Ghent University – Department of Plant Biotechnology and Bioinformatics
VIB – Center for Plant Systems Biology Research Group: Root Development
Assessing the function of MAKR4
during
main
and
lateral
root
development
Sam Vereecken
Student number: 01507164
Promoter: Prof. Dr. Tom Beeckman
Scientific supervisors: Dr. Ana I. Fernandez, Msc. Ke Xu, Dr. Barbara K. Möller Master’s dissertation submitted to Ghent University to obtain the degree of Master of Science in Biochemistry and Biotechnology. Major Plant Biotechnology
Acknowledgements
This thesis project would not have been possible without my scientific supervisors. I would like to thank Barbara for introducing me in the roots world, her guidance in the literature, the design of the experiments and constructs and her daily guidance in the lab. I would like to thank Ana and Ke, for taking the job to guide my thesis during the second semester. In particular, I would like to thank Ana for her help with the peptide treatment experiments, the discussions about the results and all the time she wanted to spend on reviewing the report, and I would like to thank Ke for his daily guidance in the lab and the support he provided. I want to take this opportunity to thank my parents, who had to pay a lot of patience during the weeks of the writing process, but also in exam periods. I owe them a lot because they made it possible to start my studies and offer me the chance to shape my own life.
Table of contents
Acknowledgements ... i
Table of contents ...ii
List of figures ... iii
List of abbreviations ... iv
Preambule ... v
Summary ... vi
Introduction ... 1
1. The Arabidopsis root system ... 1
1.1. General introduction ... 1
1.2. Anatomical structure and function ... 1
2. Lateral root formation ... 4
2.1. Morphogenesis: from nuclear migration until emergence ... 4
2.2. The function of auxin in lateral root development ... 5
2.2.1. Auxin transport and signalling ... 5
2.2.2. Auxin signalling reporters ... 6
2.2.3. Priming of lateral root founder cells in the basal meristem ... 6
2.2.3.1. Recurrent auxin signalling in the basal meristem and oscillatory gene expression in the oscillation zone correlate with LRI ... 6
2.2.3.2. Role of IBA-derived IAA released during periodic programmed cell death from the lateral root cap ... 8
2.2.4. Lateral root initiation ... 9
2.2.5. Lateral root emergence ... 10
3. The MAKR family ... 12
3.1. MAKR4 may be involved in prebranch site formation, transition from prebranch site to lateral root initiation and early stage LRP development ... 12
3.2. BKI1, MAKR2 and MAKR5 ... 13
4. Peptide signalling in root development ... 15
4.1. Peptide hormone classification and ligand-receptor signalling ... 15
4.2. The role of peptides in primary and/or lateral root development ... 15
4.2.1 RGF/GLV/CLEL peptides... 16 4.2.2 CLE40/45 peptides ... 17 4.2.3 IDA peptide ... 17 4.2.4 TOLS2/PIPL3 peptide ... 17 4.2.5 CEP5 peptide ... 18 Aim ... 20
Results ... 21
1. Assessing the function of the different MAKR4 motifs ... 21
2. Assessing the role of MAKR4 phosphorylation sites ... 24
3. Assessing the role of MAKR4 in different subcellular localisations ... 29
4. Assessing the function of MAKR4 in different tissue layers ... 32
5. Elucidating the signalling pathway where MAKR4 is in involved ... 34
Discussion ... 42
Materials and methods ... 44
References ... 47
Addendum ... 51
List of figures
Figure 1: The root system of the dicotyledonous model plant Arabidopsis thaliana. Figure 2: The role of MAKR4 in LR development.
Figure 3: The role of peptide signalling in root development.
Figure 4: The MAKR family and a detailed overview of the MAKR4 protein.
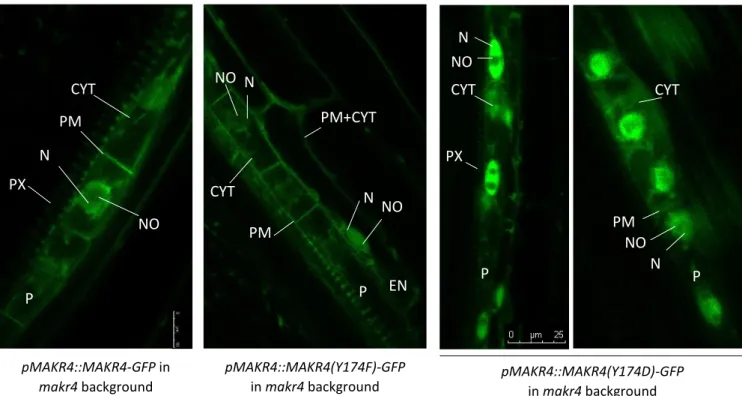
Figure 5: The subcellular localisation pattern of MAKR4-GFP and MAKR4(Y174)-GFP phosphomutants expressed in the makr4 mutant. (Barbara Möller)
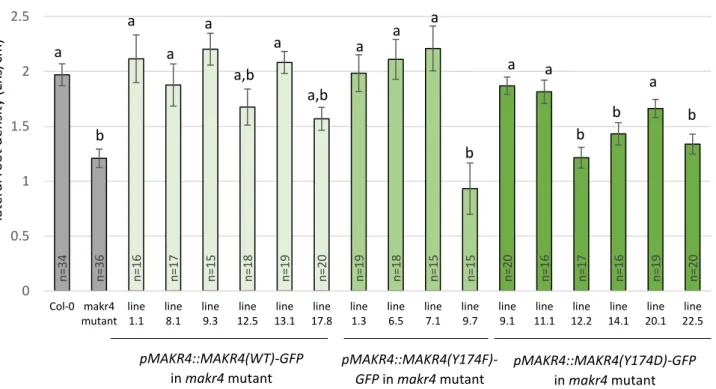
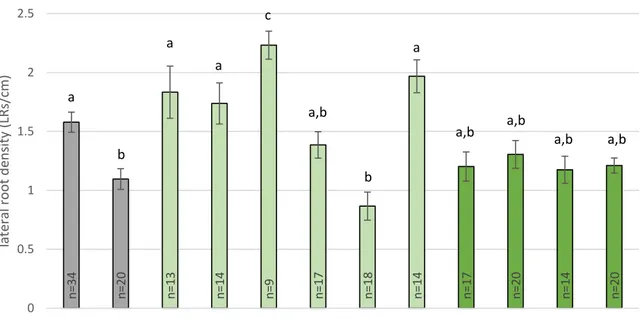
Figure 6: LR density of wild-type MAKR4-GFP and MAKR4(Y174)-GFP phosphomutants expressed in the makr4 mutant.
Figure 7: The subcellular localisation pattern of MAKR4(S235/S237)-GFP phosphomutants expressed in the makr4 mutant.
Figure 8: The subcellular localisation of MAKR4-GFP with a loss-of-function mutation in one of the two putative bipartite nuclear localisation signals (NLS).
Figure 9: The LR density of tissue specific promoter driven MAKR4-GFP expression in the
makr4 mutant background.
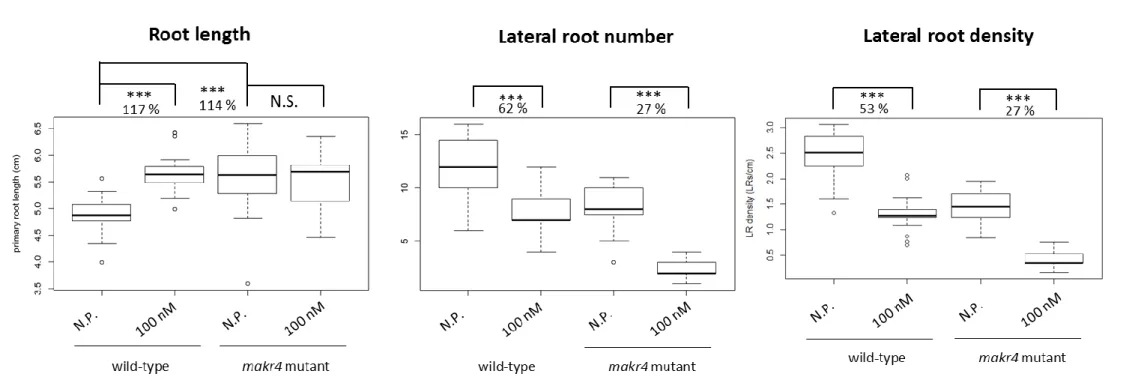
Figure 10: Effect of synthetic GLV10p peptide treatment on primary root length, LR number and LR density of wild-type Col-0 and makr4 mutant seedlings.
Figure 11: effect of synthetic CLE40p peptide treatment on primary root length, LR number and LR density of wild-type Col-0 and makr4 mutant seedlings.
Figure 12: Effect of synthetic CLE45p peptide treatment on primary root length, LR number and LR density of wild-type Col-0 and makr4 mutant seedlings.
Figure 13: Effect of synthetic IDAp peptide treatment on primary root length, LR number and LR density of wild-type Col-0 and makr4 mutant seedlings.
Figure 14: Effect of synthetic TOLS2p peptide treatment on primary root length, LR number and LR density of wild-type Col-0 and makr4 mutant seedlings.
List of abbreviations
o Arabidopsis: Arabidopsis thaliana o BKI1: BRI1 KINASE INHIBITOR 1 o BM: basal meristem
o BRI1: BRASSINOSTEROID INSENSITIVE 1 o CEP: C-TERMINALLY ENCODED PEPTIDE
o CLE: CLAVATA 3/ EMBRYO SURROUNDING REGION-RELATED
o CRISPR/Cas9: clustered regulatory interspaced short palindromic repeats/ CRISPR ASSOCIATED NUCLEASE 9
o dPCD: developmental programmed cell death o DR5: DIRECT REPEAT 5
o GATA23: GATA TRANSCRIPTION FACTOR 23 o GFP: GREEN FLUORESCENT PROTEIN
o GLV/RGF/CLEL: GOLVEN/ROOT GROWTH FACTOR/CLE-LIKE o GUS: BETA GLUCURONIDASE
o IAA: indole-3-acetic acid o IBA: indole-3-butyric acid
o IDA: INFLORESCENCE DEFICIENT IN ABSCISSION o LBD16: LATERAL ORGAN BOUNDARIES DOMAIN 16 o LR: lateral root
o LRI: lateral root (primordium) initiation o LRP: lateral root primordium
o LUC: LUCIFERASE
o MAKR4: MEMBRANE-ASSOCIATED KINASE REGULATOR 4 o makr4: CRISPR/Cas9-generated makr4 knock-out mutant o NES: nuclear exclusion signal
o NLS: nuclear localisation signal o OZ: oscillation zone
o PPP: phloem pole pericycle o PR: primary root
o QC: quiescent centre
o TOLS2: TARGET OF LBD SIXTEEN 2 o XPP: xylem pole pericycle
Preambule
Before the start of the covid-19 crisis, the thesis project had two aims: first, studying the function of MAKR4 in lateral root development and second, studying the role of GATA transcription factors during lateral root development. The study on the MAKR4 protein consisted of five subdivisions: assessing the role of motifs, phosphorylation sites, determining the function of MAKR4 in different subcellular localisations and in different tissues during lateral root development and investigate if MAKR4 is involved in peptide hormone signalling during lateral root development. Except for the last subdivision, we generated expression clones and together with expression clones that were generated before the master thesis, we transformed them in Arabidopsis in the first semester and we planned to analyse them in the first and second semester (confocal microscopy, lateral root density phenotyping). Most constructs could not be analysed in the second semester. A few constructs were analysed in the first semester. Some of the constructs that we could analyse in the first semester were already transformed in Arabidopsis before the thesis project and this material was provided by the supervisor. The GATA part of the thesis was halted at the start of the covid-19 crisis. The genotyping of a double gata mutant could not be pursued due to the current situation. We planned to phenotype the double gata mutant and depending on this result, possibly also doing further analysis.
Eventually, the thesis project is based on material that was analysed in the first semester and on the peptide treatment experiments that were performed during the second semester.
Summary
MAKR4 is suggested to be involved in multiple steps of lateral root development. It is a homologue of BKI1, which is an inhibitor of the BRI1 receptor of brassinosteroids. In this work, we analysed the role of phosphorylation in MAKR4 functioning during lateral root development. We have evidence that phosphorylation of residue Y174 in MAKR4 would result in its dissociation from the plasma membrane, indicating that the function of this residue is conserved between MAKR4 and BKI1. We propose that multiple phosphorylation sites act redundantly with Y174 during MAKR4 dissociation from the plasma membrane. Next, we also investigated in which signalling pathway MAKR4 may be involved during lateral root development. Based on the results of other MAKR proteins, MAKR4 may function at the level of or downstream of a receptor-like kinase on the plasma membrane. We tested a number of peptide hormones that coordinate diverse processes during main and lateral root development. We found that MAKR4 may be involved in the signalling pathway of GLV10, CLE45, IDA and TOLS2 peptide hormones. Further research will be useful to assess the link between these peptide hormones and the function of MAKR4 during lateral root and possibly also main root development. We also investigate the function of MAKR4 in different subcellular localisations since MAKR4-GFP was previously found to be localised on the plasma membrane, in the cytoplasm and in the nucleus. Previous analysis suggested that MAKR4 has probably no function in the nucleus. To confirm this result, we aimed to determine how MAKR4 is imported in the nucleus in order to disturb nuclear import. We found that MAKR4-GFP is likely not imported in the nucleus depending on an NLS signal. We propose that MAKR4 interacts with a protein containing an NLS that targets the protein complex to the nucleus. Further research will be useful to investigate this hypothesis. We also analysed the function of MAKR4 in different tissues during lateral root development because it was previously found that MAKR4-GFP is expressed in the pericycle and the lateral root primordium overlaying tissue layers. Pericycle-specific MAKR4-GFP expression could restore the decreased LR density of makr4 mutants to a wild-type level, indicating that MAKR4 has a main role in the pericycle during lateral root development.
Introduction
1. The Arabidopsis root system
1.1. General introduction
Roots enable plants to take up nutrients such as water and minerals to sustain life. Roots appeared during evolution independently in two clades of land plants: the lycophytes, which are closely related to ferns, and the common ancestor of the seed plants, which gave rise later to the Gymnosperms and Angiosperms. In the Angiosperms (a.k.a. flowering plants), two main root systems are present: the fibrous root system and the taproot system. The fibrous root system is predominant in monocotyledonous plants. Here, the embryonic root (radicle) is only important in the early stages of seedling development and rapidly degenerates. The growing plant mainly forms adventitious roots. The taproot system is typical for dicotyledonous plants and consists of a primary root a.k.a. taproot/main root (hereafter referred to as PR) from which smaller, lateral roots branch (hereafter referred to as LRs). LRs and adventitious roots have a different type of origin: LRs emerge from roots while adventitious roots arise from aerial parts of the plant such as the base of the stem. In dicotyledons, the root system of the model plant Arabidopsis thaliana (hereafter referred to as Arabidopsis) is studied (figure 1A). In animals, the architecture of the body is almost completely determined during the embryonic development. In contrast, plants have a plastic pattern of growth and development which may be linked to their sessile lifestyle: they need to be well-adapted to their environment to increase the likelihood to survive (e.g. availability of water, light, nutrients). During plant embryogenesis, an embryonic root and shoot are formed. Although the start point of architecture is the same for every plant (of the same species), the end point is never identical: during their entire life, plants continue to develop their root and shoot system. This post-embryonic development will take place under continuous interactions with the environment.
Identifying the mechanisms of root branching is important for targeted breeding in agriculture. For example, the root system of crops could be adapted to soil conditions. Also regarding the climate change, improved crop varieties could be developed to withstand extreme weather conditions such as drought. Important agricultural crops with a taproot system are e.g. sunflower, sugar beet and legumes but also broad-leaved trees. Fibrous root systems are present in cereal crops like maize, wheat and rice.
1.2. Anatomical structure and function
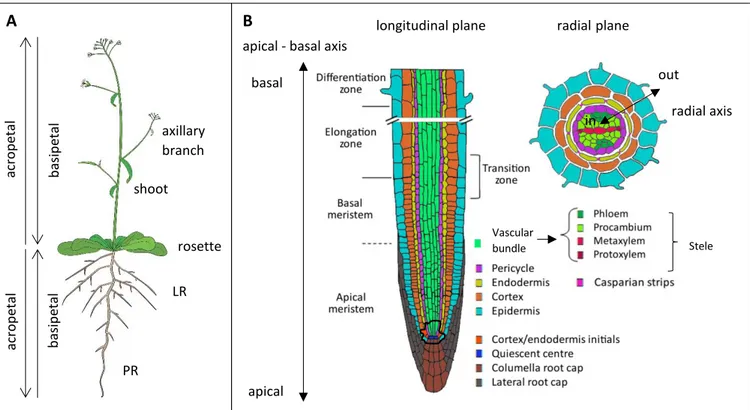
The Arabidopsis root has two main body axes: the apical-basal axis (a.k.a. the longitudinal or proximal-distal axis) and the radial axis (figure 1B). The apical-basal axis follows the longitudinal plane of the root/shoot and has the tip of the root/shoot at the apical end and the root-shoot junction at the basal end. The radial axis lies in the transverse plane of the root and has an outer and inner pole (figure 1B).
Both the PR and LR have an identical patterning of tissue layers. The radial arrangement of tissues in the PR and LR of Arabidopsis is simple: the four concentric layers, each one cell layer thick are, from the outside to the inside, the epidermis, cortex, endodermis and pericycle (figure 1B). The pericycle is the outer layer of the central cylinder/stele and includes the vascular bundles and the surrounding pericycle (figure 1B). The function of the vascular bundle is to transport molecules from root to shoot and vice versa. This traffic of molecules is
organized in separated conducting tissues. First, the xylem tissue is responsible for transporting molecules such as water and mineral nutrients from the root to the shoot. Second, the phloem tissue moves shoot-derived nutrients such as products from photosynthesis (e.g. sucrose) to the root. In Arabidopsis, the vascular bundle is organized in a diarch pattern of symmetry: the xylem and phloem tissues form two axes that are positioned perpendicular towards each other. The xylem axis consists of two poles of protoxylem cells (primary xylem) and metaxylem cells in the middle (secondary xylem). The xylem axis is flanked by two poles of phloem, each containing proto- (primary) and meta- (secondary) phloem. The presence of stem cells in the cambium layer (a lateral meristem, see paragraph below), which is situated between the xylem and phloem, enables the formation of secondary xylem and phloem. The pericycle is adjacent to both the xylem and phloem poles. Depending on their location, pericycle cells that flank the xylem are called xylem-pole pericycle cells (hereafter referred to as XPPs) and the phloem-adjacent cells are known as phloem-pole pericycle cells (hereafter referred to as PPPs).
Figure 1: The root system of the dicotyledonous model plant Arabidopsis thaliana. A: schematic overview of the plant. The aerial or above ground parts of the plant consist of rosette leaves and a reproductive shoot or
stem with axillary branches. The root system consists of a primary root (PR) and lateral roots (LR). LRs may also carry secondary LRs. Arrows indicate the orientation of the shoot and root organs: acropetal is from the base to the tip of the organ while basipetal is from the tip to the base. Figure from website of F. Bouché. B: anatomical
structure of the root system. Left: longitudinal plane on the root (in plane of apical-basal axis). Each
colour-coded tissue layer is explained in the legend at the right of the figure. Note the different zones in the root. Right: transverse view on the root (in plane of radial axis). The single layered root tissues from the outside to the inside of the root are the epidermis, cortex, endodermis (with Casparian strips), pericycle and the vascular bundle with two poles of protoxylem, metaxylem, phloem and procambium. The initials of the root apical meristem are outlined with a black line. Figure modified from (De Smet et al., 2015).
Along the apical-basal axis at the most apical side of the root lies the root cap that consists of the columella and the lateral root cap (figure 1B). The root cap protects the root tip from
longitudinal plane axillary branch shoot LR PR acro p eta l b asi p eta l b asi p eta l acro p eta l rosette apical basal radial plane out in apical - basal axis
Stele Vascular
bundle
radial axis
damage caused by particles in the soil and functions as a sensory organ that directs root growth towards e.g. gravity and water. Starch-containing organelles called amyloplasts in the columella senses gravity and mediates a response to ensure that the root tip grows in parallel to the gravity vector. This is known as the root gravitropic response. At the root tip, stem cells named initials are found (figure 1B). Each cell layer of the root is derived from a particular set of initials. Initial cells divide and give rise to two new daughter cells that maintain the capacity to divide. One daughter cell establishes a group of rapid dividing cells that are positioned in a single cell file along the apical-basal axis. The stem cell initials and the dividing daughter cells located in the root tip form together the root apical meristem and are located in the meristem zone (figure 1B). When new daughter cells are formed, older cells are pushed towards the basal end and enter the elongation zone (figure 1B). The elongation zone is characterized by one-dimensional cell stretching along the apical-basal axis. The differentiation zone is situated more basally than the elongation zone and is characterized by cell differentiation and maturation (figure 1B). The appearance of root hairs, which are tube-like extensions that are formed on epidermal cells by tip growth, and the formation of the Casparian strip, a hydrophobic barrier that seals the central cylinder from the outer root layers and the soil, in the endodermis mark the start of the differentiation zone.
The root system plays an important role for plants to acquire essential minerals from the soil and import water. The epidermal cells are in contact with the substrate and carry out the exchange of molecules from soil to root but are also important to secrete plant exudates. To improve this exchange, one type of epidermal cells (trichoblasts) initiates the formation of root hairs as to increase the surface between root and soil. Not only the root system’s capacity for nutrient uptake but also its strength through anchorage in the substrate is determined by several factors: trichoblast number, PR length, LR number and positioning and the angle between PR and LR. Also the formation of secondary LRs contributes to the plastic development of the root system.
2. Lateral root formation
2.1. Morphogenesis: from nuclear migration until emergence
Post-embryonic organs such as LRs derive from founder cells. In Angiosperms, a new LR is formed deep within the main root. In the model plant Arabidopsis, founder cells in the pericycle give rise to this post-embryonic organ. In some species including Arabidopsis, LRs are only formed from pericycle cells at the xylem poles. This was originally observed in radish, a close relative to Arabidopsis (Blakely and Evans, 1979). The pericycle is not a concentric layer with a homogeneous group of cells like the endodermis, cortex and epidermis. The diarch symmetry pattern in the vasculature is reflected in the pericycle: two xylem poles are associated with XPP cell files and two phloem poles are associated with PPP cell files. The specification of XPP and PPP cells is suggested to occur in the root apical meristem (Parizot et al., 2008). The GREEN FLUORESCENT PROTEIN (GFP) marker in the enhancer trap lines J0121 and Rm1007 is specifically expressed in XPP cell files, which are mainly three, and LRs are only formed in this expression domain (Parizot et al., 2008).
LR formation is the process whereby a new LR is developed from a group of founder cells and starts with the specification of these cells (see section 2.2.3). LR founder cells are XPP cells that are competent to give rise to a lateral root primordium (hereafter referred to as LRP). LR initiation or sometimes mentioned as LRP initiation (hereafter referred to as LRI) refers to the initial cell divisions of the founder cells. A LRP can be considered as an immature LR that develops inside the PR. Throughout LRP development, a new root apical meristem forms at the tip of the developing organ. During LR emergence, the LRP has to cross the overlaying tissue layers (endodermis, cortex and epidermis) to finally appear at the surface of the PR. The first morphological event of LRI is that nuclei of pairs of adjacent LR founder cells in a longitudinal cell file round up and migrate to the common cell wall (De Rybel et al., 2010). Simultaneous with the nuclear movement, the overlaying endodermal cells shrink to enable the volume increase of LR founder cells (Vermeer et al., 2014). Soon after, the LR founder cells start a series of anticlinal, asymmetric cell divisions in the young differentiation zone of the root (Dubrovsky et al., 2001). An asymmetric cell division (a.k.a. formative division) generates two daughter cells of unequal size. Two types of LRI have been described in Arabidopsis, namely the longitudinal unicellular and bicellular type (Dubrovsky et al., 2001). In the unicellular and bicellular LRI type, respectively one and two neighbouring founder cells per cell file simultaneously undergo asymmetric divisions and will contribute to the establishment of a LRP. The minimal number of LR founder cells to generate a LRP is suggested to be respectively three and six for the unicellular and bicellular LRI type (Dubrovsky et al., 2001). However, it is predominantly found that pairs of pericycle cells participate in LRI. Pairs of LR founder cells in three adjacent XPP cell files generate a new LRP (Casimiro et al., 2001). The LRP developmental process is divided in seven stages (stage I-VII) and depends on the number and differentiation of cells and the number of cell layers in the LRP (figure 2D) (Malamy & Benfey, 1997). The anticlinal, asymmetric cell divisions generate a single layered stage I LRP consisting of eight to ten “smaller” central cells flanked by “longer” cells. Next, the inner cells start periclinal divisions which means that the division plane is shifted from a transverse plane to a longitudinal plane, i.e. daughter cells are positioned in the radial root axis, and leads to a stage II LRP. In a later stage, the LRP crosses the endodermis and changes from a flat to a dome-shaped form. After stage VII, the LRP emerges at the root’s surface and further matures
development: LR founder cells from the middle of three XPP cell files generate daughter cells that are found in the whole LRP while the daughter cells of LR founder cells in the outer XPP cell files are primarily found at the base of the LRP (Kurup et al., 2005).
2.2. The function of auxin in lateral root development
2.2.1. Auxin transport and signalling
Auxins are a group of plant hormones that are involved in several developmental processes e.g. gravitropism, cell elongation, vascular cell differentiation, embryonic patterning and cell proliferation such as during LRI. The most prominent form of auxin present in all higher plants is indole-3-acetic acid (hereafter referred to as IAA). The main sources of IAA are the shoot apical meristem and young leaves but also the root apical meristem in the root tip of both the PR and LRs are sites of IAA production (Ljung et al., 2005). IAA is known to stimulate the formation of LRs (Torrey, 1950).
The most prominent source of IAA lies in the shoot and shoot-derived IAA eventually accumulates in different sinks such as the root. IAA is transported through the vascular tissues and through cell-to-cell movement in a process known as the polar auxin transport system. Polar transport goes from a source to a sink and is independent from the orientation of plant organs. In the root, IAA is transported from the shoot-root junction to the root apex via the central cylinder and once arrived in the root tip, IAA is transported from the root apex via the epidermis towards the shoot (Blilou et al., 2005). At the transition between the basal meristem and the elongation zone, it is proposed that auxin is recycled in the central cylinder (Blilou et al., 2005). Polar auxin transport is mediated by influx and efflux carrier proteins in the plasma membrane: influx and efflux carriers import and export their substrate from/in the apoplast in/from the cytosol, respectively. IAA efflux is mediated by protein complexes of the PIN-FORMED (PIN) and the ATP-BINDING CASSETTE-TYPE B (ABCB) protein families, the latter also known as MULTIDRUG RESISTANCE-LIKE/P-GLYCOPROTEINS (MDR/PGPs) (Blakeslee et al., 2005). IAA influx in cells is mediated by AUXIN-RESISTANT 1 (AUX1) and LIKE-AUX1 (LAX) proteins (Parry et al., 2001), and some ABCBs (Kang et al., 2011). PIN-LIKES (PILS) are located on the endoplasmatic reticulum and regulate intracellular auxin homeostasis, presumably by the conjugation of auxin to amino acid residues (Barbez et al., 2012); auxin conjugates are considered to be inactive and for some conjugates, the conjugation step is reversible.
Delivery of IAA in cells of sink tissues initiates an auxin signalling cascade that results in a biological response. The transcriptional effects of auxin perception are mediated by the Arabidopsis SKP1-like (ASK)-Cullin-Fbox-RBX1 ubiquitin E3 ligase complex, denoted as SCFTIR1/AFB, where each complex has a TIR1/AFB F-box protein that functions as the auxin
receptor. SCFTIR1/AFB-mediated auxin signalling consists of AUXIN/INDOLE-3-ACETIC ACID
INDUCIBLE (Aux/IAA) transcriptional repressors and AUXIN RESPONSE FACTOR (ARF) transcriptional activators, that bind to auxin responsive elements (AuxREs) in promoter regions of auxin-responsive genes. Auxin binding brings the TIR1/AFB F-box protein in contact with the Aux/IAAs leading to Aux/IAA ubiquitination by SCFTIR1/AFB and subsequent degradation
by the 26S proteasome. A mutation in domain II of Aux/IAA proteins prevents the binding of the auxin-TIR1/AFB complex; mutants that encode these dominant-negative Aux/IAA proteins are auxin-insensitive.
Auxin affects lateral root development at all stages. LR development is regulated by discrete auxin signalling modules. An auxin signalling module consists of a specific set of Aux/IAA repressors and ARF activators. It is proposed that auxin can exert distinct functions through multiple auxin signalling modules and most probably, each module regulates the expression of a unique set of auxin responsive genes (De Smet et al., 2010). A single developmental process may be controlled by multiple auxin signalling modules (De Smet et al., 2010).
2.2.2. Auxin signalling reporters
A highly active auxin-inducible promoter was generated, named DR5 (DIRECT REPEAT 5), that contains repeats of the synthetic AuxRE, also named DR5. The DR5 promoter is often used as a reporter to indicate sites of auxin signalling. In general, higher auxin signalling leads to higher reporter activity. DR5 consists of seven repeats of the 11 bp DR5 AuxRE including the TGTCTC sequence (Ulmasov et al., 1997). The activity of DR5 does not necessarily reflect auxin levels because auxin needs to reach a threshold level and the auxin signalling pathway can be saturated (Benková et al., 2003). The first DR5 driven reporter protein was the beta-glucuronidase (GUS) enzyme that converts the substrate 5-bromo-4-chloro-3-indolyl-beta-D-glucuronic acid (X-Gluc) in a blue precipitating product during a histochemical GUS staining assay. Although the product can diffuse in neighbouring cells, the DR5::GUS reporter has a good cellular resolution but unfortunately GUS staining kills the plant. To follow up sites with
DR5 activity over time, DR5 was also coupled to the coding sequence of fluorescent reporters
such as the GREEN FLUORESCENT PROTEIN (GFP). Fluorescent markers have a good cellular resolution but are relatively long-living proteins and have a long maturation time, which is disadvantageous to analyse fast processes (Möller et al., 2017). The DR5::LUCIFERASE (DR5::LUC) reporter allows to visualize highly dynamic auxin signalling and is based on bioluminescence (Moreno-Risueno et al., 2010). One limitation is that it cannot be resolved how many cells express the LUC reporter (Möller et al., 2017).
2.2.3. Priming of lateral root founder cells in the basal meristem
This section deals with the specification of LR founder cells in the pericycle at the xylem poles.
2.2.3.1. Recurrent auxin signalling in the basal meristem and oscillatory gene
expression in the oscillation zone correlate with LRI
The basal meristem (hereafter referred to as BM) has a recurrent DR5::GUS maximum with a time interval of approx. 15 hours and starts from 10 hours after germination (De Smet et al., 2007). Detailed analysis showed that two strands of protoxylem expressed the DR5::GUS reporter in the BM. The oscillating DR5::GUS expression in the BM is found to correlate with LRI: when the root was labelled at the prospective distance from the root tip where DR5::GUS would occur, the label was found on top of a LRP (De Smet et al., 2007). Furthermore, the time period between LRI is found to be approx. 15 hours with an approx. 20 hour interval between LRI and the auxin peak in the BM starting from 10 hours after germination (De Smet et al., 2007). The DR5::GUS peak that precedes LRI has been interpreted to specify pericycle cells as LR founder cells (a.k.a. priming of LR founder cells) (De Smet et al., 2007). However, DR5::GUS is expressed in the protoxylem while LR founder cells are found in the XPP. How auxin signalling in the protoxylem is linked to the specification of XPP cells as LR founder cells and
whether an instructive signal is transmitted from protoxylem cells to XPP cells remain unclear. In conclusion, the auxin response in the BM is suggested to determine the position where a new LRP will develop (De Smet et al., 2007). Furthermore, DR5::GFP expression in a single or pair of cells in the same XPP cell file correlates with LR founder cell specification because this auxin signalling precedes LRI (Dubrovsky et al., 2008).
DR5::LUC is periodically expressed approx. every 6 hours in the BM and the elongation zone
and this whole region is named the oscillation zone (OZ) (Moreno-Risueno et al., 2010); the time interval of DR5::GUS peaks as reported in (De Smet et al., 2007) is lower than the time interval of DR5::LUC peaks because (De Smet et al., 2007) analysed younger seedlings where the growth rate of the root apical meristem was lower (see below in paragraph). Peaks of
DR5::LUC expression in the OZ can be followed along the longitudinal axis of the root: pulses
of DR5 activity in the OZ generate static points of DR5::LUC expression which are named prebranch sites (Moreno-Risueno et al., 2010). A prebranch site correlates with the establishment of a LRP and its formation very likely correlates with LR founder cell specification but the LUC reporter system does not allow to resolve in how many cells and in which tissues DR5::LUC is expressed (Moreno-Risueno et al., 2010). Auxin is suggested to be not sufficient to trigger the formation of prebranch sites since auxin treatment of pericycle cells in the BM was not capable to induce the formation of a new prebranch in the absence of a DR5::LUC peak (Moreno-Risueno et al., 2010). In the OZ, two sets of oscillating genes were found: one set of 2084 genes is in phase with DR5::LUC oscillation peaks while the other set of 1409 genes is in antiphase with DR5::LUC oscillating expression (Moreno-Risueno et al., 2010). The oscillating expression pattern relates these genes to prebranch site formation. In conclusion, pulses of DR5::LUC expression in the BM represent an oscillating gene expression network that functions as an endogenous “clock” mechanism for regular LR positioning via the priming of LR founder cells at regular time intervals along the PR (Moreno-Risueno et al., 2010). The root apical meristem growth rate is suggested to determine the time interval between the positioning of LRs because the time interval between DR5::LUC peaks decreases the first six days after seed germination and root growth accelerates until five days after germination (Möller et al., 2017). Prebranch sites include both LR founder cells and LRPs that did not emerge from the PR (Möller et al., 2017).
The Aux/IAA SOLITARY ROOT (SLR/IAA14), which regulates LRI, is most likely not involved in auxin signalling in the BM since SLR/IAA14 is not expressed in the BM and LR founder cell specification could still take place in the dominant slr-1/iaa14 mutant, where the stabilized slr/iaa14 protein is not degraded in the presence of auxin (see also section 2.2.1) (De Smet et al., 2007). The auxin response in the BM has been shown to depend on the TIR1-/AFB-mediated auxin signalling pathway: in the presence of PEO-IAA, a compound that inhibits the TIR1/AFB auxin receptors, the oscillating DR5::GUS expression in the BM was strongly reduced or absent (De Rybel et al., 2010). IAA28 is the Aux/IAA repressor protein that acts in the auxin signalling pathway in the BM because the expression domain of IAA28 includes the BM and the percentage of seedlings that express DR5::GUS in the BM was reduced in the dominant-negative iaa28-1 mutant background (De Rybel et al., 2010). Results from a yeast-two-hybrid (Y2H) experiment indicates that IAA28 directly interacts with the auxin response factors ARF5,-6,-7,-8,-19 and possibly also ARF14 (De Rybel et al., 2010).
In conclusion, LR founder cell specification depends on oscillatory gene expression, auxin transport and auxin signalling (figure 2A) (De Rybel et al., 2010, De Smet et al., 2007, Dubrovsky et al., 2008, Moreno-Risueno et al., 2010).
GATA23 is suggested to act downstream of the IAA28-dependent auxin signalling pathway
during LR founder cell specification (figure 2A) (De Rybel et al., 2010). GATA23 promoter::GUS patches appear 10 hours after DR5::GUS expression in the BM (De Rybel et al., 2010) while expression of a fluorescent marker under GATA23 promoter control is found to coincide with
DR5::LUC peaks in the OZ (Xuan et al., 2015). GATA23 may also be involved in LRI because GATA23 promoter activity starts before the nuclear migration in LR founder cells and
continues until a stage III LRP (De Rybel et al., 2010). GATA23 knockdown results in a reduced number of early stage LRPs (De Rybel et al., 2010). GATA23 is Brassicaceae-specific (Behringer et al., 2014, Behringer & Schwechheimer, 2015) suggesting that its function(s) during LR formation cannot be conserved outside this family. Other members of the GATA23 family may act redundantly with GATA23 (B. Möller, personal communication).
2.2.3.2. Role of IBA-derived IAA released during periodic programmed cell death
from the lateral root cap
The pleiotropic functions of auxin in plant development made it difficult to unravel the molecular components that regulate LR formation. To determine how auxin acts specifically during LR development, a chemical compound screen was done. One non-auxin-like compound named naxillin was able to stimulate LR development while the effects on PR growth and aerial tissue development were more limited as compared to natural and synthetic auxins (De Rybel et al., 2012). Naxillin-responsive genes were primarily found in the BM and encompassed only a subset of auxin-responsive genes. The naxillin resistant 1 (nar1) mutant was insensitive to the increase in LR density after naxillin treatment and had a mutation in
INDOLE-3-BUTYRIC ACID RESPONSE3 (IBR3). IBR3 encodes an enzyme involved in the
indole-3-butyric acid (IBA) to indole-3-acetic acid (IAA) (IBA-to-IAA) conversion pathway in which a two carbon group is cleaved from IBA. IBA is known to stimulate LR development (Zolman et al., 2001) and acts mainly via its conversion to IAA. Gene expression analysis of genes involved in the to-IAA conversion pathway indicates that naxillin enhances the source of IBA-derived IAA in the root cap (De Rybel et al., 2012). Naxillin induces an auxin response in the BM suggesting that the IBA-to-IAA conversion pathway in the root cap is linked to the LR prepatterning process (De Rybel et al., 2012).
Blocking of the IBA-to-IAA conversion pathway leads to a decreased number of prebranch sites, LRPs and LRs but also to a reduced intensity of the DR5::LUC peaks in the OZ while the oscillation frequency remains unchanged (Xuan et al., 2015). This suggests that the amplitude of the DR5::LUC oscillation determines whether a prebranch site is formed or not (Xuan et al., 2015). Thus, it has been found that the IBA-to-IAA conversion pathway in the lateral root cap regulates the DR5::LUC oscillation amplitude, which is important for the formation of prebranch sites (Xuan et al., 2015). Furthermore, the expression of DR5::LUC in the OZ has been found to depend on auxin perception via the TIR1 and AFB2 auxin receptors since the amplitude of a DR5::LUC pulse in the OZ was strongly reduced in tir1 afb2 mutants (Xuan et al., 2015). To identify genes that act downstream of the IBA-derived IAA perception in the OZ during LR prepatterning, a transcriptome analysis was performed with two criteria: gene
expression in phase or in anti-phase with the oscillatory genes in the OZ and expression in the pericycle. One of the selected genes is MEMBRANE-ASSOCIATED KINASE REGULATOR 4 (MAKR4) (see section 3) and its expression has been found to be highly upregulated in response to exogenous IBA, naxillin or IAA (Xuan et al., 2015).
The root cap is continuously regenerated during root growth. The oldest cells in the distal lateral root cap undergo developmental programmed cell death (dPCD) at regular time intervals at the transition between the meristem and the elongation zone and are replaced by younger cells. Overlapping stripes of a DR5 driven nuclear fluorescent reporter protein and the early stage dPCD PASPA3 reporter are present at the most distal end of the lateral root cap (Xuan et al., 2016). The most distal DR5 stripe and PASPA3 stripe disappear simultaneously at regular intervals of approx. 4 hours and precede the DR5::LUC pulse in the OZ at the exact same spot on the PR (Xuan et al., 2016). This indicates that the recurrent dPCD in the lateral root cap is linked to the DR5::LUC oscillatory mechanism of LR positioning. It is the auxin that is released during periodic dPCD in the lateral root cap that is necessary for the formation of prebranch sites because in mutants where distal lateral root cap cells did not undergo dPCD, no striped DR5 expression pattern was present in the lateral root cap and the number of prebranch sites and LRs was reduced (Xuan et al., 2016). Auxin is released before the cellular integrity of lateral root cap cells is disrupted during late dPCD (Xuan et al., 2016). An in silico model confirmed that periodic release of auxin in the apoplast of epidermal cells could generate an auxin response in the stele that is necessary for the specification of LR founder cells (Xuan et al., 2016). Auxin transport in the root cap mediated by the AUX1 influx carrier and the auxin efflux carriers is necessary for the periodic DR5::LUC oscillations in the OZ (Xuan et al., 2016).
In conclusion, periodic dPCD in the distal lateral root cap cells correlates with the recurrent release of IBA-derived IAA in the PR which is suggested to be perceived in the stele and contributes to the DR5::LUC oscillations in the OZ that are necessary for prebranch site formation and LRP development (figure 2A) (Xuan et al., 2016).
2.2.4. Lateral root initiation
To investigate the molecular mechanisms that are at play during auxin-activated LRI, the transcriptional responses in wild-type were compared to that in the dominant negative
slr-1/iaa14 mutant, that has a complete absence of LRs and an agravitropic root growth. Genes
that are significantly up-/downregulated in the roots of wild-type plants but remain unchanged in slr-1/iaa14 plants are of interest for LRI. The expression of cell cycle genes is strongly upregulated after auxin treatment: cyclins and cyclin-dependent kinases (CDKs) are central regulators of cell cycle progression and are active in the different phases of the cell cycle, i.e. the G1 gap phase, S phase (DNA replication), G2 gap phase and M phase (cell division).
However, cell cycle progression is not sufficient for LRI since the slr-1/iaa14 mutant where the cyclin CYCD3;1, that stimulates cell cycle progression, is expressed, cannot produce asymmetric cell divisions and form LRPs (Vanneste et al., 2005). Polar auxin transport is necessary for auxin accumulation in the inner LR founder cells after the first asymmetric cell division and later in the tip of the LRP as visualized by DR5::GUS and anti-IAA antibodies (Benková et al., 2003). It is suggested that the local auxin gradient determines different cell fates, indicating that auxin has multiple roles in LRI (Vanneste et al., 2005). A model was proposed whereby LRI is a self-regulatory mechanism depending on the auxin concentration: a low concentration activates auxin conjugating enzymes and Aux/IAA transcriptional
repressors to dampen auxin signalling and transport whereas a high concentration is reinforced by polar auxin transport to reach a threshold level that activates LRI (Vanneste et al., 2005).
2.2.5. Lateral root emergence
The initiation and emergence of LRs is regulated by biochemical and mechanical stimuli and cell-to-cell communication between the LRP and the overlaying cells of the LRP (Vilches-Barro & Maizel, 2015). The rounding of nuclei and their movement to the common cell wall occur simultaneously with the swelling of LR founder cells and the reshaping of the adjacent endodermal cells (Vermeer et al., 2014). The responses of the endodermis to accommodate the LRP are regulated by the SHORT HYPOCOTYL2 (SHY2)/IAA3-dependent auxin signalling module (Vermeer et al., 2014). The growing LRP induces unique responses in the endodermis, and the cortex and epidermis. The shape of the endodermal cells alters to narrowly tight the emerging LRP without forming a gap in the Casparian strip network that forms the diffusion barrier between the central cylinder and the outer root layers and the soil (Vermeer et al., 2014). The cortex and epidermis cells that overlay a LRP express pectin degrading enzymes which enable cell separation in the overlaying layers upon LR emergence (Swarup et al., 2008). The expression of cell wall remodelling and degrading enzymes in the LRP overlaying tissue layers is dependent on peptide signalling (see section 4.2.3). To facilitate LR emergence, the turgor pressure in the LRP increases while it decreases in the overlaying tissue layers due to changes in the expression levels of PLASMA MEMBRANE INTRINSIC PROTEINs (PIPs) that facilitate water uptake in cells (Péret et al., 2012).
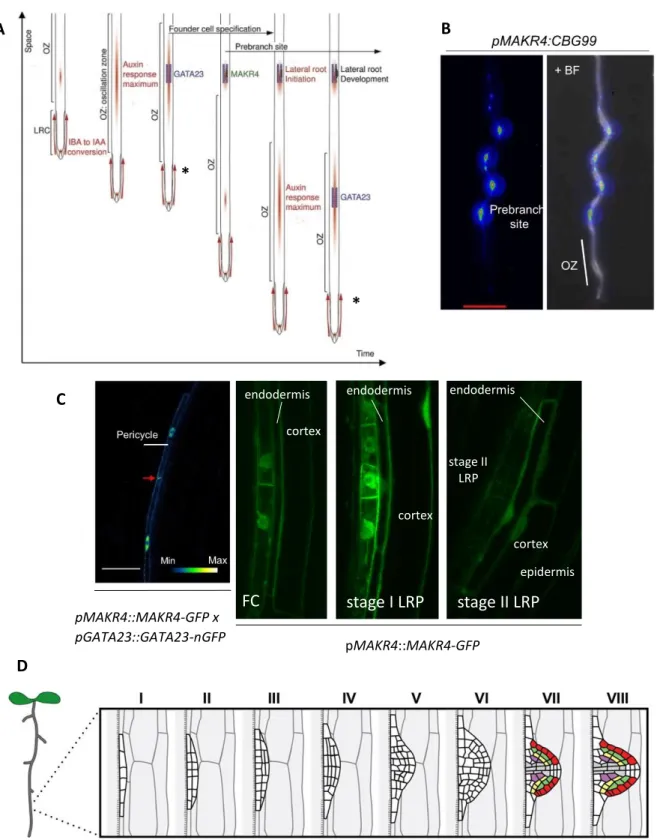
B D A * * FC stage I LRP stage II LRP endodermis cortex cortex epidermis endodermis stage II LRP cortex endodermis pMAKR4::MAKR4-GFP C pMAKR4::MAKR4-GFP x pGATA23::GATA23-nGFP
Figure 2: The role of MAKR4 in LR development. A: LR formation is a multistep process. Recurrent programmed cell death
(asterisk) in the outer layer of the lateral root cap periodically releases IBA-derived IAA in the OZ. IBA-derived IAA increases the amplitude of DR5 pulses in the OZ and this is necessary for the establishment of prebranch sites with LR founder cells.
GATA23 is an early marker for LR founder cell specification. These founder cells begin to divide during LRI and give rise to a
LRP that eventually becomes a LR. MAKR4 may function in prebranch site formation, the transition between prebranch site and LRI and LRP development. B: MAKR4 expression before LRI. Left: MAKR4 is mainly expressed in prebranch sites and very weakly in the OZ. Right: overlaying images of fluorescence and bright-field microscopy. C: MAKR4 expression during
LRI. Left: MAKR4-GFP and GATA23-nuclear GFP (nGFP) expression start before the nuclear migration of LR founder cells.
Right: MAKR4 is expressed in LR founder cells and is present at the plasma membrane, in the cytoplasm and in the nucleus. In stage I and II LRP, MAKR4 is also present in the overlaying endodermis, cortex and weakly in the epidermis. D: stages of
LRP development. Figures in A, B and left figure in C are modified from (Xuan et al., 2015), right figure in C is from B. Möller
3. The MAKR family
3.1. MAKR4 may be involved in prebranch site formation, transition from
prebranch site to lateral root initiation and early stage LRP development
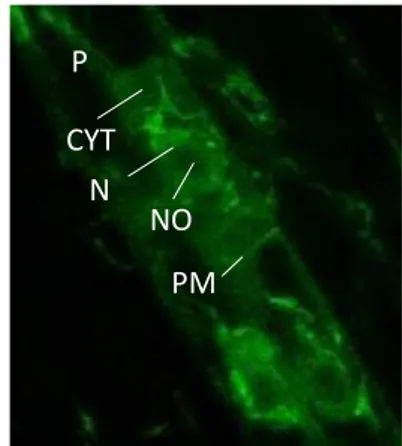
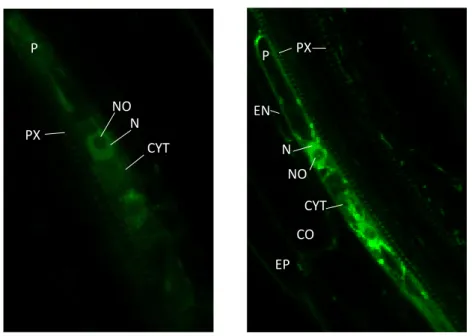
Transcriptional reporters indicate that MAKR4 is expressed at different time points and in different tissues during LR development: pMAKR4::CLICK BEETLE LUCIFERASE (CBGr99) is very weakly expressed in the OZ but has a strong periodic expression along the longitudinal axis of the root with the same frequency as DR5::LUC, which indicates that MAKR4 is expressed in prebranch sites (figure 2B), and pMAKR4::MAKR4-GFP (MAKR4 fused to the fluorescent reporter GFP) is expressed in pericycle cells from before nuclear migration of LR founder cells until the formation of a stage II LRP, and in the LRP overlaying tissue layers, primarily in the endodermis but also in the cortex and weakly in the epidermis (Xuan et al., 2015)(figure 2C). MAKR4-GFP is localized on the plasma membrane, in the cytoplasm and in the nucleus in pericycle cells (figure 2C). Furthermore, MAKR4 is also expressed in the protoxylem cells in the root apical meristem, as determined by the pMAKR4::GUS reporter (Xuan et al., 2015), and in the quiescent centre (hereafter referred to as QC) as determined by single cell transcriptome analysis (B. Möller, unpublished data).
Previous analysis in the lab showed that the CRISPR/Cas9-generated makr4 knock out mutant (hereafter referred to as makr4) has a decreased number of LRs and prebranch sites comprising LR founder cells and non-emerged LRPs, and the DR5::LUC oscillation frequency and amplitude in the OZ remains unchanged (B. Möller, unpublished data). Moreover, it has been shown that the makr4 mutant has a reduced number of DR5::VENUS expressing cells, which are suggested to be LR founder cells, and a delayed LRP formation after root bending-induced LR formation (Péret et al., 2012)(B. Möller, unpublished data). These results indicate that MAKR4 may be involved in prebranch site formation, i.e. LR founder cell specification, and LRP development (B. Möller, unpublished data). Xuan et al., 2015 also suggested that MAKR4 may be involved in the transition from prebranch site to LRI (figure 2A).
MAKR4 is found to be a downstream factor of LATERAL ORGAN BOUNDARIES DOMAIN 16 (LBD16), an auxin-inducible transcription factor that is one of the key regulators that connects LR founder cells to the formation of a new LRP (Goh et al., 2019). It is suggested that LBD16 is involved in nuclear migration, asymmetric cell divisions during LRI and early LRP development (Goh et al., 2012). LBD16 functions in the gene regulatory network that regulates LRI and activates target genes such as MAKR4, GATA23 and PUCHI (Goh et al., 2019). LBD16 expression starts as early as in the OZ (Moreno-Risueno et al., 2010). MAKR4 orthologues have been identified in species members of the Cucurbitaceae based on protein phylogenetic analysis and gene transcriptional activation in response to IBA and NAA treatment (Kiryushkin et al., 2019). Seven family members of the Angiosperms, including the Cucurbitaceae, have an alternative mechanism of LR branching: LRPs initiate in the root apical meristem. In squash (Cucurbita pepo), CpMAKR4 is expressed, like MAKR4 in Arabidopsis, in the XPP cells before the first asymmetric cell division of LR founder cells and suggests a similar function for both MAKR4 orthologues in LRI and/or LRP development (Kiryushkin et al., 2019). However,
CpMAKR4 expression also differs from that of Arabidopsis MAKR4 as the expression is found
in all protoxylem cells in the meristem and the expression is maintained in LRPs until stage V (Kiryushkin et al., 2019).
3.2. BKI1, MAKR2 and MAKR5
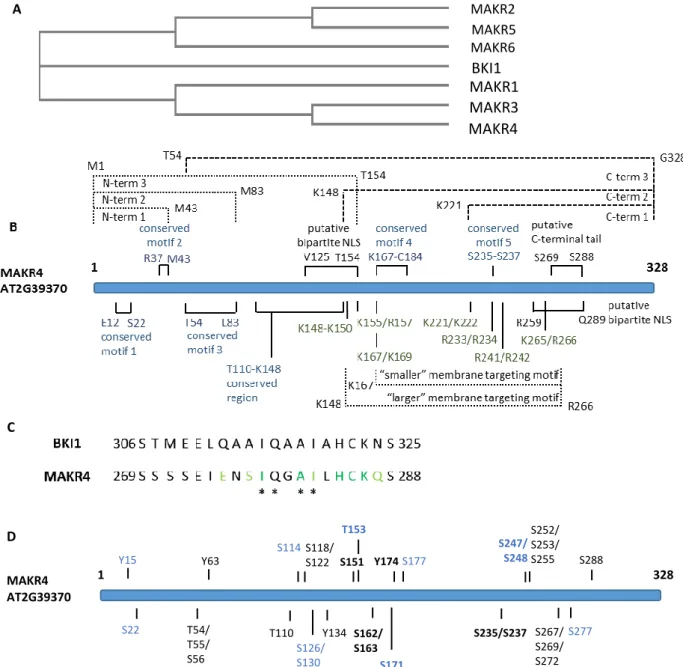
MAKR4 is a member of a family with seven members named BRASSINOSTEROID INSENSITIVE 1 (BRI1) KINASE INHIBITOR 1 (BKI1) and MAKR1-6 (Jaillais et al., 2011). BKI1 is the founding member of this family: two conserved motifs in BKI1 (see below) are found in all members of the MAKR family (Jaillais et al., 2011). BKI1 is a negative regulator of BRI1, the receptor of brassinosteroids, which are a group of plant steroid hormones (Wang & Chory, 2006). BRI1 is a leucine-rich repeat receptor-like kinase with an extracellular domain that binds the receptor’s ligand, a transmembrane domain and a cytosolic domain containing a kinase domain that is activated after ligand binding. When brassinosteroid levels are low, the BRI1 kinase domain is kept in a basal state by an auto-inhibitory region and by the interaction with the kinase inhibitor BKI1 (Wang & Chory, 2006). BKI1 blocks the interaction of BRI1 with its co-receptor BRI1 ASSOCIATED KINASE1 (BAK1) (Jaillais et al., 2011). Both BRI1 and BAK1 are dual-specificity kinase, i.e. their kinase domain phosphorylates serines/threonines and tyrosines (Oh et al., 2009). Brassinosteroid binding to the extracellular domain of BRI1 leads to BRI1-BAK1 heterodimerization which brings their kinase domains in close proximity. This allows
trans-phosphorylation of BRI1 and BAK1 which causes the BRI1-BAK1 pair to form a highly
active signalling complex and releases BKI1 inhibition. BKI1 has two domains that are evolutionary conserved in BKI1 orthologues and are found in all members of the MAKR family: a twenty amino acid residue segment called the C-terminal tail that causes the interaction between BKI1 and BRI1 kinase domain and a motif of repeating pairs of basic amino acid residues arginine (R) and/or lysine (K) (hereafter referred to as membrane targeting motif) that targets BKI1 to the plasma membrane (Jaillais et al., 2011). Wild-type BKI1 is associated with the plasma membrane and is also found in the cytosol (Jaillais et al., 2011). BKI1 plasma membrane localisation is caused by electrostatic interactions between the positive charges in the basic residues of the membrane targeting motif and negative charges on the plasma membrane (Simon et al., 2016).
BRI1 activation leads to BKI1 phosphorylation at highly conserved residues: first serine residues at positions 270 and 274 (S270/274) and second the tyrosine residue at position 211 (Y211) are phosphorylated (Jaillais et al., 2011, Wang et al., 2011). It is most likely that BRI1 directly phosphorylates Y211 and S270/274 since BKI1 is shown to be phosphorylated on these residues in an in vitro assay with BRI1 kinase domain but not with BAK1 kinase domain (Wang et al. 2014?). Y211 is located in the membrane targeting motif. BKI1(Y211) phosphorylation in response to BRI1 activation has a major effect on BKI1 translocation from the plasma membrane to the cytosol since mutating Y211 into a non-phosphorylizable residue (Y211F) leads to BKI1 retention at the plasma membrane while mutating Y211 in a residue that mimics the negative charge of a phosphate group (Y211D) results in complete absence of BKI1 at the plasma membrane even without brassinosteroid treatment (Jaillais et al., 2011). It also suggests that dissociation of phosphorylated BKI1 from the plasma membrane is the result of electrostatic repulsion with the plasma membrane. It is suggested that S270/274 is phosphorylated prior to Y211 and that besides the phosphorylation of Y211, S270/274 phosphorylation has a substantial effect on BKI1 translocation (Wang et al., 2011). The phosphopeptide binding protein(s) 14-3-3 bind(s) to BKI1 in a S270/274-dependent manner (Wang et al., 2011) and is likely important for plasma membrane-to-cytosol translocation of phosphorylated BKI1. The BKI1-14-3-3 interaction may also positively regulate downstream signalling because 14-3-3 proteins block downstream transcription factors (Wang et al., 2011).
It is also proposed that 14-3-3 proteins prevent dephosphorylation and the recycling of BKI1 from the cytosol to the plasma membrane (de Boer et al., 2013). The binding of BKI1 to BRI1 is suggested to be necessary but not sufficient to inhibit BRI1 kinase activity since BKI1 with a mutation in the conserved HCK motif in the C-terminal tail could bind BRI1 but overexpression of this mutated BKI1 did not lead to a dwarf phenotype, which indicates that brassinosteroid signalling was not blocked (Jaillais et al., 2011).
MAKR2, another member of the MAKR family, is suggested to control the speed of root gravitropism. The root gravitropic response is initiated when the root tip does not lie in parallel with the gravity vector. An asymmetric auxin accumulation on the lower side of the root tip due to auxin redistribution causes the root tip to bend and align with the gravity vector. Rho-GTPase of Plants 6 (ROP6) regulates the asymmetric auxin accumulation during the gravitropic response via decreased PIN2 endocytosis and thus more PIN2 auxin efflux activity at the plasma membrane of epidermal cells on the lower side of the root (Armengot et al., 2016). TRANSMEMBRANE KINASE (TMK) receptors were identified upstream of ROP6 activation in a fast auxin response (Xu et al., 2014). Auxin signalling at the plasma membrane is suggested to initiate a fast non-transcriptional auxin response but how this process may activate ROP6 and what the role of TMKs in this activation may be, is still unclear (Armengot et al., 2016). MAKR2 is a negative regulator of the leucine-rich repeat receptor-like kinase TMK1 (Marquès-Bueno et al., unpublished results). MAKR2 binds to and is phosphorylated by TMK1 because MAKR2 has been found to be phosphorylated on serine residues in an in vitro assay with the TMK1 kinase domain (Marquès-Bueno et al., unpublished results). MAKR2 is also translocated from the plasma membrane to the cytosol in response to IAA treatment in a TMK1-dependent manner (Marquès-Bueno et al., unpublished results). It is suggested that MAKR2 translocation from the plasma membrane to the cytosol acts downstream of a fast non-transcriptional auxin signalling pathway because the protein synthesis inhibitor cycloheximide and the 26S proteasome inhibitor MG132, both of which inhibit the transcriptional auxin signalling pathway, could not block the translocation after naphthalene-acetic acid (NAA, a synthetic auxin) or IAA treatment (Marquès-Bueno et al., unpublished results).
MAKR5 was found in a screen for suppressors of the brx phenotype: makr5 could rescue the loss-of-function in BREVIS RADIX (BRX), a gene that is involved in protophloem differentiation (Kang & Hardtke, 2016). BRX is antagonized by the peptide hormone CLAVATA3/EMBRYO SURROUNDING REGION 45 (CLE45)(see also section 4.2.2): CLE45 binds to its receptor BARELY ANY MERISTEM3 (BAM3) in the developing protophloem and prevents the premature differentiation of protophloem cells (Kang & Hardtke, 2016). The MAKR5 protein is found to be required for BAM3-dependent CLE45 signalling and implicates that MAKR5 is a positive effector of CLE45-BAM3 signalling, which is opposite to the functions ascribed to other MAKR proteins. Redundancy with MAKR5 may explain why bam3 mutants are fully CLE45 insensitive and makr5 mutants are only partially CLE45 insensitive (Kang & Hardtke, 2016). A domain swap experiment with the C-terminal parts of BKI1 and MAKR5 indicated that the C-terminal part of MAKR5 determines the specificity of the protein in the CLE45-BAM3 signalling module (Kang & Hardtke, 2016). CLE45 signalling increases the ratio of plasma membrane-to-cytosol localisation of MAKR5 (Kang & Hardtke, 2016). MAKR5 does not directly interact with BAM3 and this mechanism is not analogous to BRI1/BKI1 and TMK1/MAKR2 signalling (Kang & Hardtke, 2016). In conclusion, the MAKR family contains both positive (MAKR5) and negative regulators (BKI1, MAKR2) of receptor signalling.
4. Peptide signalling in root development
4.1. Peptide hormone classification and ligand-receptor signalling
Peptide hormones have been described as mobile signals that mediate cell-to-cell communication and is one of several types of signalling molecules next to the classical plant hormones. Peptide hormones coordinate a diverse range of processes such as reproduction, growth, development and plant defence. Peptide hormones are first classified depending on whether they are secreted, i.e. if a secretion signal is present at the N-terminus (Matsubayashi, 2014). Most peptide hormones act extracellularly (Matsubayashi, 2014). Secreted peptides are further divided based on their structure in the cysteine-poor posttranslationally modified peptides and the cysteine-rich peptides (Matsubayashi, 2014). The cysteine-poor posttranslationally modified peptides are embedded in large inactive preproproteins: preproproteins have a regular structure with an N-terminal secretion signal, a central variable region and a C-terminal region with the functional peptide hormone. After cleaving the signalling peptide, the proprotein receives at least one posttranslational modification such as tyrosine sulphonation, proline hydroxylation and/or hydroxyproline arabinosylation (addition of the monosaccharide arabinose) and undergoes proteolysis to release the mature peptide. However, the order of posttranslational modifications and proteolysis during processing is unclear in plants. The mature peptide is approx. 5 to 20 amino acid residues in length. The chemical nature and location of the posttranslational modification(s) as well as the chain length are critical for the function of the mature peptide and the interaction with its receptor (Matsubayashi, 2014). Cysteine-rich peptides contain an even number of cysteine pairs (between 2 and 16 and typically 6-8) that allows the formation of intramolecular disulphide bridges after protein synthesis. Cysteine-rich peptides may vary in length between 40 and more than 100 residues and are in most cases not synthesized as an inactive precursor (Matsubayashi, 2014).
Secreted peptide hormones bind to the extracellular domain of receptors in the plasma membrane and initiate a biological response (biochemical, physiological) inside the cell. Most identified peptide receptors in plants belong to the group of LEUCINE-RICH REPEAT RECEPTOR-LIKE KINASEs (LRR-RLKs). LRR-RLK receptors usually require a coreceptor that activates the receptor via trans-phosphorylation in the presence of the peptide signal. BAK1 or another SOMATIC EMBRYOGENESIS RELATED KINASE (SERK) is mainly involved as coreceptor (Han et al., 2014). In some cases, a LRR-receptor like protein may function as receptor and a LRR-RLK then functions as coreceptor (Liebrand et al., 2014).
4.2. The role of peptides in primary and/or lateral root development
Multiple peptide signal families have been described that affect root development. Within the same family, several members may have an identical function in a particular process and thus act redundantly. A family of peptide hormones has a characteristic conserved motif that is present in all members of that family. Hereafter, only peptides that may be related to MAKR4 are mentioned (see results).
4.2.1 RGF/GLV/CLEL peptides
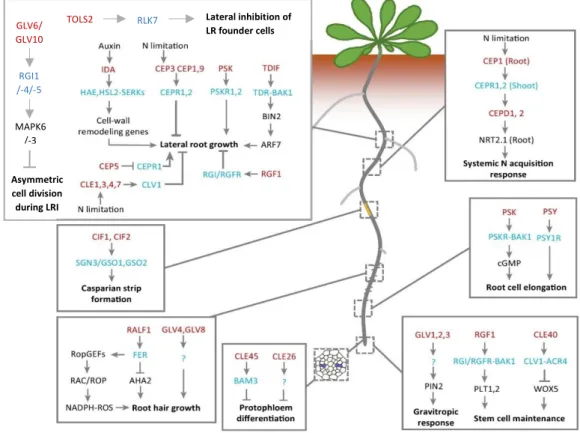
The ROOT MERISTEM GROWTH FACTOR (RGF)/GOLVEN (GLV)/ [CLAVATA 3 (CLV3)/EMBRYO-SURROUNDING REGION (ESR)-RELATED] (CLE)-LIKE (CLEL) signalling peptide family is involved in different developmental processes (Matsuzaki et al., 2010, Meng et al., 2012, Whitford et al., 2012). The RGF/GLV/CLEL (hereafter referred to as GLV) peptide hormone family is now considered to have eleven members (Fernandez et al., 2013b). GLV peptides have two conserved motifs: an N-terminal secretion signal and a C-terminal conserved motif of 13 to 16 amino acids that defines the RGF/GLV/CLEL family and is suggested to be the core sequence of the secreted, functional peptide hormone (Fernandez et al., 2013b). The C-terminal conserved motif is found in all higher plants indicating the existence of homologous proteins.
GLV genes are classified in three groups depending on their expression domain: GLV5,-7,-10
and -11 are expressed in the QC and/or in columella cells (domain I); GLV3,-6,-9 are expressed above the QC in the meristem in most cell layers but with limited expression in the epidermis (domain II); GLV6 is expressed in domain I and II ; GLV4 and -8 are expressed in the epidermis above the meristem (domain III) (Fernandez et al., 2013a). GLV peptides expressed in domain I function to maintain root meristematic activity and probably act by stabilizing the PLETHORA (PLT) gradient in the meristem which is essential for maintaining the initial cell stem cell niche and the proliferation of daughter cells (figure 3)(Matsuzaki et al., 2010). GLV peptides expressed in domain II act non-cell autonomous to establish an auxin gradient in the epidermis during the gravitropic response (figure 3)(Fernandez et al., 2013a). Ten of the eleven GLV genes are expressed in different stage of LR development (Fernandez et al., 2013a). A reduction of the transcriptional activity of GLV genes that are expressed in early LRP stages, i.e. between stage I and IV, more severely inhibits LR development than a reduced transcription of GLV genes that are expressed from stage V onwards (Fernandez et al., 2013a).
GLV6 expression is the earliest of GLVs during LR development and starts before the nuclear
migration of LR founder cells, continues throughout LRP development and is also found in the overlying endodermis (Fernandez et al., 2015). GLV6 and GLV10 act redundantly during LR development and inhibit the initial asymmetric divisions in LR founder cells during LRI because the glv6 glv10 double knock-out mutant generated via CRISPR/Cas9 has an increased density of stage I LRPs, that passed through the first and/or the second round of asymmetric cell divisions (figure 3)(Fernandez et al., 2020). It is suggested that the inhibition of asymmetric cell divisions functions as one of the checkpoints during LR formation that enables plants to regulate the density of LRs (Fernandez et al. 2020). A GLV6/-10 gradient in the LR founder cells may also be important for patterning in the LRP, i.e. to control which LR founder cells undergo asymmetric cell division (Fernandez et al., 2020). Some GLV peptides have been shown to be tyrosine sulphonated and proline hydroxylated but all GLV peptides are predicted to have these posttranslational modifications since the tyrosine and proline residues are highly conserved in the GLV peptide motif. TYROSYL SULFOTRANSFERASE (TPST) is the only enzyme in Arabidopsis that catalyses tyrosine sulphonation of peptide hormones including GLVs (Matsuzaki et al., 2010). Five receptors of RGFs were identified and were named RGF INSENSITIVE/RGF RECEPTOR 1-5 (RGI-/RGFR1-5). It is suggested that SERKs act as coreceptors for RGFRs (Song et al., 2016). RGI1, RGI4 and likely RGI5 are the receptors of GLV6/GLV10 peptides and MITOGEN-ASSOCIATED KINASE REGULATOR 6 (MAPK6) and possibly MAPK3 act downstream of GLV6/GLV10-RGI1/-4/-5 signalling (Fernandez et al., 2020).
4.2.2 CLE40/45 peptides
The CLAVATA 3/ EMBRYO SURROUNDING REGION-RELATED (CLE) family of secreted signalling peptides have functions in root development. The thirty-two family members are classified in two groups: the A-type CLEs regulate root meristem development and B-type CLEs regulate vascular development (Whitford et al., 2008). The conserved CLE motif is suggested to be the core sequence of CLE peptides (Fiers et al., 2005). Treating seedlings with most CLEs reduces the primary root length as a consequence of decreasing the root apical meristem size (Whitford et al., 2008). The A-type CLE40 stimulates columella stem cell differentiation (figure 3)(Stahl et al., 2009) and is also suggested to inhibit differentiation in meristematic region above the QC (Pallakies & Simon, 2014). In the root apical meristem, the QC maintains stem cell identity in the surrounding initials via the QC-specific expression of the homeobox domain protein WUSCHEL-RELATED HOMEOBOX 5 (WOX5). CLE40 is expressed in the differentiated columella cells and antagonizes the effect of WOX5 in the columella stem cells. CLE40 binds to the RLK receptors ARABIDOPSIS CRINCKLY 4 (ACR4) and CLAVATA 1 (CLV1) in the columella stem cells and the adjacent layer of columella cells (figure 3)(Stahl et al., 2013, Stahl et al., 2009). The B-type CLE45 binds to the LRR-RLK BARELY ANY MERISTEM3 (BAM3) and inhibits protophloem differentiation (figure 3)(Depuydt et al., 2013). CLE45 treatment leads to a short root and small root apical meristem phenotype, like in the brevis radix (brx) mutant, suggesting that CLE45/BAM3 antagonizes BRX. BRX, CLE45 and BAM3 may regulate the timing when protophloem cells switch from proliferation to differentiation (Depuydt et al., 2013).
4.2.3 IDA peptide
INFLORESCENCE DEFICIENT IN ABSCISSION (IDA) has been first described in floral organ abscission but also facilitates the emergence of a LRP through the overlaying cell layers. IDA acts cell-autonomously and binds to the LRR-RLKs HAE in the endodermis and HAE and HSL2 in the cortex and epidermis (figure 3)(Kumpf et al., 2013). In the endodermis, IDA induces the expression of cell wall remodelling enzymes (e.g. xyloglucan endotransglycosylases/ hydrolases (XTHs) and expansines (EXPs)) that make the rigid cell wall more flexible and allow the endodermis cells to accommodate the emerging LRP. In the cortex and epidermis, IDA induces the expression of pectin degrading enzymes that facilitates cell separation when the LRP crosses these layers. In floral organ abscission, IDA induces the heterodimerization of HAE/HSL2 and SERKs which form a signalling complex (Meng et al., 2016) suggesting the same to occur to LR development.
4.2.4 TOLS2/PIPL3 peptide
The peptide hormone TARGET OF LBD SIXTEEN 2 (TOLS2)/PAMP-INDUCED SECRETED PEPTIDE-LIKE 3 (PIPL3) (hereafter referred to as TOLS2) and its close homolog PLASMA MEMBRANE INTRINSIC PROTEIN 2 (PIP2) repress the specification and/or maintaining of LR founder cells in closely neighbouring LR founder cells (figure 3)(Toyokura et al., 2019). This lateral inhibition mechanism ensures the regular spacing of LRs along the main root axis. The reduced number of DR5::LUC sites and the decreased number of LRs and LRPs in all developmental stages upon TOLS2 excess suggest that TOLS2 reduces the number of LR founder cells and does not affect LRP development (Toyokura et al., 2019). The transcription factor LBD16 activates TOLS2 expression in LR founder cells downstream of auxin (Toyokura et al., 2019). The mature TOLS2 peptide has a length of eleven amino acid residues and is proline hydroxylated at the fourth residue (Toyokura et al., 2019). TOLS2 binds to the LRR-RLK RECEPTOR-LIKE KINASE 7 (RLK7)
in the neighbouring cells of a pair of LR founder cells and induces the expression of the transcription factor PUCHI. PUCHI has dual roles: downstream of LBD16, it is required for LRP development but downstream of TOLS2-RLK7, it inhibits LRI in cells that flank LR founder cells (Toyokura et al., 2019). The lateral inhibitory mechanism of TOLS2 is linked to the LR founder cell specification process in the OZ. In the OZ, the DR5::LUC expressing area comprises several cells although only a pair of LR founder cells become competent to form a LR (Toyokura et al., 2019). Sometimes, two prebranch sites in wild-type are closely spaced but eventually the prebranch site with the weakest DR5::LUC expression disappears (Toyokura et al., 2019). In contrast, these adjacent prebranch sites persist and remain expressing DR5::LUC in the rlk7 mutant indicating that TOLS2-RLK7 signalling is necessary for guaranteeing single LR founder cell patches, thus preventing irregularly spaced LRs (Toyokura et al., 2019). There is evidence that TOLS2 leads to RLK7 translocation to the vacuole which may lead to attenuation of TOLS2 signalling in the TOLS2-producing LR founder cells (Toyokura et al., 2019).
4.2.5 CEP5 peptide
Several peptide-encoding genes from the C-TERMINALLY ENCODED PEPTIDE (CEP) family are expressed during LR development. CEP peptides have been described for their role during nitrogen (N) starvation responses in the root (figure 3)(Ohyama et al., 2008) and the associated inhibition of LR elongation (figure 3)(Delay et al., 2013), but some members are suggested to function during LRI. CEP5 is expressed in the BM in PPP cells and to a less extent in the phloem cells and is suggested to be negatively regulated by auxin (Roberts et al., 2016). Reduced CEP5 expression leads to a strong increase in stage I LRP density and a faster progression through the LRP developmental stages while CEP5 overexpression or exogenous peptide treatment results in a decreased density of early stage LRP (Roberts et al., 2016). CEP5 overexpression also causes the development of pairs of LRPs in close proximity (Roberts et al., 2016). The LRR-RLK CEP RECEPTOR 1 (CEPR1)/XYLEM INTERMIXED WITH PHLOEM (XIP1) is the receptor of several CEP members (Tabata et al., 2014). CEPR1/XIP1 expression starts in the BM in PPP and phloem cells (Roberts et al., 2016). CEP5 is likely the ligand of CEPR1 because the cepr1 mutant is partially insensitive to CEP5 treatment (Roberts et al., 2016). The decreased density of total LRPs in cepr1 is in contrast with the cep5 phenotype and indicates that CEP5 is a negative regulator of CEPR1 and that CEPR1 signalling in PPP cells stimulates LRI in the XPP cells (figure 3)(Roberts et al., 2016).