, the mucus layer produced by the combination of the three cell types in the absence of bacteria or inflammatory stimuli was compared to a mucus layer secreted by solely T84 cells. Mucus was visualised using an anti-MUC2 antibody with a confocal microscope. The thickness of the mucus layer was quantified through optical sectioning and analysed with an in-house developed ImageJ macro. The addition of the LS174T cells to the T84 cells resulted in the production of a thicker and more homogenously distributed mucus layer. In conclusion, the multicellular co-culture model is a promising model to study the host-microbe interactions during inflammation. In future research, a differential gene expression analysis can give more insight into the underlying molecular pathways and genes involved. The model can be personalised through the use of complex microbial samples derived from CD patients to perform mechanistic studies as well as therapeutic screening assays.
VII
SAMENVATTING
De ziekte van Crohn (CD) behoort tot de groep van de chronische inflammatoire darmziekten (IBD) en wordt voornamelijk gekenmerkt door een chronische en terugkerende inflammatie van de dikke darm, hoewel de ontsteking zich ook kan verspreiden over het volledige gastro-intestinale stelsel. Sterker nog, de ziekte wordt vaak geassocieerd met extra-intestinale manifestaties en auto-immuunziekten. Vaak voorkomende klinische symptomen zijn bloederige diarree, abdominale pijn, koorts, braken, vermoeidheid en gewichtsverlies. Aangezien er nog geen behandeling bestaat voor CD die tot volledige genezing leidt, heeft de ziekte een levenslange impact op zowel het fysieke, mentale en financiële welzijn van de patiënt. Wereldwijd stijgt het aantal nieuwe diagnoses dagelijks, waardoor de ziekte globaal een groot gezondheidsprobleem vormt. Hoe IBD zich exact ontwikkelt, is nog niet duidelijk, maar wetenschappelijk onderzoek wijst in de richting van een complexe wisselwerking tussen de genetische ontvankelijkheid, de omgeving en het darmmicrobioom van de patiënt wat zou leiden tot een verstoring van het aangeboren en adaptieve immuunsysteem. Gedurende de laatste 20 jaar werd er aanzienlijk meer aandacht besteed aan de bijdrage van het darmmicrobioom tot het ontstaan van de ziekte, aangezien wetenschappelijk onderzoek steeds meer IBD geassocieerde genen kon linken aan de interactie tussen de mens en zijn darmmicrobioom. Bovendien werden bij veel IBD patiënten onregelmatigheden opgemerkt in de samenstelling van hun darmmicrobioom.
De meeste wetenschappelijke bevindingen over microbe-gastheer interacties zijn gebaseerd op associaties afkomstig van genomische en metagenomische analyses, terwijl eigenlijk nog weinig geweten is over de onderliggende moleculaire processen. Er is vandaag de dag een gebrek aan representatieve in vitro modellen waarmee mechanistische studies kunnen worden uitgevoerd. Het doel van deze masterproef was om een multicellulair co-cultuurmodel van het dikke darm epithelium te optimaliseren en te valideren om de microbe-gastheer interacties tijdens inflammatie te onderzoeken. Het co-cultuurmodel is gebaseerd op een indirect interactiemodel tussen bacteriën en een combinatie van T84 epitheliale, LS174T mucus producerende en THP-1 macrofaag-achtige cellen in een Transwell®
setup. De bacteriën werden toegevoegd aan het model bovenop een agarmucinelaag in het apicaal gedeelte met als doel een biofilm te vormen, terwijl de humane cellen werden gekweekt in het basolateraal compartiment. Direct contact tussen het bacteriële compartiment en de humane cellen werd verhinderd door het toevoegen van een agarmucinelaag bovenop verplaatsbare filters met een semipermeabel membraan.
Het doel tijdens de optimalisatie was de bacteriële groei te limiteren tot representatieve aantallen om de
in vivo situatie zo goed mogelijk na te bootsen. Een adhesie experiment werd uitgevoerd om de
adhesiecapaciteit van de probiotische stam, Lactobacillus rhamnosus GG (LGG), aan de agarmucinelaag te bepalen. Ongeveer 20 % van de LGG bacteriën was in staat om zich vast te hechten aan de agarmucinelaag wanneer één wasstap werd uitgevoerd na 2 uur incubatie. De wasstap werd
VIII
toegevoegd aan het protocol van de co-cultuurexperimenten om de planktonische bacteriën te vervangen door anaerobe PBS zodat enerzijds de vastgehechte bacteriën een biofilm konden vormen tijdens de co-cultuur en anderzijds de groei van de planktonische cellen gelimiteerd werd. Het effect van een Triton X-100 behandeling werd geëvalueerd op de levensvatbaarheid van de LGG bacteriën met als doel na te gaan of de enumeratie van intacte biofilm bacteriën kon uitgevoerd worden met flow cytometrie in de plaats van het bepalen van het totaal kiemgetal. De levensvatbaarheid van de LGG bacteriën werd niet significant gereduceerd door de Triton X-100 behandeling waardoor de oppervlakte-actieve stof kon worden gebruikt om de biofilm cellen los te maken van de agarmucinelaag en ze op te lossen als individuele cellen voor flow cytometrie metingen. Verschillende incubatie tijdstippen werden uitgetest om de overleving en groei van de LGG bacteriën en van een complexe microbiële gemeenschap op de agarmucinelaag te analyseren onder aerobe omstandigheden. Zowel de LGG bacteriën als de complexe microbiële gemeenschap waren in staat – in afwezigheid van de humane cellen – om te groeien op de filters, maar na 16 uur incuberen werden al hogere microbiële concentraties bereikt dan in vivo. Om het risico op cytotoxiciteit te verminderen, werd daarom voor de co-cultuurexperimenten een co-incubatietijd van 16 uur gekozen. Tijdens de co-co-cultuurexperimenten werd de groei van de LGG bacteriën en de complexe microbiële gemeenschap onder controle gehouden door de aanwezigheid van de humane cellen. De LGG bacteriën bereikten een concentratie representatief aan de in vivo situatie. De complexe microbiële gemeenschap daarentegen groeide nog steeds uit tot te hoge bacteriële aantallen na 16 uur co-incuberen, maar er werd geen cytotoxiciteit waargenomen bij de humane cellen.
Inflammatie in het model werd biochemisch geïnduceerd door het toevoegen van het pro-inflammatoire cytokine TNFα en microbiologisch door het aanbrengen van een complexe microbiële gemeenschap. Daarnaast werd het effect van LGG, dat bekend staat om het immuunsysteem te beïnvloeden, geëxploreerd op de aangebrachte inflammatie. De viabiliteit en cytotoxiciteit van de humane cellen werd gecontroleerd met een lactaat dehydrogenase (LDH) en resazurine test. Er werd geen reductie waargenomen in de levensvatbaarheid van de cellen van het multicellulaire co-cultuurmodel na het aanbrengen van bacteriën of inflammatoire stimuli. Bovendien konden de drie verschillende celtypes herkend worden op de microscopiebeelden, waren ze in staat om te overleven op de collageenmatrix en verhinderden ze elkaars groei niet. De ontstekingsreactie door het aanbrengen van de bacteriën of de inflammatoire stimuli werd geëvalueerd na 16 uur incuberen door het kwantificeren van de cytokinevrijstelling via ELISA testen en de resultaten werden vergeleken met een T84 monocellulaire model. Het multicellulaire model reageerde accurater op de inflammatoire stimuli en bacteriën in vergelijking met het monocellulaire model. Een dalende trend in IL-8 en een stijgende trend in IL-1β productie werd waargenomen wanneer LGG aan het multicellulaire model toegevoegd werd, wat overeenkwam met bevindingen in de literatuur. Als laatste doelstelling werd de gesecreteerde mucuslaag door de combinatie van humane cellen in afwezigheid van bacteriën of inflammatoire stimuli vergeleken met een mucuslaag gesecreteerd door enkel T84 cellen. Mucus werd gevisualiseerd met een anti-MUC2 antilichaam door middel van een confocale microscoop. De dikte van de mucuslaag
IX werd gekwantificeerd door het creëren van optische secties die werden geanalyseerd met ImageJ. Het combineren van LS174T cellen met de T84 cellen resulteerde in de productie van een dikkere en meer homogene mucuslaag.
Uit het bovenstaande kunnen we besluiten dat het multicellulaire co-cultuurmodel een veelbelovend model is om microbe-gastheer interacties te bestuderen tijdens inflammatie. In toekomstig onderzoek kan een differentiële genexpressieanalyse meer inzicht bieden in de onderliggende moleculaire processen en de betrokken genen. Het model kan worden gepersonaliseerd door complexe microbiële stalen afkomstig van CD patiënten te includeren als om zowel mechanistische studies als therapeutische screeningstesten uit te voeren.
1
LITERATURE REVIEW
Discovery and definition of inflammatory bowel disease
Inflammatory bowel disease (IBD) is an umbrella term for a group of disorders characterised by chronic and relapsing inflammation of the gastrointestinal tract (GIT) (Torres et al., 2017). The IBD entity manifests itself in diverse subtypes with Crohn’s disease (CD) and ulcerative colitis (UC) as the two major forms (Baumgart & Sandborn, 2012). UC is the most frequently occurring form of IBD worldwide. Inflammation is restricted to the mucosal layer and it generally initiates in the rectum from where it can further spread out to more proximal colon regions. Typical histologic features of the inflammation are crypt abscesses or distorted crypt architecture, goblet cell mucin depletion and superficial ulcerations (Danese & Fiocchi, 2011; Xavier & Podolsky, 2007). CD on the other hand is characterised by a patchy, transmural inflammation. Afflicted areas, extending into the deeper layers of the GIT wall, alternate with healthy tissue (Gravina et al., 2018). Inflammation usually takes place at the terminal ileum, however the whole GIT can be involved (Figure 1) (Shanahan, 2002). The histologic features of CD are similar to UC but CD is also typified by the aggregation of macrophages (granulomas), fissuring abscesses, a thickened submucosa layer and a cobblestone appearance of the mucosal layer (Khor et al., 2011). Patients of UC and CD regularly experience bloody diarrhoea with or without mucus. Other clinical symptoms can occur depending on the severity of the disease including abdominal pains, fever, vomiting, fatigue and weight loss (Stange et al., 2008). Because of the similar clinical manifestations, the two subtypes have been mistakenly diagnosed in the past (de Souza & Fiocchi, 2016)
.
FIGURE 1 | The two major forms of IBD. With Crohn’s disease (left), the inflammation (red parts) is known to be patchy and can be widespread throughout the whole GIT. Inflammation in case of ulcerative colitis (right) is usually restricted to the colon. Adapted from The Hospital for Sick Children (2013).
UC was probably first reported in 1859 by an English physician, Sir Samuel Wilks, in the medical paper “Morbid appearances in the intestines of Miss Bankes” (Kirsner, 2001), whereas CD was considered to be already described in several reports from as early as 1769 (Aufses, 2001). In 1932, CD was defined as regional ileitis by Burrill B. Crohn, Leon Ginzburg and Gordon Oppenheimer at the American Medical
2
Association annual meeting (Crohn et al., 1932). One year later, the term Crohn’s disease was officially used in the United States in an article written by F.I. Harris (Kirsner, 2001).
The incidence of IBD – the number of new cases of IBD that occur during a given period of time – is increasing globally, turning IBD into a major global public health problem. Since 1900, the number of new IBD cases has stabilised in Europe, North America and Oceania. Nevertheless, the prevalence – the percentage of individuals suffering from IBD at a given time – remains high because of the low mortality rate and the primarily young age of onset (Ng et al., 2017). The majority of IBD patients are diagnosed at the age of 20 to 40 (Molodecky et al., 2012). According to the European Federation of Crohn’s & Ulcerative Colitis Associations, approximately 3.4 million people are living with IBD in Europe nowadays (EFCCA, 2019). IBD does not only cause a life-long burden to the individual – physically, mentally, and economically – the disease also evokes direct costs to society such as medication, hospitalisation and surgery as well as indirect costs such as lost productivity, premature retirement or premature death (Windsor & Kaplan, 2019). The yearly average direct cost per patient during follow-up, on the health care system in Europe, is estimated by € 3542 for CD and € 2088 for UC patients (Burisch et al., 2020).
Factors influencing the aetiology of inflammatory bowel disease
Many research studies have tried to unravel the aetiology of IBD, however the exact cause remains unclear (Molodecky et al., 2012). Nowadays a complex interplay of three factors – the genetic background, environmental factors and the gut microbiota – is believed to result in a disturbed mucosal innate and adaptive immune response (Torres et al., 2017). As a result of this multifactorial aspect, it is very difficult to fully understand the biological mechanisms behind the development of the disease i.e. the pathogenesis (de Souza & Fiocchi, 2016).
2.1 Impact of the host’s genetic background
In Germany, a nationwide study was performed by Spehlmann and his research group with questionnaires for mono- and dizygotic twins to investigate the concordance rates of CD and UC. In other words, they tried to assess the probability that both twin siblings have IBD when one of the pair was diagnosed with IBD. With these probabilities, useful information was obtained to investigate the heritability of IBD. The results showed that 35 % of the monozygotic twins and 3 % of the dizygotic twins were concordant for CD. Only 16 % of the monozygotic and 2 % of the dizygotic twins were concordant for UC (Spehlmann et al., 2008). A population based cohort study in Denmark from Moller et al. (2015) declared that 12 % of CD patients had a family history in CD (Moller et al., 2015). Similar results were obtained with family and twin concordance studies in Sweden (Halfvarson et al., 2003), Norway (Bengtson et al., 2009), United Kingdom (Thompson et al., 1996), Denmark (Jess et al., 2005) etc. Despite the relatively low concordance rates, all studies are suggesting a contribution of genetics in CD and to a lesser extent in UC.
3 Diverse ethnicities have also been related to differences in risk to develop CD. It has been observed for example that Ashkenazi Jews have a three to four times increased risk of CD in comparison to non-Jewish populations (Ananthakrishnan, 2015). People descending from African-Americans and Asians show the lowest risk (Huang et al., 2015). To better understand the relationship between the aetiology of IBD and the genetics of an individual, several studies focused on the identification of IBD susceptibility genes through linkage mapping (Brant & Shugart, 2004). Only a few genes could be detected with this method, including one of the major susceptibility genes NOD2, also known as CARD15 (Hugot et al., 2001; Hugot et al., 1996). With the development of genome-wide association studies and the collaboration of different research groups to create larger data sets, more than 200 risk loci have been confirmed (Verstockt et al., 2018). In the most recent analysis, 241 IBD susceptibility genes were identified (Perez-Alamino et al., 2016). Interestingly, many of these loci increase the risk for both CD and UC. Furthermore, these studies have linked susceptibility loci of IBD to inflammatory extraintestinal symptoms such as ankylosing spondylitis, non-drug induced osteoporosis and associated autoimmune diseases such as asthma, multiple sclerosis, type 1 diabetes and autoimmune thyroid disease (Perez-Alamino et al., 2016; Verstockt et al., 2018). Nevertheless, the susceptibility genes only explain approximately 20 % of the heritability of Crohn’s disease, which emphasises, together with the low concordance rates, the role of the gut microbiome, the disturbed immune response, the epigenetic and other environmental factors (Baumgart & Sandborn, 2012; Matsuoka & Kanai, 2015).
2.2 Link with the environment
Since the 20th century, the incidence of IBD has been the highest in the western world. These
observations led to the belief that IBD was only affecting those of Caucasian descent in industrialised nations (Windsor & Kaplan, 2019). However, at the beginning of the 21st century, IBD incidence accelerated in the newly industrialised countries of Asia, South America and Africa (Ng et al., 2017). Similarly, in multiple epidemiologic studies, a higher incidence has been noticed in urban regions in comparison to rural regions (Molodecky et al., 2012). Both observations suggest a crucial role of the environment on the pathogenesis of IBD. Individuals living in an industrialised or urbanised environment are exposed to changes in sanitation, microbial communities, diet, occupations, pollution exposure, medication, lifestyle behaviour etc. These changes have been identified as potential environmental risk factors for IBD (Molodecky et al., 2012; Piovani et al., 2019). In addition to risk factors, protective factors have been discovered as well (Table 1).
4
TABLE 1 | Possible risk and protective factors to develop Crohn’s disease or ulcerative colitis. Adjusted from Piovani
et al., (2019)
Crohn’s disease Ulcerative colitis Study
Risk
Factors smoking urban living sucrose vitamin D deficiency oral contraceptive use appendectomy tonsillectomy soft drinks urban living sucrose vitamin D deficiency oral contraceptive use
Mahid et al., 2006; Nie & Zhao, 2017 Soon et al., 2012
L. Zeng et al., 2017; Wang et al., 2017 Del Pinto et al., 2015
Ortizo et al., 2017 Kaplan et al., 2008 Sun et al., 2016
Protective
Factors physical activity bed sharing high vitamin D, A, K & E levels
pets
tea
bed sharing
high folate, vitamin D & A levels
pets smoking
Wang et al., 2016; Nie & Zhao, 2017 Cholapranee & Ananthakrishnan, 2016 Sadeghian et al., 2016; Fabisiak et al., 2017; Lu et al., 2015; Pan et al., 2017 Cholapranee & Ananthakrishnan, 2016 Mahid et al., 2006
2.3 Importance of the gut microbiota
Rutgeerts et al. documented in 1991 that the diversion of a faecal stream – creating a terminal ileostomy to leave out the colon of the intestinal transit – mitigates intestinal inflammation. Additionally, antibiotics are to a certain extent effective as a treatment for IBD (Khan et al., 2011). Several identified IBD susceptibility genes have been related to host responses to the gut microbiota (Liu et al., 2015). The
NOD2 gene for example codes for a pattern recognition receptor (PRR) that distinguishes commensal
bacteria from pathogens in order to promote the correct innate and adaptive immune response (Sidiq et al., 2016). One of the consequences of mutations in the gene, is a heightened immune response due to improper bacterial clearance (Shaw et al., 2011).
Multiple studies have reported differences in the gut bacterial (Walker et al., 2011), fungal (Sokol et al., 2017) and viral (Wagner et al., 2013) communities between patients with IBD and healthy individuals, including reduced diversity (Hansen et al., 2012; Manichanh et al., 2006) and differences in relative abundances (Gophna et al., 2006; Wang et al., 2014). Most consistently reported is the decrease in relative abundance of the phylum Firmicutes, whereas both increases and decreases in relative abundance of the phyla Bacteroidetes and Proteobacteria have been observed (Nishida et al., 2018). The observed disbalance in the gut microbiota, often described as dysbiosis, is associated with a disturbed immune response as well as with an impaired mucosal barrier function, also known as the leaky gut theory (Petersen & Round, 2014). A deficient mucosal barrier gives rise to bacterial translocation from the gut lumen to the lamina propria and eventually to extraintestinal sites, causing local and systemic inflammation. However, it is unclear whether dysbiosis contributes to the onset of the
5 disease or whether it is merely a result of the environmental stress caused by the chronic intestinal inflammation (Ni et al., 2017), which will be further discussed in section 5. Pathogenesis of Crohn’s
disease.
Anatomy and physiology of the intestinal wall
The GIT wall is divided into four layers: the mucosa, the submucosa, the muscularis propria and the serosa. The submucosa and serosa are layers of loose, fibrous connective tissue containing nerves, blood and lymphatic vessels. The muscularis propria consists of 2 layers of smooth muscle fibres that both regulate the peristalsis (Insel et al., 2007). The mucosal layer is composed of a single-cell epithelial layer, a layer of connective tissue (the lamina propria) and a thin double layer of smooth muscle (the muscularis mucosa) that modulates local movement of the mucosa (Stipanuk, 2019).
The mucosal layer of the small intestine and the colon is characterised by transverse folds and crypts of Lieberkühn in which stem cells reside (Schmitt et al., 2018). The mucosa of the small intestine also contains villi and microvilli structures to increase the absorptive surface. The intestinal stem cells continuously proliferate and generate transit-amplifying cells. These cells move upwards out of the intestinal crypt while differentiating into enterocytes, goblet cells or enteroendocrine cells (Yen & Wright, 2006). In the small intestine some stem cells differentiate into Paneth cells that remain in the crypt and help maintaining the stem cell niche through paracrine signalling. Moreover, they regulate the stem cell proliferation and differentiation into the other intestinal cell types and play a role in the innate immunity (Barker et al., 2007). In the colonic crypts, cells expressing CD24+ have been found which display similar functions to Paneth cells and which are located in between the stem cells (Sato et al., 2011).
Paracellular transport through the epithelium is regulated by protein complexes known as tight junctions. Only small molecules, ions and water molecules are allowed to pass by diffusion processes. Tight junctions can be found near the apical side of the intestinal epithelial cells (IECs) (Ulluwishewa et al., 2011). Below these protein complexes adherens junctions, desmosomes and gap junctions are located which play a role in cell-cell adhesion and intracellular signalling. Integrins connect the basolateral membrane of the epithelial cells to the extracellular matrix of the lamina propria (Wells et al., 2017).
From the stomach to the colon, mucins – highly O-glycosylated proteins – are continuously produced by goblet cells, enterocytes and glands which form the major building blocks of the mucus layer adherent to the epithelial surface (Johansson & Hansson, 2016). The mucus layer traps and transports digested food components and bacteria outwards the GIT. By lubricating the digestive content, transport is facilitated and the mechanical stress is lowered (Atuma et al., 2001). In the small intestine and the colon, mucus is primarily composed of the gel-forming mucin type II (MUC2) produced by goblet cells (Wells et al., 2017). The mucus layer of the small intestine is easily removable from the epithelial surface and relatively porous to increase the efficiency of the nutrient uptake. To avoid the penetration of bacteria through the mucus layer, immunoglobulin A (IgA) and antimicrobial peptides (AMPs) such as defensins,
6
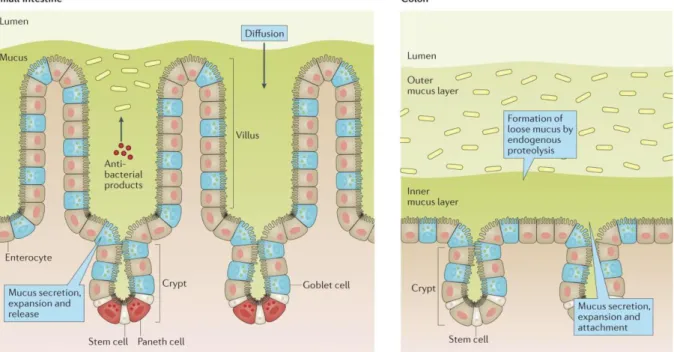
lysozyme and cathelicidines produced by IECs are distributed along the layer (Ermund et al., 2013). The mucus layer of the colon on the contrary is composed of a dense inner and loose outer layer with a lower concentration of IgA and AMPs. The inner layer is firmly attached to the epithelium and is almost impenetrable to bacteria – acting as a physical barrier –, while the outer layer is colonised by a group of mucosal microorganisms that can metabolise and adhere to host-derived mucins (Figure 2) (Tailford et al., 2015). The deeper the microorganisms penetrate into the colonic mucus layers, the more the concentration of AMPs, IgA and oxygen increases (Van den Abbeele et al., 2011). As a result of the proximity to the IECs, the mucosal microbiota plays an important role in regulating the mucosal immune system.
FIGURE 2 | The intestinal epithelial barrier and the production of mucus in the small intestine compared to the colon.
Enterocytes, goblet cells and glands continuously produce and shed mucus which traps bacteria. The mucus layer in the small intestine (left) is diffuse. High loads of antibacterial products: antimicrobial peptides (AMPs) and secreted immunoglobulin A (sIgA) inhibit the growth of bacteria inside the layer. The mucus layer in the colon (right) is compartmentalised and contains less antibacterial products. The loose outer layer provides a stable environment for mucosal bacteria, while the inner dense layer is almost impenetrable (Johansson & Hansson, 2016).
The gastrointestinal tract microbial community
4.1 Composition of the gut microbiota
The human body is colonised by bacteria, archaea, fungi, viruses and some protozoa. A study in 1972 estimated the total number of microbes residing in the human body at 1014, in comparison to solely 1013
human cells (Luckey, 1972). This 10:1 ratio has been subsequently reported in various research articles, although the calculations were only roughly made and therefore unreliable. A more recent study estimated the bacterial/human cell ratio as 1.3 and the amount of human cells in a 70 kg “reference man” as 3.8 x 1013 instead of 1013 (Sender et al., 2016). However, this calculation did not take into
account the eukaryotes, archaea and viruses living in the various body environments (Gilbert et al., 2018).
7 From all the currently identified bacteria of the human gut, 99% belongs to four phyla, namely the
Firmicutes, Bacteroidetes, Proteobacteria and Actinobacteria. In healthy adults, the phyla Firmicutes and Bacteroidetes dominate the gut microbiota (Nishida et al., 2018), although the composition of the gut
microbiome is unique for every individual. Genetics (Goodrich et al., 2014) and environmental factors like diet (Costello et al., 2009; Gilbert et al., 2018), xenobiotics (Maurice et al., 2013) and diurnal rhythm (Thaiss et al., 2014) have been proven to influence the community composition. Over time, the gut community structure of an adult is highly dynamic due to biochemical changes, however the rate of change is individual-specific (Costello et al., 2009; Gilbert et al., 2018). Some sections of the tract contain more bacteria than other parts, related to several factors including the luminal pH, retention time and luminal secretions (Sender et al., 2016). Microbial colonisation in the upper digestive tract is limited. The ileum and colon, on the other hand, are characterised by a long retention time and other more favourable environmental conditions, resulting in a high microbial load. The colon harbours the largest and most complex microbial community of the whole body with a concentration of 1011 bacterial cells/mL (Sender
et al., 2016; Van den Abbeele et al., 2011).
4.2 Beneficial functions of the gut microbiota
In return for the nutrient-rich environment and the residence, gut bacteria contribute to humans through multiple ways. The three main benefits for humans are the provision of energy and nutrients, the maturation and persistent training of the immune system and the protection against pathogens (Nishida et al., 2018).
4.2.1
Supply of nutrients and energy
Some commensal bacteria populating the GIT produce short chain fatty acids (SCFA) through the saccharolytic fermentation of non-digestible carbohydrates and resistant starch. These products are mainly acetate, propionate and butyrate. Lactate can also be produced from the fermentation of non-digestible carbohydrates and can be further metabolised into acetate, propionate and butyrate by a number of cross-feeding bacteria (Morrison & Preston, 2016). Next to saccharolytic fermentation, protein-derived branched chain amino acids can be fermented to generate branched chain fatty acids or acetate and propionate (Russell et al., 2011). Butyrate is primarily used as an energy source for the colonic epithelial cells (Roediger, 1982), while acetate and propionate are mainly absorbed by passive diffusion across the colonic epithelium and used systemically as an energy source (Morowitz et al., 2011). Beside SCFA production, the commensal bacteria of the Bacteroides, Bifidobacterium and
Enterococcus genera are known to synthesize complex vitamins such as the vitamin B and K family
8
4.2.2
Training and maturation of the immune system
The human body learns the difference between commensal and pathogenic bacteria through the sampling of microbial macromolecules from the gut lumen. This sampling process is executed by different antigen-sampling cells which pass on the microbial antigens to dendritic cells (DCs) or macrophages distributed along the lamina propria or in specific lymphoid tissue. Microfold (M) cells are highly specialised antigen-sampling cells which transcytose luminal macromolecules across the epithelium into lymphoid structures. These M cells are located at the intestinal epithelium overlying the gut-associated lymphoid tissue, which includes Peyer’s patches in the small intestine, isolated lymphoid follicles and mesenteric lymph nodes (Mabbott et al., 2013). Goblet cells are another example of antigen-sampling cells that allow the passage of low-molecular-weight molecules through the epithelial layer (Buffie & Pamer, 2013; Ley et al., 2006). At last, macrophages and DCs are able to directly sample gut microbial antigens with long transepithelial dendrites (Figure 3) (Farache et al., 2013; Maloy & Powrie, 2011).
FIGURE 3 | Intestinal sampling procedures through the epithelial layer with its different cell types in the small
intestine (left), above lymphoid follicles (middle) and in the colon (right). Dendritic cells (DCs) and macrophages sample macromolecules from the gut with transepithelial dendrites. Microfold (M) cells transcytose gut luminal particles into the gut-associated lymphoid tissue and goblet cells allow diffusion of low-molecular-weight molecules into the lamina propria to DCs and macrophages. IEC: intestinal epithelial cell; IESC: intestinal epithelial stem cell; AMP: antimicrobial peptide; sIgA: secreted immunoglobulin A (Peterson & Artis, 2014).
Mucosal DCs, macrophages and IECs express pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) embedded in the cell and endosome membrane and NOD-like receptors (NLRs) in the cytosol (Chu & Mazmanian, 2013). These receptors recognise conserved structures of microorganisms or microbe-associated molecular patterns (MAMPs) e.g. peptidoglycan and capsular polysaccharides
9 which induce the expression of multiple genes leading to the production of anti- or pro-inflammatory cytokines, type I interferons, AMPs and regulators of the host metabolism and tissue regeneration (Lazar et al., 2018). Upon stimulation of TLRs, naive CD4+ T cells resident in the lamina propria can either differentiate into regulatory T cells (Treg cells) – promoting tolerance – or into T helper cells (TH1, TH2 and
TH17) which induce an inflammation cascade. During steady state, Treg cells suppress the activity of the
effector T cells (Torres et al., 2017). There are less TLRs located on the apical (luminal) side of the IECs in comparison to the basolateral side. TLRs on the basolateral side mainly promote pro-inflammatory responses to protect the host from invading microorganisms, whereas prolonged or repeated exposure of ligands from gut bacteria prime the low amount of TLRs on the apical side towards transient tolerance responses (Chistiakov et al., 2015). Nevertheless, host cells are able to re-induce the expression of pro-inflammatory factors in response to these ligands over time. The exposure of a newborn to diverse microbial species induces the maturation of the mucosal innate and adaptive immune system (Torow & Hornef, 2017). Studies have demonstrated that the mucosal immune system is gravely underdeveloped in germfree mice. They develop multiple deficiencies including an impaired T-cell maturation (Chung et al., 2012) and a dysfunction of regulatory immune cells (Mishima et al., 2019). Hence, a stable gut community is necessary to maintain a homeostatic host/commensal relationship (Butcher et al., 2018).
4.2.3
Protection against pathogens
To survive in the gut, microbes have to contend with several conditions. For example, they need the right enzymes for the available nutrients, the ability to grow rapidly to avoid washout, contain enough genetic flexibility to mutate and stay well adapted, survive the physical and chemical stresses etc. (Ley et al., 2006; Sekirov et al., 2010). The microbial diversity is influenced by two selective pressures. The
top down selection on the microbiota supports stable communities with a high degree of functional
redundancy. This means that there are diverse species present in the gut who can execute the same function. The circumstances define which species dominate at a given time point. On the other hand, the bottom up pressure stimulates the microbes to become more and more specialised into one specific functional niche. Due to this force, microbial cells will compete with each other to occupy a functional niche. Because of the prevalent competition among the microbes and the high occupation of all niches and habitats, pathogens have fewer chances to settle in the gut and become virulent, a mechanism which is better known as colonisation resistance (Buffie & Pamer, 2013; Ley et al., 2006).
Some gut microbes directly inhibit the colonisation of pathogens through the production of antimicrobial compounds (Nishida et al., 2018). Bacillus thuringiensis for example produces a bacteriocin that targets the pathogen Clostridium difficile and other spore-forming Clostridia and Bacilli (Huang et al., 2016). Other commensal bacteria and their microbial products protect the host against pathogens indirectly by inducing immune responses. Bacteroides fragilis produces polysaccharide A which induces the secretion of IL-12 by DCs leading to the differentiation of naive CD4+ T cells into TH1 cells (Mazmanian
10
microbes, are known to promote the production of IgA by B cells as well as the secretion of AMPs and the differentiation of naive CD4+ T cells into TH17 cells (Ivanov et al., 2009; Talham et al., 1999).
Pathogenesis of Crohn’s disease
Individuals with CD do no longer promote tolerance responses against commensal gut bacteria. The balance between Treg cells and effector T cells is disturbed. Local inflammation is induced through TH1
and TH17 mediated responses which overrule the activity of the Treg cells (Baumgart & Sandborn, 2012).
Upon TLR signalling, IL-23 and IL-12 are produced by DCs and macrophages to stimulate the differentiation of naïve CD4+ T cells into TH17 and TH1 cells (Neurath, 2019). TH17 cells secrete cytokines
such as IL-17, IL-21 and IL-22 which trigger the production of pro-inflammatory cytokines like IL-6, TNFα and IL-1β (Neurath, 2014). Moreover, IL-23 induces memory T cells and neutrophils to secrete similar pro-inflammatory cytokines (Neurath, 2019). TH1 cells mainly secrete the pro-inflammatory cytokine
IFN-γ (Damsker et al., 2010). The exact biological mechanism responsible for this shift in T cell balance remains unknown. In the following paragraphs, three hypotheses are discussed which possibly contribute to the pathogenesis of the disease.
5.1 Intestinal dysbiosis
Reduced gut community diversity has been observed in mucosal biopsies (Tong et al., 2013) and in stool samples (Manichanh et al., 2006) from CD patients in comparison to healthy individuals. The most consistent observation is the decreased abundance of members belonging to the Firmicutes (Sheehan et al., 2015). Some studies analysing mucosal biopsies of CD patients have also reported increased relative abundances of Bacteroidetes and Proteobacteria (Gophna et al., 2006; Walker et al., 2011), especially Enterobacteriaceae (Baumgart et al., 2007). In a study from Png et al. (2010), the relative abundances of mucolytic bacteria like Ruminococcus gnavus and Ruminococcus torques were observed to be raised in mucosal biopsies of CD patients. The authors suggest that the enrichment of mucolytic bacteria could lead to an enhanced degradation of MUC2 and hence more mucin metabolites, resulting in an overgrowth of cross-feeding bacteria in the mucus layer (Png et al., 2010).
Reduction in relative abundance of members belonging to the Clostridium clusters IV (e.g.
Faecalibacterium prausnitzii), XIVa and XVII, known for their SCFA production, are also numerously
described (Halfvarson et al., 2017; Takahashi et al., 2016,; Wang et al., 2014). SCFAs promote the
mucosal barrier function. Butyrate and propionate are known to stimulate the intestinal MUC2 expression of goblet cells (Burger-van Paassen et al., 2009). In addition, butyrate enhances the tight junction assembly (Peng et al., 2009) and fuels the colonic IECs (Roediger, 1982). Furthermore, butyrate works anti-inflammatory through the suppression of the pro-inflammatory transcription factor nuclear factor kappa B (NF-κB) (Segain et al., 2000) and the stimulation of a Treg response (Atarashi et al., 2013). A
multi-omics study of Lloyd-Price et al. (2019) reported a depletion of SCFA in CD stool samples in comparison to healthy stool samples consistent with the observed reduction of SCFA producers. In
11 general, they observed an increase of facultative anaerobes like the Enterobacteriaceae and a decrease in obligate anaerobes (including the SCFA producer). As inflammation is an oxidative state, higher oxygen levels are observed in the gut lumen due to elevated blood flow and presence of haemoglobin. Moreover, an increase in reactive oxygen species creates a hypoxic environment in the mucosa (M. Y. Zeng et al., 2017). These environmental changes give respiratory flexible bacteria such as the
Enterobacteriaceae a fitness advantage over the obligate anaerobes. This would implicate that dysbiosis
is not a direct cause of CD, but rather a consequence of the chronic inflammation (Ni et al., 2017).
The decrease of beneficial commensals and the increase of pathobionts – opportunistic commensal bacteria e.g. E. coli – enhances the risk of pathogen or pathobiont overgrowth and invasion. These can damage the mucosal barrier and cause an inflammation response. There is no strong evidence that CD is caused by a single pathogen or pathobiont. A relative increase of the pathobiont adherent invasive
E.coli (AIEC) has been documented several times in CD patients (especially in ileal CD)
(Martinez-Medina et al., 2009), however a direct causal relationship has never been proven (Palmela et al., 2018). Therefore it is more probable that a pathogen or a pathobiont acts in concert with an unbalanced gut microbiota to evoke the disease state (Ni et al., 2017).
The obtained results in the gut microbial community studies are sometimes heterogeneous. The discrepancies can be partly explained by an inconsistency in experimental design and interindividual variability. The sampling method (biopsies versus faecal) as well as the sample location in the GIT, the disease activity and the analysation technique have an important impact on the obtained results (Matsuoka & Kanai, 2015). Considering the importance of the mucosal microbiota in the pathogenesis of CD, mucosal biopsies are probably more interesting to analyse.
5.2 The leaky gut hypothesis
During the active phase of CD, increased intestinal permeability has been regularly observed in patients (Benjamin et al., 2008; Hollander et al., 1986). Moreover, when the enhanced gut permeability is noticed during remission, it is a reliable predictor of relapse (Wyatt et al., 1993). A mucosal barrier defect is also observed in up to 10 % of healthy first-degree relatives, suggesting that the defect could precede the onset of the disease and that the permeability is influenced by genetical as well as environmental factors (Hollander et al., 1986). It has been reported in CD patients that the expression and redistribution of the tight junction proteins between IECs, claudin 5 and claudin 8, is diminished. Furthermore, the expression of claudin 2, a pore-forming tight junction protein, was upregulated (Zeissig et al., 2007). The pro-inflammatory cytokines TNFα (Mankertz et al., 2009) and IL-6 (Suzuki et al., 2011) are identified as contributors of the promoted expression of claudin 2. A reduction of SCFA-producers, as mentioned in paragraph 5.1, contributes as well to a loss of intestinal barrier function. Next to the increased epithelial layer permeability, a decrease in β-defensins (Wehkamp et al., 2003) and a reduction in the mucus layer thickness (Fyderek et al., 2009) was observed in inflamed biopsies from CD patients compared to healthy
12
gut tissues. The enrichment of mucolytic bacteria could be correlated to the deterioration of the intestinal mucus layer.
Enhanced penetration of microorganisms, food antigens and other noxious particles from the gut lumen into the lamina propria can provoke local inflammation responses. Moreover, increased paracellular transport of solutes and water diffused from the blood stream to the intestinal lumen can cause leak-flux diarrhoea (de Souza & Fiocchi, 2016). Since healthy individuals can also demonstrate a weakened mucosal barrier function (Hollander et al., 1986), a leaky gut probably needs external triggers to develop into CD or UC.
5.3 Disrupted bacterial clearance
It has been noticed in CD patients that intestinal macrophages display an aberrant cytokine production (Na et al., 2019). The secretion of chemokines like IL-8 is reduced, which delays the attraction of neutrophils to clear invaded bacteria or debris of damaged tissue (Smith et al., 2009). The impaired bacterial and cellular clearance can stimulate local inflammation and cause accumulation of T cells and macrophages, resulting in granuloma formation (Segal, 2019). On the other hand, CD macrophages produce more pro-inflammatory cytokines than macrophages of healthy individuals, which could assist the excessive inflammation response (Kamada et al., 2008).
Mutations in the genes ATG16L1 and IRGM lead to an impaired autophagy process, which is a process responsible for the degradation and recycling of cellular components, therefore maintaining cellular homeostasis (Hoefkens et al., 2013). The pathway also plays an important role in the mucosal immune system by clearing intracellular bacteria. Defects in the autophagy process could lead to the accumulation of malfunctioning host cells, cellular components and intracellular pathogens which evoke an inflammatory response (Kabat et al., 2016). Mutations in the NOD2 gene could as well lead to enhanced inflammation through an impaired bacterial and cellular clearance. The gene is responsible for the NLR recognition of bacteria and the induction of autophagy (Shaw et al., 2011).
Current therapeutic treatments of Crohn’s disease
Since no cure has been found yet for CD, current treatments focus on obtaining and maintaining deep remission, which means that the clinical symptoms as well as the signs of tissue inflammation should be strongly diminished upon treatment (Siegel et al., 2018). With multiple treatment strategies available, the choice of strategy is based on the severity of the disease as well as the response to previous therapies (Torres et al., 2017).
Aminosalicylates are a first type of drugs belonging to the classic anti-inflammatory drug class. The active compound 5-aminosalicylic acid inhibits the inflammation response through several pathways including the suppression of pro-inflammation mediators such as TNFα and IL-1, the neutralisation of
13 free radicals and oxidants and the inhibition of leukocyte chemotaxis (Curkovic et al., 2013). One of the greatest challenges of this drug type is the delivery to the appropriate site along the GIT to improve its efficacy (Pithadia & Jain, 2011). While aminosalicylates have been proven to be effective in patients with mild or moderately active UC to induce and maintain remission, the efficacy in CD patients is only moderate (especially in small intestine CD) (Gisbert et al., 2011).
The second type of the classic anti-inflammatory drugs are the corticosteroids. These drugs prevent the release of prostaglandins and leukotrienes (lipid inflammatory mediators) and downregulate the transcription factors NF-κB and activator protein-1 (AP-1) (Ramamoorthy & Cidlowski, 2016). Long-term use of corticosteroids can lead to several severe adverse effects: osteonecrosis or osteoporosis, hyperglycaemia, glaucoma or cataract and increased susceptibility towards fungal, viral and bacterial infections (Curkovic et al., 2013). Some novel drug formulations (e.g. budesonide) show an increased specificity for the glucocorticoid receptor which diminishes the adverse effects (Silverman & Otley, 2011). However, a considerable amount of CD patients show either no responsiveness towards the drugs or they experience a relapse of symptoms after lowering the dose. Therefore, aminosalicylates are often prescribed in combination to maintain remission (Steinhart et al., 2003). Because of the lack of responsiveness and the side effects, other therapeutic treatments are more often prescribed than this drug class (Pithadia & Jain, 2011).
A second class of drugs are the classic immunosuppressants including methotrexate and the thiopurines, azathioprine and 6-mercaptopurine. Methotrexate inhibits the proliferation and induces apoptosis of immune cells, while thiopurines suppress the activation of RAC1, a small signalling protein that plays a role in various pathways of the innate and adaptive immune system (Neurath, 2017). Both drug types are effective for inducing and maintaining remission in CD patients. Despite significant adverse effects (including increased infection risk, nausea and vomiting) immunosuppressants are still better tolerated than long-term corticosteroid therapy. However, several studies have observed an elevated risk of malignancies – lymphomas (Beaugerie et al., 2009), non-melanoma skin cancers (Peyrin-Biroulet et al., 2011) and urinary tract cancers (Bourrier et al., 2016) – after immunosuppressant therapy.
Since the late 1990s, humanised monoclonal antibodies or biologicals entered the market that target the cytokine TNFα. In the treatment of moderate to severe CD, anti-TNF agents have proven to induce and uphold remission (Colombel et al., 2010). The first biological, infliximab, contains proteins derived from mice. As a consequence, acute immunogenicity reactions (fever, chills, hives) and subacute (serum sickness-like) reactions have occurred after administration (Pithadia & Jain, 2011). Additionally, the production of antibodies against the drug results in a loss of response over time (Ben-Horin et al., 2013). With the development of fully humanised antibodies (e.g. adalimumab), these problems were reduced (Panaccione et al., 2010). However side effects such as skin reactions, sinusitis, nausea, upper respiratory infections or symptoms of nervous system inflammation still manifest (Pithadia & Jain, 2011). Approximately 20 % of CD patients show no responsiveness towards this biological therapy and the
14
efficacy over time is known to decrease in some patients (Wong & Cross, 2017). To increase the effectiveness of the monoclonal antibodies, biological therapy is often combined with immunosuppressants (Colombel et al., 2010).
The most recent therapeutic strategies have been focusing on monoclonal antibodies targeting other biochemical pathways than blocking TNFα. Natalizumab and vedolizumab for example are humanised antibodies that bind specific integrins expressed on the surface of B and T cells to interfere with leukocyte homing. Nevertheless, only vedolizumab is approved as a drug for CD in Europe, because of the safety issues with natalizumab (Colombel et al., 2017; Pagnini et al., 2017). Etrolizumab is also a monoclonal antibody with a similar mode of action which holds promising results, but the clinical trials are still ongoing (Pagnini et al., 2019; Vermeire et al., 2014). Next to inhibiting leukocyte homing, novel drugs have been developed that target other pro-inflammatory cytokines aside from TNFα. For example, ustekinumab binds to the p40 subunit of IL-12 and IL-23 and prevents the interaction between natural killer cells and T cells. This antibody is also already market approved (Barré et al., 2018). At last, antibodies have been developed that target the downstream activity of cytokine signalling pathways e.g. the JAK-STAT pathway. Most of them still need to undergo several clinical trials before approval is granted (Pagnini et al., 2019).
Although promising improvements are made with pharmaceutical drugs, the poor response rate, high cost and adverse effects have been stimulating research towards alternative approaches (Singh et al., 2012). The increasing evidence of the involvement of the gut microbiota into the pathogenesis of CD has raised the interest in the last decades in using probiotics, prebiotics or synbiotics – a combination of the two – to restore the gut microbial balance and attenuate the intestinal mucosal inflammation (Abraham & Quigley, 2017). In case of UC, many studies obtain positive results while proving efficacy (Tamaki et al., 2016; Tursi et al., 2010). The results for CD, however, are less clear. A small study with a synbiotic – Bifidobacterium longum combined with a prebiotic – improved the clinical symptoms of CD, but it did not significantly attenuate mucosal inflammation on the long-term (Steed et al., 2010). The administration of VSL#3 – a combination of 4 strains of Lactobacillus, 3 strains of Bifidobacterium and 1 strain of Streptococcus salivarius subspecies thermophilus – did not prevent endoscopic recurrence after a surgical resection in comparison to a placebo group. Although a decrease in mucosal inflammatory cytokines was observed among patients who received VSL#3 for 365 days (Fedorak et al., 2015). One of the first generation probiotics Lactobacillus rhamnosus GG was not able to significantly induce or maintain remission in CD patients (Schultz et al., 2004). The controversial results can be partly explained by the interindividual variability among people, the small sample sizes of the trials, differences in pro- and prebiotics, time and type of administration etc. The number of trials is limited, which makes it hard to derive a conclusion (Dore et al., 2019). Furthermore, the survival of the administered bacteria could be restricted by the hostile inflammation environment (Bellaguarda & Chang, 2015).
15
In vitro
models to study host-microbe interactions
Despite the numerous scientific indications of the involvement of the host’s gut microbiota into the pathogenesis of IBD, evidence of causality remains sparse. A better understanding of the exact mechanisms behind the host-microbial crosstalk could lead to more effective therapeutic strategies. In
vivo human trials are biased by confounding factors such as interindividual variability and the
environment, which makes it hard to investigate underlying mechanistic processes of host-microbe interactions (Benson et al., 2010). In animal models, these parameters can be controlled; nevertheless the models are ethically constrained, expensive and translation to humans is limited by physiological differences (Jiminez et al., 2015). Consequently, in vitro models, which are relatively cheap, highly reproducible and limited in ethical constraints, are useful alternatives to give insight into the host-microbe interactions (von Martels et al., 2017).
Different in vitro models have been developed that mimic situations in the GIT as closely as possible. Each system has its limitations and the choice of model should be made according to the research question. To investigate the host-microbe interface interactions in the context of CD, cell culture models simulating the mucosal environment and the intestinal epithelium are the most relevant. These models use either primary cell lines, which are cells directly derived from human or animal tissue, secondary cell lines – subcultures of primary cell lines –, immortalised cell lines or stem cells (Costa & Ahluwalia, 2019). The most straightforward models only use one cell type – primarily the immortalised epithelial cell lines Caco-2, T84 or HT-29 – adhered to a flat, artificial plastic or glass surface (Antoni et al., 2015). However, the use of only one cell type oversimplifies the in vivo situation. Co-culture models using different cell types allow cell-cell communication and provide a more accurate representation of the intestinal epithelium (Hilgendorf et al., 2000). Intestinal in vitro co-cultures mainly consist of epithelial cells combined with mucus-producing cells (Chen et al., 2015; Nollevaux et al., 2006), fibroblasts (Koh et al., 2019) and/or immune cells (Kämpfer et al., 2017; Leonard et al., 2010; Noel et al., 2017). Furthermore, the simple 2D monoculture model does not take into account the in vivo extracellular matrix (ECM) and its interaction with the cells. Cells cultured in a 3D conformation demonstrate more in vivo-like physiological responses, creating their own ECM for example, in comparison to cells grown in monolayers (Kim et al., 2014; Yi et al., 2017). Some methods to obtain a 3D structure are the use of scaffolds (e.g. silk fibroin, synthetic polymers) (Bhang et al., 2007; Mandal & Kundu, 2009), hydrogels (e.g. collagen, gelatine) (Dosh et al., 2019; Ogawa et al., 2010) or bioreactors (e.g. rotating wall vessel to create spheroids/organoids) (Massai et al., 2016).
One of the golden standard tools for in vitro models simulating the intestinal epithelium is the use of Transwell® filter inserts. Transwell® inserts create two compartments, an apical and a basolateral one,
through separation by a microporous filter (Altay et al., 2019). The pore size and the membrane polymer can be varied according to the experimental requirements (Tan et al., 2019). Direct and indirect