of polyethylene films
plasmas on the physicochemical surface modification
Effect of isopropanol addition to N , Ar and air DBD
Academic year 2019-2020
Master of Science in de industriële wetenschappen: chemie
Master's dissertation submitted in order to obtain the academic degree of
Supervisors: Prof. dr. Rino Morent, Dr. Rouba Ghobeira
Student number: 01502387Ilia Goemaere
of polyethylene films
plasmas on the physicochemical surface modification
Effect of isopropanol addition to N , Ar and air DBD
Academic year 2019-2020
Master of Science in de industriële wetenschappen: chemie
Master's dissertation submitted in order to obtain the academic degree of
Supervisors: Prof. dr. Rino Morent, Dr. Rouba Ghobeira
Student number: 01502387Ilia Goemaere
Impact of COVID-19
March 2020 was marked by the introduction of measurements taken to combat/slow down the SARS-CoV-2-caused pandemic. These regulations were still enforced by the deadline of this master’s dissertation. Among these measurements, the access to University buildings was restricted for students. This meant that experiments could no longer be performed and no real-life contact between students and Ghent University personnel was possible from March onward. Despite the fact that all experiments were performed during the first semester and early on in the second semester, this pandemic still had an influence on the realization of this master’s thesis. As it was the intention to gradually collect the data and subsequently discuss it on-site as progress was being made in writing this master’s dissertation, immediate processing of the results and/or exporting the data to a personal storage space was not always done. With all computers not being connected to the internet or shut-off, there was no way of obtaining some of said data. More specifically, it concerned the created atomic force microscopy (AFM) images (and accompanying roughness values) to study the surface morphology of treated samples, and Lissajous figures and voltage-current waveforms to support the results of the electrical characterization of the DBD. Moreover, some of the high resolution XPS spectra were to be deconvoluted in order to gain insight on the types of bonds/functional groups present on the surfaces of (treated) samples, but this could not be performed as real-time/real-life assistance and experience was crucial. Only the AFM images were to be considered essential for the completion of this master’s dissertation.
In an effort to minimize the influence of the pandemic on this master’s thesis, it was decided to discuss what was expected from the AFM images and the expected surface functionalities – based on water contact angle (WCA) and X-ray photoelectron spectroscopy (XPS) results, and relevant literature –, and to leave out both the Lissajous figures and the voltage-current waveforms that were to be included as examples. In this way, a relatively complete work could be still delivered. All relevant information on the experiments is described where it would otherwise have been, with a reminder of the troubles caused by the pandemic in place where necessary.
This preamble was drafted in consultation between student and promotor, and approved by both parties.
Preface
This work was performed at the Department of Applied Physics under the supervision of dr. Rouba Ghobeira and Prof. dr. Rino Morent.
First, I want to briefly address the readers to give a background on why and how I structured my thesis the way it is. From the very beginning, I set out to write a readable dissertation that is not only clear to people with a general scientific background, but that also gives a nudge in the direction of seeing the bigger picture. Achieving this was quite a considerable task. In the end, all chapters were ordered in such a way that they organically flow from one to the other, which was at least one step towards my personal objective. The very first section may be a bit too introductory for most readers, but it serves the purpose as a refresher and context in which the following sections/chapters are set. Readers are strongly encouraged to reflect on the supplied information and apply it in other cases. A lot of effort went into writing this dissertation to the best of my abilities and I sincerely hope to have obtained my goals, all the while adhering to the general purpose and guidelines of a master’s thesis.
Several people deserve my genuine appreciation for helping me realize this thesis. My deepest thanks and gratitude go out to dr. Rouba Ghobeira, my main supervisor, who taught me how to use the DBD reactor and all surface characterization techniques, shared her wealth of knowledge, was always there when I had questions or wanted to discuss some of my results, provided valuable suggestions, was very supportive and was incredibly kind to me. Both Tim Egghe and Parinaz Saadat Esbah Tabaei also deserve words of appreciation for the expertise/tips they shared and for welcoming me with questions I had. Tim Poelman, thank you for solving any of the more technical problems with the equipment throughout this year. Of course, I also want to thank Prof. dr. Rino Morent for giving me the opportunity to perform my master’s thesis at the Research Unit Plasma Technology (RUPT) group. All other members of the group who supported or helped me, naturally deserve my gratitude, too. Finally, I want to express my appreciation for my family and friends who supported me over the span of this thesis.
Deze pagina is niet beschikbaar omdat ze persoonsgegevens bevat.
Universiteitsbibliotheek Gent, 2021.
This page is not available because it contains personal information.
Ghent University, Library, 2021.
Abstract
In this thesis, the influence of isopropanol addition (up to 5 g/h) to medium pressure argon, air and nitrogen dielectric barrier discharge (DBD) plasmas on the physicochemical surface properties of low density polyethylene (LDPE) is extensively investigated. Water contact angle (WCA) and X-ray photoelectron spectroscopy (XPS) measurements reveal that plasma-induced surface properties depend on the used working gas and discharge power. Despite increasing the surface oxygen content, an isopropanol addition to both argon and air plasmas results mainly in a decreased wettability compared to pure argon and air plasma treatments. However, nitrogen/isopropanol plasma treatments exhibit an extremely enhanced wettability at high and medium powers when compared to pure nitrogen plasmas. In fact, super-hydrophilic surfaces with WCAs reaching 0° are detected upon addition of 2,5 g/h and 5 g/h of isopropanol at high power. XPS results show an increase in the incorporation of both nitrogen and oxygen functionalities, suggesting the occurrence of a plasma polymerization. Fourier transform infrared spectroscopy (FTIR) results come to confirm this hypothesis as they reveal the deposition of a film. Analysis of FTIR spectra suggests that the plasma-polymerized films mostly consist of amides, amines, ketones and oximes/imines. Ageing experiments reveal that only nitrogen/isopropanol plasmas at high or medium power trigger a retention of the (enhanced) wettability. Overall, nitrogen/isopropanol plasma treatments at optimized power setting prove themselves useful in the long-term enhancement of polymeric surface energy.
Keywords: dielectric barrier discharge (DBD), isopropanol addition, low density polyethylene (LDPE) film, ageing, plasma treatment.
Effect of isopropanol addition to N
₂
, Ar and air DBD
plasmas on the physicochemical surface modification
of polyethylene films
Ilia Goemaere
Supervisors: dr. Rouba Ghobeira and Prof. dr. Rino Morent
Abstract—In this thesis, the influence of isopropanol addition
(up to 5 g/h) to medium pressure argon, air and nitrogen dielectric barrier discharge (DBD) plasmas on the physicochemical surface properties of low density polyethylene (LDPE) is extensively investigated. Water contact angle (WCA) and X-ray photoelectron spectroscopy (XPS) measurements reveal that plasma-induced surface properties depend on the used working gas and discharge power. Despite increasing the surface oxygen content, an isopropanol addition to both argon and air plasmas results mainly in a decreased wettability compared to pure argon and air plasma treatments. However, nitrogen/isopropanol plasma treatments exhibit an extremely enhanced wettability at high and medium powers when compared to pure nitrogen plasmas. In fact, super-hydrophilic surfaces with WCAs reaching 0° are detected upon addition of 2,5 g/h and 5 g/h of isopropanol at high power. XPS results show an increase in the incorporation of both nitrogen and oxygen functionalities, suggesting the occurrence of a plasma polymerization. Fourier transform infrared spectroscopy (FTIR) results come to confirm this hypothesis as they reveal the deposition of a film. Analysis of FTIR spectra suggests that the plasma-polymerized films mostly consist of amides, amines, ketones and oximes/imines. Ageing experiments reveal that only nitrogen/isopropanol plasmas at high or medium power trigger a retention of the (enhanced) wettability. Overall, nitrogen/isopropanol plasma treatments at an optimized power-setting prove themselves useful in the long-term enhancement of polymeric surface energy.
Keywords—dielectric barrier discharge (DBD), isopropanol
addition, low density polyethylene (LDPE) film, ageing, plasma treatment.
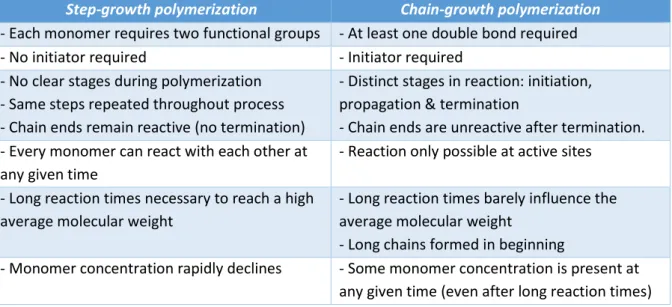
I. INTRODUCTION
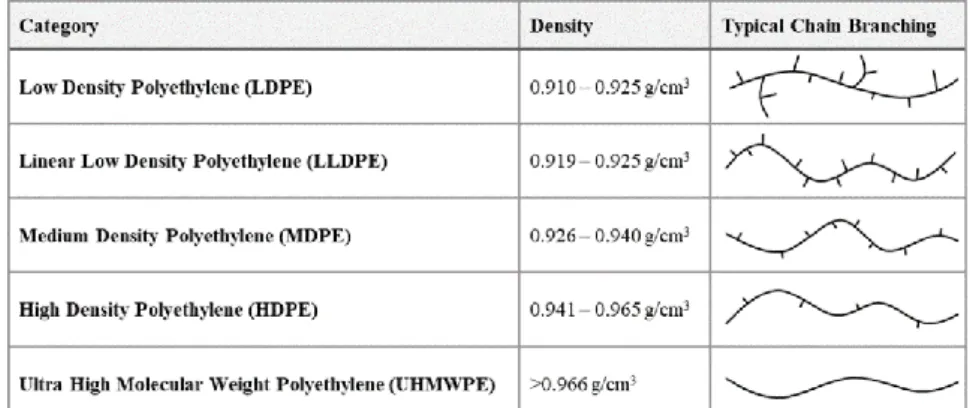
Polyethylene (PE) is often selected for a variety of applications based on its excellent material properties (e.g. toughness, chemical resistance and flexibility). [1] However, given its low surface energy, PE is unsuitable for most applications where its surface is involved in bonding, adhesion, printing, etc. [2] Luckily, a plethora of surface modification techniques can impart the desired properties on the PE surface while maintaining its bulk properties. [3]
Among them, plasma treatments are an excellent choice because of their relatively low energy consumption, near absence of (toxic) waste and flexibility. [4] Commonly used, are dielectric barrier discharges (DBDs), which are usable across a wide pressure range and are reactors presenting a good scalability (relative to other implementations). [2, 5]
Plasma treatments using simple gaseous molecules (e.g. O2,
N2, NH3 and CO2) have been shown to introduce vast amounts
groups/esters and peroxides) on polymer surfaces by the interaction between the active plasma species and substrates, enhancing the surface energy by plasma activation. [5, 6] However, the ameliorated wettability partially reverts back to its original state over time in a process called ageing. [3]
Therefore, solvent vapors of more complex molecules are sometimes added to achieve plasma polymerization, which generally yields a very stable amorphous and highly functionalized/crosslinked deposited film with superior ageing properties. [6, 7] Alcohol vapor addition is of specific interest since most common alcohols (e.g. ethanol and isopropanol) are considered to be environmentally safe/green and relatively cheap solvents, with their hydroxyl group potentially triggering an (extensive) incorporation of oxygen functionalities – likely leading to an enhanced wettability. [2, 7-9]
In this thesis, the influence of (up to 5 g/h) isopropanol vapor addition to various carrier gases on the physicochemical surface modifications of a low density polyethylene (LDPE) film treated by a medium pressure DBD at different discharge powers, is extensively investigated.
To do so, several surface analysis techniques are used of which water contact angle (WCA) measurements to evaluate the wettability, X-ray photoelectron spectroscopy (XPS) to analyse the surface atomic composition, Fourier transform infrared spectroscopy (FTIR) to study the type of functional groups on the potentially deposited plasma-polymerized films and atomic force microscopy (AFM) to gain information on the surface morphology. The ageing effect of treated LDPE exposed to air is also evaluated using WCA and XPS. All this is preceded by a brief study on the influence of the discharge power and the treatment time, with the purpose of defining standard treatment times for different power-settings.
II. MATERIALS & METHODS
A. Polyethylene
Acquired from Goodfellow Cambridge Ltd., the substrate subjected to the different plasma treatments was a linear low density polyethylene (LDPE) foil with a thickness of 0,18 mm (± 20%). The LDPE film that did not undergo any treatments prior to the plasma treatments, is biaxially oriented and additive free. All samples were cut in rectangular pieces of roughly 1 cm x 5 cm.
B. DBD set-up and characterization
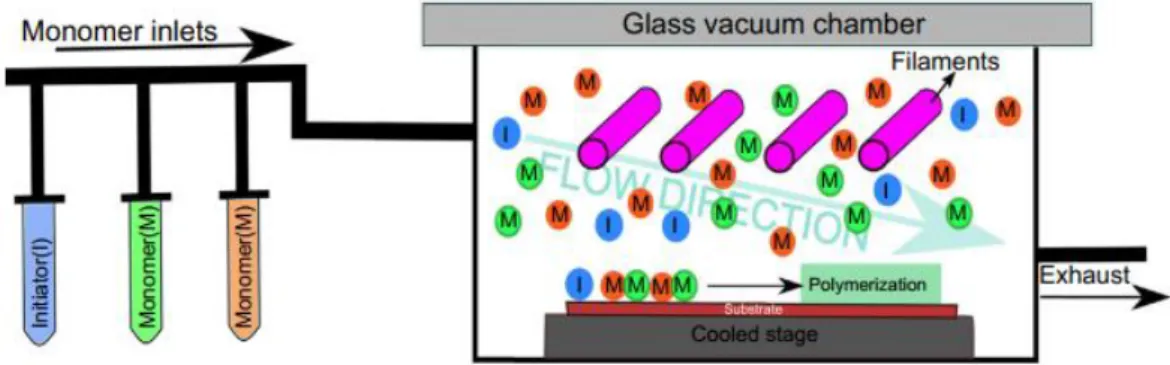
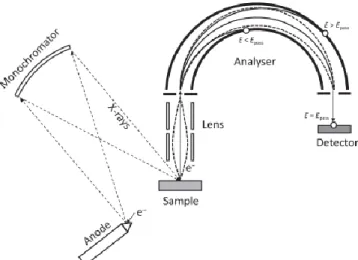
Plasma treatments of the LDPE samples were performed using an in-house built DBD reactor (Figure II-1). Encompassed by a cylindrical enclosure, the discharge itself takes place between two copper electrodes (diameter = 4 cm). Each of the electrodes is covered with a ceramic (Al2O3) plate
(5 cm x 5 cm – thickness = 0,7 mm) serving as the dielectric. The inter-electrode distance is 4 mm. Connected to the upper electrode is the high frequency (kept constant at 50 kHz) AC power source, while the lower electrode is grounded. The samples that were cut out of the LDPE foil were placed in the center of the lower electrode with the long side parallel to the gas inlet.
Once the sample was placed in the reactor, the pressure in the discharge chamber was pumped down to 3 mbar and consequently filled with the pure selected carrier gas at 3 standard litres per minute (slm) until a pressure of 850 mbar was reached, at which the discharge chamber was purged for 3 min. Upon completion of the purging step, the reactor was pumped down to a pressure of 50 mbar after setting the flow rate to 1 slm. Carrier gases used in this thesis were argon, (dry) air and nitrogen (Air Liquide – Alphagaz 1).
After reaching 50 mbar, the desired isopropanol (>99,99% purity, Sigma-Aldrich) vapor flow rate was added to the selected carrier gas (total gas flow of 1 slm). The isopropanol vapor flow rates were varied between 0 g/h and 5 g/h at four points: 0 g/h, 0,5 g/h, 2,5 g/h and 5 g/h. Addition of the isopropanol vapor was obtained by using a CEM® (Controlled Evaporation and Mixing) system purchased from Bronkhorst.
Once the desired gas/vapor mixture was achieved, the AC power supply was turned on, initiating the treatment. After finishing the treatment, the mixture flow rate was set to 0 slm and the reactor was pumped down to 3 mbar, followed by opening the reactor valve and removing the sample.
Electrical characterization of the DBD was attained in order to evaluate the discharge modes and powers the DBD operated at. Voltage-current waveforms were obtained by measuring the applied high voltage [1000:1 high voltage probe (Tektronix P6915A) connected to the upper electrode] and the discharge current [across a 50 Ω resistor connected in series to the lower electrode and the ground (Figure II-1)]. A digital oscilloscope (Picoscope 3204A) recorded both waveforms, which were then visualized using Picoscope 6 software. Discharge powers were obtained by replacing the 50 Ω resistor with a 10,4 nF capacitor and multiplying the area of the Lissajous figure with the AC frequency.
C. Surface characterization
The evaluation of the wettability was performed by static WCA measurements under ambient laboratory conditions using a KRÜSS Easy Drop system (KRÜSS GmbH). Distilled water droplets (2 µl) were deposited on the surface, after which the WCAs were obtained by applying Laplace-Young fitting. The reported values are the average and the standard deviation of the WCAs of 6 droplets deposited on different locations per sample.
Detailed quantitative information about the surface atomic composition of the (un)treated LDPE was gained by employing a PHI 5000 Versaprobe II spectrometer (XPS-system). The device is equipped with a monochromatic Al Kα x-ray source (hν = 1486,6 eV) and operates at a power of
25 W. A surface area of 500 x 500 µm² was irradiated by an incident X-ray beam (size = 100 µm) under an angle of 45° with respect to the sample surface, just like the detector. The XPS main chamber was unceasingly held at a pressure < 10-7
mbar when in operation. A pass energy of 187,85 eV and step size of 0,8 eV was set for survey scans, which were analysed using the Multipak (version 9.6) software. During the analysis, Shirley background is considered in accordance to the relative sensitivity parameters provided by the spectrometer manufacturer. All binding energy scales were calibrated with regards to the hydrocarbon component of the C1s spectrum, located at 285 eV. The reported values are the average and standard deviation of 4 measurements taken at 4 random surface locations per sample.
A Bruker Tensor 27 (Bruker) spectrometer fitted with a single reflection ATR accessory (germanium crystal; MIRacle™ Pike technology) was used to perform FTIR measurements. The spectra (4 cm-1 resolution) were recorded
using a liquid nitrogen cooled MCT-detector (mercury – cadmium – telluride). All acquired spectra are the result of the integration of 64 scans and three separate measurements.
Analysis of the surface morphology of the LDPE samples was conducted using an XE-70 AFM (Park Systems) with a silicon cantilever (NANOSENSORS™ PPP-NCHR). The scan size was set to 15 µm by 15 µm and the images were recorded in noncontact mode. The surface roughness was evaluated using XEP software, after performing an X- and Y- plane autofit procedure. Should the surface roughness values have been reported, they would have been the average of three measurements taken at different sample locations.
III. RESULTS & DISCUSSION
A. Discharge powers and treatment times
In order to investigate the influence of the discharge power on the effect of isopropanol addition, three different powers for each working gas were defined: high power (HP), medium power (MP) and low power (LP). LP was the minimal power for the plasma to be formed, HP was the highest power reached before plasma becomes not contained between the electrodes anymore (e.g. showed arcing to the cylindrical electrodes enclosure) and the MP was in between the other 2 powers. Each power for every individual power-setting was chosen in such a way that it is somewhat similar for every carrier gas. Table I presents the selected average powers for each setting following from the discharge power determination. No HP-setting was defined for argon as no comparable powers to the HP-settings of air and nitrogen could be reached because of plasma arching.
Figure II-1 Schematic diagram of the DBD reactor (1 - Carrier gas
cylinder; 2 - Carrier gas mass flow controller; 3 - Isopropanol container; 4 – Isopropanol liquid flow controller; 5 - CEM® system; 6 - DBD reactor; 7 - Pressure gauge; 8 – Valve; 9 - Pump). [4]
Table I Summary of used average powers for each working gas and
power-setting.
Setting Argon Air Nitrogen
HP – 15,1 W 17,2 W
MP 4,2 W 8,4 W 7,2 W
LP 2,5 W 2,3 W 1,8 W
The voltage-current characteristics corresponding to every working gas/power-setting combination, indicated that the discharge modes were either pseudoglow or glow, implying that a homogenous surface treatment could be achieved, which is desirable. [2] Moreover, these results corroborate the obtained discharge modes that Van Deynse et al. [2] obtained using the exact same DBD set-up but with the addition of similar ethanol vapor concentrations.
Due to the ongoing pandemic at the time of writing, the already created Lissajous figures, and voltage-current waveforms could not be extracted from the laboratory’s computer, making it impossible to show them here.
Having defined the powers to be used for every carrier gas, the influence of the treatment time could now be analysed.
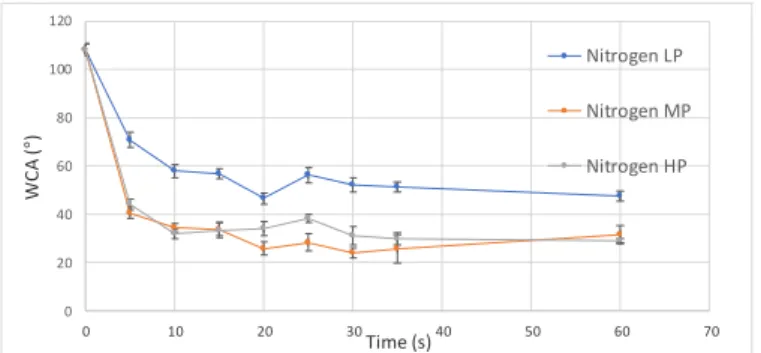
Figure III-1 shows the WCA changes in function of treatment
time for LDPE treated with a nitrogen plasma at the three power-settings. Very similar trends were observed for all argon and air plasmas at every power-setting.
Untreated LDPE had a WCA of 108,1 ± 2,42°. Shortly after the LDPE came into contact with a plasma, a sharp decrease in WCA was noticed, until a constant WCA was reached, indicating that the surface became saturated with incorporated functionalities and longer treatment times were of no use. [10] A relatively stable WCA was comfortably reached after 20 to 30 s for all carrier gas/power-setting combinations, which made it possible to define standard treatment times. However, to ensure a more objective comparison between the different plasmas at every power-setting, constant energy densities (EDs) were defined in such a way that the saturated WCA region was reached.
Table II gives an overview of the defined EDs for the three
power-settings and the WCAs of the different corresponding plasma treatments, which are similar to the ones measured by Cools et al. [10] A dependability on the working gas regarding the saturated WCAs is clearly implied. Moreover, the obtained WCAs indicate an enhanced wettability and presence of a large number of incorporated hydrophilic groups when the LDPE was plasma treated, regardless of the carrier gas or power-setting. [5] Both XPS and AFM measurements were also performed and gave more insight into the processes giving rise to the enhanced wettability, but these will be cited later on (where applicable) in combination with the
Table II Overview of the selected energy densities and
corresponding WCAs for each working gas/power-setting.
Setting ED Argon Air Nitrogen
HP 30 J/cm² – 61,6 ± 2,56° 31,7 ± 2,08°
MP 15 J/cm² 40,2 ± 2,07° 36,5 ± 2,44° 25,7 ± 2,68°
LP 5 J/cm² 41,3 ± 2,31° 48,0 ± 1,96° 46,4 ± 2,13°
B. Influence of isopropanol addition on wettability, surface composition and surface morphology
1) WCA measurements
The examination of the effect of isopropanol addition on the surface properties of treated LDPE was attained by using static WCA, XPS and AFM measurements. The treatments were identical to the ones previously defined using constant EDs. Every treatment was performed four separate times using the following isopropanol flow rates: 0 g/h, 0,5 g/h, 2,5 g/h and 5 g/h. Only four different isopropanol flow rates were used as to limit the amount of measurements. Figure III-2 presents the change in WCA in function of the isopropanol flow rate for the different carrier gases and discharge powers.
As mentioned earlier, pure carrier gas plasma treatments of LDPE already enhanced the wettability of LDPE significantly, which follows from the vast reduction in WCAs (Table II) but can also be observed in Figure III-2.
Regarding the use of argon as carrier gas, the addition of isopropanol at any of the flow rates and powers reduced the wettability of the LDPE films when compared to pure argon plasma treated samples. The increase in WCA was most noticeable with an isopropanol flow rate of 2,5 g/h for an MP plasma treatment. Both flow rates of 0,5 g/h and 5 g/h resulted in less dramatic WCA increases. At LP, the effect of isopropanol addition on the increase of the WCAs was more or less the same for any isopropanol flow rate. The reduced wettability was smaller than at MP when compared to pure argon plasma treated samples at their respective powers.
Isopropanol addition to an air plasma resulted in different effects. First and foremost, at HP, only the addition of 2,5 g/h of isopropanol significantly decreased the WCA with respect to a pure air HP plasma treatment – albeit it a small decrease – , whereas both the addition of 0,5 g/h and 5 g/h of isopropanol vapor resulted in an insignificant increase in the WCA. All used isopropanol flow rates with an air plasma at MP yielded a slightly increased WCA. At LP, the addition of isopropanol seemed to marginally improve wettability.
The most substantial effects were obtained when adding isopropanol to a HP or MP nitrogen plasma – all WCAs for every added concentration (including 0 g/h) are more or less the same at LP. In fact, the introduction of 0,5 g/h of 0 20 40 60 80 100 120 0 10 20 30 40 50 60 70 W C A (° ) Time (s) Nitrogen LP Nitrogen MP Nitrogen HP
Figure III-2 Evolution of the WCA of plasma treated LDPE in
function of the flow rate of isopropanol added to the pure carrier gas.
Figure III-1 Evolution of the WCA of nitrogen plasma treated LDPE
isopropanol vapor to a pure nitrogen HP plasma, triggered a 90% reduction in WCA compared to untreated LDPE, which was an extra 68% decrease with respect to a pure nitrogen plasma treatment. A complete wettability (100% reduction in WCA) was obtained by adding both 2,5 g/h and 5 g/h of isopropanol vapor to the pure HP nitrogen plasma – the WCAs were below the limit of quantification, thus they were recorded as 0°. Irrespective of the isopropanol flow rate added to an MP nitrogen plasma, a 87% decrease in WCA was achieved with respect to untreated LDPE. This was an additional 52% decrease relative to pure nitrogen plasma treated LDPE at MP.
2) XPS measurements
All WCA results of plasma treated samples were a first indication of a functionalized hydrophilic surface. [2] XPS measurements were performed to further investigate the changes in the surface atomic compositions which led to the increased wettability. The multitude of results makes it impossible to present all of them here graphically/in tables, which is why they will be discussed with respect to the surface composition of the pure carrier gas plasma treated samples and the untreated sample (Table III).
It has to be noted that the untreated LDPE did not solely consist out of carbon atoms. A small amount of nitrogen (0,40 ± 0,39%) and oxygen (4,06 ± 0,32%) is also present on the surface. Most likely, this can be attributed to surface contaminations (e.g. by water vapor). [2]
Pure argon plasma treatments led to a significant increase in oxygen surface content, with the nitrogen surface content being equal to that of untreated LDPE. Possible sources of the oxygen content are residual air in the plasma chamber, post-treatment oxidation, desorbed molecules from the reactor wall and working gas impurities. [5, 11] Upon addition of isopropanol, an increase in incorporated oxygen was noticed while the nitrogen content remained the same (both at MP & LP) – with respect to a pure argon plasma treatment. This increase in oxygen content can be attributed to the oxygen atom present in the added isopropanol. [7] In any of the cases, the improved wettability is thus caused by an increase in surface oxygen functionalities. However, despite increasing the oxygen content, argon/isopropanol treatments negatively impacted the wettability, regardless of the discharge power.
Very similar atomic surface compositions to a pure argon plasma treatment were obtained using a pure air plasma at MP and LP. However, at HP, there was an increase in both the nitrogen and oxygen content (with regards to MP- and LP-treatments), caused by the higher density of active species. [12] The minor increase in nitrogen on the surface is caused
by the nitrogen fraction in the air carrier gas. [11] Again, an increase in oxygen content was perceived upon addition of isopropanol, with more oxygen incorporated and smaller negative impact on the WCA as the discharge power increased.
Applying a pure nitrogen plasma treatment led to an increase in nitrogen-containing groups, with more nitrogen incorporated as the power increases due to higher active species densities. [12] All samples showed a similar (increased) oxygen content, with the same origins as described before. The (high) polarity of nitrogen-containing functional groups and the net increase in oxygen relative to the other carrier gas plasma treatments, led to the observed better wettability. The addition of isopropanol led to considerable increases in both surface oxygen and nitrogen content. Only at HP and MP, this induced a strongly enhanced wettability. The surface content of 0,5 g/h and 2,5 g/h isopropanol addition to an MP nitrogen plasma treatment correlated well with the excellently improved wettability (strong increases in N & O), contrary to the results obtained after 5 g/h of isopropanol addition at MP, and all nitrogen/isopropanol mixtures at HP (smaller N & O increases).
The deconvolution of the XPS spectra would have been able to explain the observations in more detail, but was not performed, partly due to the ongoing pandemic at the time of writing. A literature review did provide some insight, though.
Common incorporated oxygen functionalities are C-O (hydroxyl, ether), C=O (carbonyl) and O-C=O (carboxyl, ester), while C-NHx (amines), C=N (imines/oximes) and
N-C=O (amides) are commonly introduced nitrogen functionalities, all enhancing surface wettability. [2, 5] The addition of isopropanol favors the incorporation of ethers ketones and esters instead of hydroxyl, aldehydes or carboxyl groups, thus lowering the polarity and wettability. [13, 14] This effect is believed to be rendered almost negligible by increasing the discharge power (i.e. more fragmentation) or incredible crosslinking densities at the top atomic layers. [7, 15-17]
3) AFM measurements
While all AFM measurements were performed, the resulting images were not able to be processed. The ongoing pandemic at the time of writing restricted access to them. Therefore, what was expected from the AFM images will be discussed based on the WCA and XPS results, and available literature. This is all rather hypothetical and has its limits as such.
Previous studies have shown that both pure argon and nitrogen plasmas have a (very) limited etching effect that is normally enhanced by the presence of oxygen and higher discharge powers. [11, 12, 18] Thus, only a slightly rougher surface is expected for these treatments – except at HP, where the discharge power might have been strong enough to evenly etch surfaces (i.e. smoothing it), albeit no longer uniformly in some cases, which decreased the wettability. [19]
Oxygen-containing plasmas have been shown to readily etch polymer surfaces, increasing the surface roughness and thus having a positive effect on surface wettability – if the discharge power is not too high. [18]
Discussing the effects of the addition of a monomer vapor to various plasmas is infinitely more complex and largely dependent on the operating parameters, reactor set-ups, monomer and whether or not plasma polymerization occurs – which, in turn, depends on the operating parameters. [6]
Table III Overview of atomic surface compositions after pure carrier
One aspect that seems to be rather constant, is the enhanced etching effect by oxygen atoms in plasmas, which is why the oxygen-containing isopropanol could also result in slightly roughened LDPE surfaces. [18] This has been confirmed for an ethanol addition using the same set-up, operating parameters, substrate and carrier gases as long as no plasma polymerized film was deposited. In the case of film deposition, the surface became smoother. [2] However, if the discharge power was too high, a similar roughness or even a mildly rougher surface than untreated samples was observed. [8] Moreover, plasma polymerization of isopropanol and subsequent film deposition to treat a polystyrene dish, has been shown to faintly increase the surface roughness. [13] No clear hypothesis can be made.
C. Evaluation of film deposition
The detection of potential film deposition and the analysis of its chemical structure were performed using FTIR. The expected penetration depth is approximately 600 nm with the used set-up, which is deeper than with XPS and is why film deposition can be studied. [8] All carrier gas/isopropanol plasma treatments were performed for two min instead of the previously defined standard treatment times, with the goal of obtaining sufficient film growth. Film deposition is proof of elaborate plasma polymerization. [2]
Of all performed treatments, only nitrogen/isopropanol discharges at HP had clear changes in the FTIR spectra for all added isopropanol flow rates, with the most extreme result for an isopropanol addition of 2,5 g/h (Figure III-3). This suggests that only nitrogen/isopropanol HP plasma treatments resulted in plasma polymerization, while all other treatments were only capable of plasma activation. [2]
The broad absorption band (Figure III-3) at 3300 cm-1 can
be attributed to OH and NH stretching vibrations. Between 1750 cm-1 and 1500 cm-1, there is another broad peak with a
maximum at. 1660 cm-1 and a shoulder-type maximum at
1550 cm-1, denoting C=O/C=N, and NH bending vibrations,
respectively. [20] This suggests that the deposited coating mostly consisted out of amides, amines, ketones and oximes/imines, corroborating previously made assumptions. Bear in mind that the low resolution spectra are the result of all peaks, some with intrinsic weak intensities, superimposed on each other, which is why there is probably a variety of other functional groups also present in the coating, albeit in smaller numbers.
D. Ageing
Well established, is the phenomenon in which the imparted surface hydrophilicity reverses, to some degree, back to the untreated state after plasma treatment in a process called ageing or hydrophobic recovery. [2, 5] As the focus lies on the creation of a (stable) hydrophilic LDPE surface, ageing is undesirable.
The investigation of the ageing effect was performed by storing the treated samples in ambient laboratory conditions for a period of 1 day and 7 days and analyzing them by means of WCA and XPS measurements.
Because of the plethora of results, only a representative figure for the WCA results of the aged samples treated with an isopropanol addition of 5 g/h is shown here (Figure III-4).
On a general note, all WCAs obtained after ageing were still notably lower than the WCA of untreated LDPE (108,1 ± 2,42°), thus showing a retention of the wettability to some degree. For treated samples showing pronounced ageing, most of the increase in WCA seemed to happen in the first day of exposure to air. Moreover, the reduction in wettability can be more or less quantified by using the loss in treatment efficiency L (%):
where θs1 is the saturated WCA after plasma treatment, θs2 is
the reached WCA after storage under ambient conditions and θuntreated is the WCA of the untreated substrate.
From Figure III-4 and data not shown here, it followed that isopropanol addition to argon and air plasmas did not positively influence the ageing behaviour. This was reflected by the losses in treatment efficiency and a reduction in surface oxygen content over time, the latter explaining the increase in WCA. This was to be expected, as plasma activation does not lead to an extensively crosslinked surface, which would reduce chain mobility and thus diffusion/reorientation of polar functionalities. [7] However air/isopropanol treatments at MP and LP did show slightly improved and strongly worsened Ls. Likely, this is the result of a more crosslinked/stable surface and higher driving forces caused by an increased oxygen incorporation. [21]
Notwithstanding the changed surface compositions, both HP and MP nitrogen/isopropanol treated samples showed no ageing at all after storage under ambient condition for 7 days for all added isopropanol flow rates (Ls ≤ 0%). Only extreme crosslinking densities at the top atomic layers, quite certainly linked to plasma polymerization, can cause this. [16, 17] This raises some questions regarding the used treatment time for
Figure III-4 Evolution of the WCA (°) of plasma treated LDPE
samples subjected to the different plasma treatments with an isopropanol addition of 5 g/h in function of the storage time.
Figure III-3 FTIR spectra of untreated LDPE and LDPE treated for
2 min with a HP nitrogen/isopropanol plasma.
𝐿 = 𝜃𝑠2− 𝜃𝑠1 𝜃𝑢𝑛𝑡𝑟𝑒𝑎𝑡𝑒𝑑 − 𝜃𝑠1
the FTIR measurements, as those only confirmed plasma deposition for nitrogen/isopropanol HP plasma treatments. LP-treatments with any of the nitrogen/isopropanol mixtures, however, gave rise to Ls and WCA plateaus almost identical to those described for a pure nitrogen LP-treatment, and isopropanol addition to air and argon at LP (Ls ≈ 25%).
IV. CONCLUSION
In this master thesis, the effects of the addition of isopropanol vapor to medium pressure nitrogen, argon and air DBD plasmas on the physicochemical surface properties of LDPE films have been investigated. This was achieved by an evaluation of the wettability (WCA), an analysis of the surface atomic composition (XPS), a study of surface morphology (AFM) and an evaluation of the type of functional groups on the potentially deposited plasma-polymerized films (FTIR). AFM measurements were hypothesised based on relevant literature, as the results became unavailable due to the ongoing pandemic at the time of writing.
Analysis of the effect of treatment time and discharge power revealed that treating LDPE for longer than 20 s resulted in a constant WCA, regardless of carrier gas and power-setting. Thus, standard treatment times based on constant EDs were defined for all ensuing experiments, eliminating treatment time as a parameter to study.
Despite the detected increased surface oxygen content, isopropanol addition to both argon and air plasmas resulted in a decreased wettability (except for an isopropanol addition of 2,5 g/h) at HP, relative to pure argon and air plasma treatments.
Nitrogen/isopropanol plasma treatments, however, exhibited an extremely enhanced wettability at HP and (slightly less at) MP in comparison to the corresponding pure nitrogen plasma treatments. In fact, super-hydrophilic surfaces with WCAs reaching 0 ° were detected upon addition of 2,5 g/h and 5 g/h of isopropanol at HP. XPS results showed an increase in the incorporation of both nitrogen and oxygen functionalities, suggesting the occurrence of a plasma polymerization. FTIR results came to confirm this hypothesis as they revealed the deposition of a film. Analysis of FTIR spectra suggested that the plasma-polymerized films mostly consisted of amides, amines, ketones and oximes/imines.
Ageing experiments revealed that only nitrogen/isopropanol plasmas at high or medium power trigger a retention of the (enhanced) wettability.
Overall, nitrogen/isopropanol plasma treatments at an optimized power-setting prove themselves useful in the long-term enhancement of polymeric surface energy.
ACKNOWLEDGEMENTS
This master’s dissertation was performed at the Department of Applied Physics under the supervision of dr. Rouba Ghobeira and Prof. dr. Rino Morent, whom I would like to thank for their guidance.
REFERENCES
[1] S. Ronca, "Polyethylene," in Brydson's Plastics Materials, 2017, pp. 247-278.
[2] A. Van Deynse, R. Morent, C. Leys, and N. De Geyter, "Influence of ethanol vapor addition on the surface modification of polyethylene in a dielectric barrier discharge," Applied Surface
[3] S. K. Nemani et al., "Surface Modification of Polymers: Methods and Applications," Advanced Materials Interfaces, vol. 5, no. 24, 2018.
[4] H. Dave, L. Ledwani, and S. K. Nema, "Nonthermal plasma: A promising green technology to improve environmental performance of textile industries," in The Impact and Prospects of Green Chemistry for Textile Technology, 2019, pp. 199-249. [5] N. De Geyter, C. p. Leys, and R. p. Morent, "Plasma modification
of polymer surfaces in the subatmospheric pressure range," 2008., 2008.
[6] R. Bitar, P. Cools, N. De Geyter, and R. Morent, "Acrylic acid plasma polymerization for biomedical use," Applied Surface Science, vol. 448, pp. 168-185, 2018.
[7] K. S. Siow, L. Britcher, S. Kumar, and H. J. Griesser, "Plasma Methods for the Generation of Chemically Reactive Surfaces for Biomolecule Immobilization and Cell Colonization - A Review," Plasma Processes and Polymers, vol. 3, no. 6‐7, pp. 392-418, 2006/08/15 2006.
[8] A. Van Deynse, C. Leys, R. Morent, and N. De Geyter, "Plasma Polymerization in a Nitrogen/Ethanol Dielectric Barrier Discharge: A Parameter Study," Plasma Chemistry and Plasma Processing, vol. 39, no. 5, pp. 1317-1342, 2019.
[9] F. P. Byrne et al., "Tools and techniques for solvent selection: green solvent selection guides," Sustainable Chemical Processes, vol. 4, no. 1, p. 7, 2016/05/23 2016.
[10] P. Cools, S. Van Vrekhem, N. De Geyter, and R. Morent, "The use of DBD plasma treatment and polymerization for the enhancement of biomedical UHMWPE," Thin Solid Films, vol. 572, pp. 251-259, 2014/12/01/ 2014.
[11] N. De Geyter et al., "Plasma modification of polylactic acid in a medium pressure DBD," Surface and Coatings Technology, vol. 204, no. 20, pp. 3272-3279, 2010/07/15/ 2010.
[12] J. M. Grace and L. J. Gerenser, "Plasma Treatment of Polymers," Journal of Dispersion Science and Technology, vol. 24, no. 3-4, pp. 305-341, 2003.
[13] S. A. Mitchell, M. R. Davidson, N. Emmison, and R. H. Bradley, "Isopropyl alcohol plasma modification of polystyrene surfaces to influence cell attachment behaviour," Surface Science, vol. 561, no. 1, pp. 110-120, 2004/07/10/ 2004.
[14] D. C. Guerin, D. D. Hinshelwood, S. Monolache, F. S. Denes, and V. A. Shamamian, "Plasma Polymerization of Thin Films: Correlations between Plasma Chemistry and Thin Film Character," Langmuir, vol. 18, no. 10, pp. 4118-4123, 2002/05/01 2002. [15] J. Friedrich, G. Kühn, R. Mix, A. Fritz, and A. Schönhals,
"Polymer surface modification with monofunctional groups of variable types and densities," Journal of Adhesion Science and Technology, vol. 17, no. 12, pp. 1591-1617, 2003/01/01 2003. [16] R. C. Chatelier, X. Xie, T. R. Gengenbach, and H. J. Griesser,
"Effects of plasma modification conditions on surface restructuring," Langmuir, vol. 11, no. 7, pp. 2585-2591, 1995/07/01 1995.
[17] H. J. Griesser, Y. Da, A. E. Hughes, T. R. Gengenbach, and A. W. H. Mau, "Shallow reorientation in the surface dynamics of plasma-treated fluorinated ethylene-propylene polymer," Langmuir, vol. 7, no. 11, pp. 2484-2491, 1991/11/01 1991.
[18] A. Terriza, R. Alvarez, A. Borras, J. Cotrino, F. Yubero, and A. Gonzalez-Elipe, "Roughness assessment and wetting behavior of fluorocarbon surfaces," Journal of colloid and interface science, vol. 376, pp. 274-82, 03/22 2012.
[19] M. Minařík et al., "Preparation of Hierarchically Structured Polystyrene Surfaces with Superhydrophobic Properties by Plasma-Assisted Fluorination," Coatings, vol. 9, no. 3, 2019. [20] M. A. Mohamed, J. Jaafar, A. F. Ismail, M. H. D. Othman, and M.
A. Rahman, "Fourier Transform Infrared (FTIR) Spectroscopy," in Membrane Characterization, 2017, pp. 3-29.
[21] U. Oran, S. Swaraj, J. F. Friedrich, and W. E. S. Unger, "Surface Analysis of Plasma Deposited Polymer Films, 5," vol. 2, no. 7, pp. 563-571, 2005.
Invloed van isopropanol additie bij N
2
, Ar en lucht
DBD-plasma's op de fysicochemische
oppervlaktebehandeling van polyethyleen folies
Ilia Goemaere
Begeleiders: dr. Rouba Ghobeira en Prof. dr. Rino Morent
Abstract—In deze thesis wordt de invloed van tot 5 g/h
isopropanoldamp toegevoegd aan een medium druk argon, lucht en stikstofgasdieëlektrische barrière ontlading (DBD) plasma’s op de fysicochemische oppervlaktebehandeling van lage dichtheid polyethyleen (LDPE) bestudeerd. Watercontacthoek- (WCA) en X-straal foto-elektron spectroscopie- (XPS) metingen onthullen dat het effect van isopropanoladditie zowel van het dragergas als het ontladingsvermogen afhankelijk is. Ondanks een toename in oppervlaktezuurstofgehalte, resulteert isopropanoladditie bij argon en lucht plasma’s voornamelijk in een verlaagde bevochtbaarheid in vergelijking met pure argon en lucht plasmabehandelingen. Stikstofgas/isopropanol plasma-behandelingen, daarentegen, vertonen een sterk toegenomen bevochtbaarheid op hoog en medium vermogen in vergelijking met de overeenkomstige pure stikstofgas plasmabehandelingen. Hier bereikt de WCA zelfs 0° bij additie van 2,5 g/h en 5 g/h aan isopropanol op hoog vermogen. XPS-metingen tonen een stijging in zuurstof- en stikstofhoudende functionaliteiten, wijzend op plasmapolymerisatie. Fouriertransformatie infrarood-spectroscopie (FTIR) bevestigt deze hyopthese door het aantonen van filmdepositie. Analyse van de spectra indiceert de aanwezigheid van voornamelijk amiden, ketonen en oximen/iminen. Verouderingsexperimenten onthullen dat enkel stikstofgas/isopropanol plasmabehandelingen op hoog en medium vermogen leiden tot een verbeterde retentie van het aangebrachte hydrofiel karakter. Ze zijn dan ook de enige behandelingen die hun nut bewezen hebben voor de langdurige verbetering van de oppervlakte-energie van polymeren bij een geoptimaliseerd vermogen.
Kernwoorden—diëlektrische barrière ontlading (DBD), isopropanol additie, lage dichtheid polyethyleen (LDPE) folie, veroudering, plasmabehandeling.
I. INTRODUCTIE
Polyethyleen (PE) wordt vaak gekozen voor verscheidene toepassingen vanwege zijn uitstekende materiaal-eigenschappen (e.g. flexibiliteit, chemische bestendigheid en taaiheid). [1] Echter, zijn lage oppervlakte-energie zorgt ervoor dat PE vaak niet gebruikt kan worden waar het oppervlak betrokken is in bindingen, adhesie, printen, etc. [2] Oppervlaktebehandelingen kunnen alsnog de gewenste oppervlakte-eigenschappen aanbrengen zonder de benodigde bulkeigenschappen te veranderen. [3]
Een van deze behandelingen zijn de plasmabehandelingen, die een goede keus zijn door o.a. hun lage energieconsumptie, afwezigheid van (toxisch) afval en flexibiliteit. [4] Vaak gebruikt zijn diëlektrische barrière ontladingen (DBD’s), die toepasbaar over een groot drukbereik en relatief goed op te schalen reactoren zijn. [2, 5]
Plasmabehandelingen met eenvoudige gassen (e.g. O2, N2,
groepen (e.g. aminen, alcoholen/ethers, carbonzuren/esters en peroxiden) op polymeeroppervlakken te incorporeren door de interactie tussen actieve plasma species en de substraten, om zo de oppervlakte-energie te doen toenemen (= oppervlakteactivatie). [5, 6] Deze verandering gaat echter gedeeltelijk teloor in de loop van de tijd in een proces genaamd veroudering. [3]
Daarom worden soms solventdampen van complexere moleculen toegevoegd om plasmapolymerisatie tot stand te brengen, wat resulteert in een normaliter stabiele en sterk gefunctionaliseerde/vernette film met superieure verouderingseigenschappen. [6, 7] Alcoholdampadditie is hierbij zeer interessant aangezien de meeste (courante) alcoholen (e.g. ethanol en isopropanol) milieuvriendelijke solventen en vrij goedkoop zijn, terwijl de hydroxylgroep tot uitgebreide incorporatie van zuurstofhoudende functionaliteiten kan zorgen – mogelijks resulterend in een sterk verbeterde bevochtbaarheid. [2, 7-9]
In deze thesis wordt de invloed van (tot 5 g/h) isopropanol- additie bij verschillende medium druk dragergasplasma’s op de fysicochemische oppervlaktebehandeling van een lage dichtheid polyethyleen (LDPE) folie behandeld met een DBD op verschillende vermogens uitvoerig onderzocht.
Een gedegen kennis van het effect van isopropanoladditie op plasma behandelde LDPE stalen wordt mogelijk gemaakt door watercontacthoekmetingen (WCA) om de bevochtbaarheid te bestuderen, X-straal foto-elektron spectroscopie (XPS) om de atomaire oppervlaktecompositie te analyseren, atoomkrachtmicroscopie (AFM) voor de evaluatie van de oppervlaktemorfologie en Fouriertransformatie infraroodspectroscopie om filmdepositie na te gaan. Het verouderingsproces van behandelde LDPE stalen bewaard in lucht wordt eveneens bestudeerd door middel van WCA en XPS. Dit wordt allen voorafgegaan door een korte studie van de invloed van het vermogen en behandelingstijd, met als doel standaardbehandelingstijden te definiëren.
II. MATERIALEN & METHODEN
A. Polyethyleen
Aangekocht van Goodfellow Cambridge Ltd., het substraat blootgesteld aan de plasmabehandelingen was een LDPE folie met een dikte van 0,18 mm (± 20%). De niet op voorhand behandelde LDPE film, is bi-axiaal georiënteerd en additief-vrij. Alle stalen werden in rechthoekige stukken van ongeveer 1 cm x 5 cm gesneden.
B. DBD set-up en karakterisering
Plasmabehandelingen werden uitgevoerd met een in-house gemaakte DBD reactor (Figuur II-1). Omsloten door een cilindrisch omhulsel vindt de ontlading plaats tussenin twee koperen elektroden (diameter = 4 cm). Beide elektroden zijn bedekt met een keramische (Al2O3) plaat (5 cm x 5 cm – dikte
= 0,7 mm), dienstdoende als diëlektricum. De inter-electrode afstand is 4 mm. Verbonden aan de bovenste elektrode is de hoogfrequentie (constant op 50 kHz gehouden) AC energiebron, terwijl de onderste elektrode geaard is. De uit de LDPE folie gesneden stalen werd in het centrum van de onderste elektrode geplaatst met de lange zijde parallel aan de gasingang.
Eens het staal in de reactor geplaatst was, werd de druk in de ontladingsruimte naar 3 mbar gebracht en daaropvolgend gevuld met het geselecteerde puur dragergas aan 3 standaard liters per minuut (slm) tot een druk van 850 mbar bereikt werd, waar de ontladingsruimte 3 min lang gespoeld werd. Na het voltooien van de spoeling werd de reactor tot een druk van 50 mbar gebracht na het volumedebiet op 1 slm in te stellen. De in deze thesis gebruikte dragergassen waren argon, droge lucht en stikstofgas (Air Liquide – Alphagaz 1).
Na het bereiken van 50 mbar werd de gewenste isopropanol (>99,99% zuiverheid, Sigma-Aldrich) damp flowsnelheid toegevoegd aan het dragergas (totaal volumedebiet 1 slm). De flowsnelheden werden gevarieerd tussen 0 g/h en 5 g//h op vier punten: 0 g/h, 0,5 g/h, 2,5 g/h en 5 g/h. Additie van isopropanol werd bekomen door middel van een CEM® (Controlled Evaporation and Mixing) systeem, aangekocht van Bronkhorst.
Bij het bekomen van het stabiel gewenste gas/damp mengsel, werd de AC energiebron aangeschakeld (aanvang behandeling). Bij het voltooien van de behandeling werd het debiet op 0 slm ingesteld en de kamer naar 3 mbar gebracht, waarna het ventiel geopend en het staal verwijderd werd.
De elektrische karakterisering van de DBD werd uitgevoerd om de ontladingsregimes en -vermogens te bepalen. Stroom-spanning golfvormen werden opgemeten door de aangebrachte spanning [1000:1 hoge spanningsprobe (Tektronix P6915A) aan de bovenste electrode] en de ontladingsstroom [over een 50 Ω weerstand in serie met de onderste electrode en aarding (Figuur II-1)] te meten. Een digitale oscilloscoop (Picoscope 3204A) registreerde beide golfvormen, welke werden gevisualiseerd m.b.v. Picoscope 6 software. Ontladingsvermogens werden gemeten door de 50 Ω weerstand met een 10,4 nF condensator te vervangen en de oppervlakte van de Lissajous-figuur met de AC frequentie te
C. Oppervlaktekarakterisering
Evaluatie van de bevochtbaarheid werd uitgevoerd a.d.h.v. statische WCA-metingen onder omgevingsomstandigheden met een KRÜSS Easy Drop systeem (KRÜSS GmbH). Druppels gedistilleerd water (2 µl) werden op het oppervlak aangebracht, gevolgd door bepaling van de WCA’s d.m.v. Laplace-Young fitting. De gerapporteerde waarden zijn het gemiddelde en standaardafwijking van 6 metingen per staal.
Gedetailleerde kwantitatieve informatie omtrent de atomaire oppervlaktesamenstelling van de stalen werd bekomen d.m.v. een PHI 5000 Versaprobe II spectrometer (XPS-systeem). Een oppervlakte van 500 x 500 µm² werd bestraald door een x-straal (grootte = 100 µm) afkomstig van een monochromatische Al Kα x-straalbron (hν = 1486,6 eV).
Deze opereerde op een vermogen van 25 W en stond, evenals de detector, onder een hoek van 45° t.o.v. het staaloppervlak. Een maximumdruk van 10-9 mbar werd aangehouden in de
meetruimte tijdens de metingen. Een pass energie van 487,85 eV en stapgrootte van 0,8 eV werd gehanteerd voor de survey scans, welke werden geanalyseerd m.b.v. Multipak (versie 9.6) software. Tijdens de analyse werd rekening gehouden met de Shirley achtergrond in overeenstemming met de door de spectrometerfabrikant meegeleverde relatieve sensitiviteits-parameters. Alle bindingsenergieschalen werden gekalibreerd o.b.v. de koolwaterstofcomponent van het Cs1 spectrum (op 285 eV). Alle waarden in deze thesis zijn het gemiddelde en de standaardafwijking van 4 metingen per staal.
Een Bruker Tensor 27 (Bruker) spectrometer uitgerust met een enkelvoudige reflectie ATR-accessoire (germaniumkristal; MIRacle™ Pike technology) werd gebruikt voor de FTIR-metingen. De spectra (4 cm-1 resolutie)
werden opgenomen a.d.h.v. een vloeibare stikstof-gekoelde MCT-detector (kwik – cadmium – telluur). Alle spectra zijn het resultaat van de integratie van 64 scans en evaluatie op drie verschillende staallocaties.
Analyse van de oppervlaktemorfologie van de LDPE-stalen werd gedaan m.b.v. een XE-70 AFM (Park Systems) met een silicone probe (NANOSENSORS™ PPP-NCHR). De scangrootte was 15 x 15 µm en de afbeelding werden opgenomen in noncontact mode. Oppervlakteruwheid werd geëvalueerd met XEP software na het uitvoeren van een X- en Y-vlak autofit procedure. Hadden ze hier gepresenteerd geweest, zouden de gerapporteerde oppervlakte-ruwheden het gemiddelde van drie metingen per staal zijn.
III. RESULTATEN & DISCUSSIE
A. Ontladingsvermogens en behandelingstijden
Om de invloed van het ontladingsvermogen op het effect van de isopropanoladditie te bestuderen, werden drie vermogens gedefinieerd voor ieder dragergas om alle isopropanoladdities bij te evalueren: hoog vermogen (HP), medium vermogen (MP) en laag vermogen (LP). LP werd gebaseerd op het minimumvoltage nodig om plasma te vormen, HP op het maximumvoltage waarbij plasma zich buiten de elektroden begaf en MP als een waarde tussenin. Elk vermogen voor iedere individuele setting werd zo gekozen dat ze min of meer gelijk waren voor ieder dragergas. Tabel I toont de geselecteerde gemiddelde vermogens voor iedere setting, afkomstig van de ontladingsvermogen bepalingen. Er werd geen HP-setting voor argon gekozen, daar geen vergelijkbaar vermogen met die van de HP-setting van lucht en stikstofgas bereikt kon worden vanwege boogontladingen.
Figuur II-1 Schematisch diagram van de DBD reactor (1 – Draaggas
cilinder; 2 - Draaggas massaflow controller; 3 - Isopropanol houder; 4 – Isopropanol vloeistofflow controller; 5 - CEM® systeem; 6 - DBD reactor; 7 - Drukmeter; 8 – Ventiel; 9 - Pomp). [4]
Tabel I Overzicht van de gebruikte gemiddelde vermogens voor elk
dragergas en iedere vermogen-setting.
Setting Argon Lucht Stikstofgas
HP – 15,1 W 17,2 W
MP 4,2 W 8,4 W 7,2 W
LP 2,5 W 2,3 W 1,8 W
De stroom-spanning karakteristieken overeenkomstig met elke dragergas/vermogen-setting combinatie suggereerde dat de ontladingsmodes waren of pseudoglow of glow, wat inhoudt dat een homogene oppervlaktebehandeling bereikt werd. [2] Deze resultaten werden ook waargenomen door Van Deynse et al. [2], die gebruik maakten van dezelfde DBD set-up, maar wel (vergelijkbare concentraties) ethanol toevoegden.
Vanwege de voortdurende pandemie tijdens het schrijven, konden de reeds gecreëerde Lissajous-figuren en stroom-spanning golfvormen niet van de laboratorium pc gehaald worden, waardoor ze hier niet getoond worden.
Analyse van de behandelingstijdinvloed kon nu plaatsvinden aangezien de nodige vermogens geselecteerd waren. Figuur III-1 toont de veranderingen in de WCA’s in functie van de behandelingstijd voor LDPE behandeld met stikstofgas plasma’s. Zeer gelijkaardige resultaten werden behaald bij argon en lucht plasmabehandelingen.
Onbehandeld LDPE had een WCA van 108,1 ± 2,42°. Kort na blootstelling van LDPE aan een plasma, werd een sterke WCA-daling gezien tot een constante WCA bereikt werd, wijzend op verzadiging van geïncorporeerde functionaliteiten en dat langere behandelingstijden geen nut hadden. [10]
Een relatief stabiele WCA werd comfortabel bereikt na 20 tot 30 s voor alle dragergas/vermogen-combinaties, wat het mogelijk maakte standaardbehandelingstijden te definiëren. Een meer objectieve vergelijking tussen verschillende plasma’s op een bepaalde setting werd mogelijk gemaakt door constante energiedichtheden (ED’s) te kiezen zodanig dat de verzadigde WCA’s werden bereikt.
Tabel II weergeeft de gedefinieerde ED’s voor de drie
vermogen-settings en de WCA’s van de overeenkomstige plasmabehandelingen, welke gelijken op deze gemeten door Cools et al. [10] Een afhankelijkheid van het dragergas m.b.t. de verzadigde WCA’s is duidelijk waarneembaar. Verder nog, de bekomen WCA’s wijzen op een verbeterde bevochtbaarheid en aanwezigheid van hydrofiele groepen in grote getallen die gevormd werden door de plasmabehandelingen, losstaande van het vermogen en dragergas. [5] Zowel XPS- als AFM-metingen werden uitgevoerd op deze stalen, maar zullen enkel verderop aangehaald worden waar toepasselijk.
Tabel I Overzicht van de geselecteerde energiedichtheden en
corresponderende WCA’s voor elk dragergas/vermogen-setting.
Setting ED Argon Lucht Stikstofgas
HP 30 J/cm² – 61,6 ± 2,56° 31,7 ± 2,08°
MP 15 J/cm² 40,2 ± 2,07° 36,5 ± 2,44° 25,7 ± 2,68°
LP 5 J/cm² 41,3 ± 2,31° 48,0 ± 1,96° 46,4 ± 2,13°
B. Invloed van isopropanoladditie op bevochtbaarheid, oppervlaktesamenstelling en -morfologie
1) WCA-metingen
Het effect van isopropanoladditie op de oppervlakte-eigenschappen van behandeld LDPE werd bestudeerd d.m.v. statische WCA-, XPS- en AFM-metingen. De behandelingen zijn identiek aan degene die eerder opgesteld werden a.d.h.v. constante ED’s. Elke behandeling werd viermaal afzonderlijk uitgevoerd, elk met een van volgende isopropanol-flowsnelheden: 0 g/h, 0,5 g/h, 2,5 g/h en 5 g/h. Slechts vier flowsnelheden werden gebruikt om het aantal metingen te drukken. Figuur III-2 toont de WCA-verandering in functie van de toegevoegde isopropanolflow voor de verschillende dragergassen en vermogens.
Zoals eerder vermeld, verbeterden de pure dragergas plasmabehandelingen de bevochtigbaarheid van LDPE al aanzienlijk, wat volgt uit de sterk gereduceerde WCA’s (Tabel II) die waar te nemen zijn in Figuur III-2.
Betreffende het gebruik van argon als dragergas, gaf isopropanoladditie in eender welke concentratie en gebruikt vermogen aanleiding tot een daling in bevochtigbaarheid t.o.v. pure argon plasmabehandelingen. De WCA-stijging was het duidelijkst bij de additie van 2,5 g/h aan isopropanol bij de MP plasmabehandeling. Beide addities van 0,5 g/h en 5 g/h aan isopropanol resulteerden in minder dramatische WCA-stijgingen. Op LP was het effect van isopropanoladditie op de WCA voor zo goed als ieder geval gelijk. Hier was de reductie in bevochtigbaarheid kleiner dan op MP, wanneer vergeleken met de pure argon plasmabehandelingen op hun respectievelijke vermogens.
Isopropanoladditie bij een lucht plasma vertoonde andere effecten. Het meest voorname, op HP, is dat enkel de 2,5 g/h isopropanoladditie significant de WCA reduceerde in vergelijking met de pure lucht HP plasmabehandeling – al is het verschil klein –, terwijl additie van 0,5 g/h en 5 g/h isopropanoldamp voor een insignificant gestegen WCA zorgde. Alle toegevoegde isopropanolconcentraties bij een MP lucht plasma leverden licht verhoogde WCA’s op. Op LP leek de isopropanoltoevoeging de bevochtigbaarheid iets te verbeteren bij lagere concentraties.
De meest aanzienlijke effecten werden bekomen wanneer isopropanol aan een HP of MP stikstofgas plasma toegevoegd 0 20 40 60 80 100 120 0 10 20 30 40 50 60 70 W C A (° ) Time (s) Nitrogen LP Nitrogen MP Nitrogen HP
every 5 seconds for three different powers.
Figuur III-2 Evolutie van de WCA van plasma behandeld LDPE in
functie van de isopropanolflow toegevoegd aan zuiver draaggas.
Figuur III-1 Evolutie van de WCA van stikstofgas plasma behandeld
werd – op LP waren alle WCA’s voor iedere toegevoegde flow (inclusief 0 g/h) min of meer gelijk. Introductie van 0,5 g/h aan isopropanol bij een HP stikstofgas plasma, leverde een WCA-reductie van 90% op t.o.v. onbehandeld LDPE, wat een extra 68% vermindering was ten aanzien van een pure stikstofgas plasmabehandeling op HP. Volledige bevochtiging (100% WCA-reductie) werd bekomen bij additie van 2,5 g/h en 5 g/h aan isopropanoldamp bij de pure HP stikstofgas plasma. De WCA’s lagen onder de detectielimiet en werden aangeduid als 0°). Ongeacht de toegevoegde isopropanolflow bij een MP stikstofgas plasma, werd een 87% WCA-vermindering waargenomen t.o.v. onbehandeld LDPE. Dit was een additionele 52% reductie ten aanzien van puur MP stikstofgas plasma behandeld LDPE.
2) XPS-metingen
Alle WCA-resultaten van de plasma behandelde stalen zijn een eerste indicatie van een gefunctionaliseerd hydrofiel oppervlak. [2] XPS-metingen werden uitgevoerd om meer inzicht in de veranderingen van de atomaire oppervlaktecompositie te krijgen. Vanwege het grote aantal resultaten kan niet alles hier grafisch (of in tabellen) gepresenteerd worden. Daarom worden de resultaten t.o.v. de oppervlaktecomposities van de met zuivere dragergassen plasma behandelde stalen besproken (Tabel III).
Het dient aangehaald te worden dat het onbehandelde LDPE kleine hoeveelheden stikstof (0,40 ± 0,39%) en zuurstof (4,06 ± 0,32%) bezat op het oppervlak. Hoogstwaarschijnlijk is dit te wijten aan oppervlaktecontaminaties (e.g. door waterdamp). [2]
Zuivere argon plasmabehandelingen leidden tot een aanzienlijke stijging in incorporatie van zuurstof terwijl het stikstofgehalte vergelijkbaar bleef met dat van onbehandeld LDPE. Mogelijke bronnen van de zuurstofatomen zijn residuele lucht in de ontladingsruimte, oxidatie na behandeling, van de reactorwand gedesorbeerde moleculen en dragergasonzuiverheden. [5, 11] Bij isopropanoladditie werd een toename in zuurstofgehalte waargenomen bij zowel MP als LP t.o.v. puur argon plasma behandelde stalen. Deze toename in zuurstofgehalte kan aan het zuurstofatoom in isopropanol toegeschreven worden. [7] In alle gevallen was de toename in bevochtigbaarheid te danken aan geïncorporeerde zuurstofhoudende functionele groepen op het oppervlak. Een relatief slechtere bevochtigbaarheid werd bekomen wanneer isopropanol aan een argon plasma toegevoegd werd op eender welk vermogen, ondanks stijgingen in het oppervlaktezuurstofgehalte.
Zeer gelijkaardige oppervlaktecomposities aan die van zuivere argon plasma behandelde stalen werden bekomen bij het gebruik van pure lucht plasma’s op MP en LP. Wel werd
een duidelijke stijging in zuurstof- en stikstofgehalte gezien op HP t.o.v. MP- en LP-behandelingen, als resultaat van de hogere dichtheid actieve species. [12] De kleine toename in stikstof op het oppervlak wordt verklaard door de stikstoffractie in lucht. [11] Opnieuw werd een stijging in zuurstofgehalte gezien bij additie van isopropanol, met een grotere stijging en kleinere negatieve impact op de WCA tien het vermogen groter werd.
Pure stikstof plasmabehandelingen resulteerden in een gestegen oppervlaktestikstofgehalte, met sterkere stijgingen bij een toenemend vermogen (hogere dichtheid actieve species). [12] Alle stalen vertoonden een gelijkaardige stijging in zuurstofgehalte. De polariteit van de stikstof-functionaliteiten en de netto toename in zuurstof t.o.v. de plasmabehandelingen met de andere dragergassen is de oorzaak van de waargenomen sterkere bevochtigbaarheid. Isopropanoladditie leidde tot aanzienlijke toenames in geïncorporeerd zuurstof en stikstof. Enkel op HP en MP uitte zich dit in een drastisch verbeterde bevochtigbaarheid. De oppervlaktecompositie van LDPE behandeld met een MP stikstofgasplasma met 0,5 g/h of 2,5 g/h aan isopropanol correleert goed met de verlaagde WCA’s (sterke N & O toenames), in tegenstelling tot de andere MP/HP stikstofgas/isopropanol behandelingen (kleinere N & O toenames).
De deconvolutie van de XPS-spectra zou enkele waarnemingen meer hebben kunnen verduidelijken, maar dit werd (grotendeels) door de actieve pandemie op het tijdschip van schrijven niet uitgevoerd. Een literatuurstudie kon echter enkele zaken opklaren.
Vaak geïncorporeerde zuurstoffunctionaliteiten zijn C-O (hydroxyl, ether), C=O (carbonyl) en O-C=O (carboxyl, ester), terwijl C-NHx (amines), C=N (imines/oximes) en
N-C=O (amides) vaak geïntroduceerde stikstoffunctionaliteiten zijn – allen bevorderend voor de bevochtigbaarheid. [2, 5] Additie van isopropanol bevoordeelt de incorporatie van ethers, ketonen en esters, en verlaagt zo de polariteit/bevochtigbaarheid van het oppervlak. [13, 14] er wordt geloofd dat dit effect (deels) tenietgedaan kan worden door een verhoogd vermogen (i.e. meer fragmentatie) of een zeer hoge vernettingsgraad in de bovenste atomaire lagen. [7, 15-17]
3) AFM-metingen
Alle AFM-metingen werden voltooid, maar de bekomen beelden konden niet verwerkt worden aangezien er geen toegang was tot de pc door de actieve pandemie op het moment van schrijven. In plaats daarvan wordt hier gespeculeerd over wat van de resultaten verwacht werd a.d.h.v. de WCA en XPS resultaten, en toepasselijke literatuur. Deze bespreking heeft dus zijn beperkingen.
Eerdere studies hebben reeds aangetoond dat zuivere argon en stikstofgas plasma’s slechts een (zeer) beperkt etsend effect hebben – versterkt door de aanwezigheid van zuurstof(gas) en hogere vermogens. [11, 12, 18] Daarom wordt slechts een licht geëtst oppervlak voor deze plasma behandelingen verwacht – behalve op HP, waar het vermogen mogelijks groot genoeg is om het oppervlak gelijkmatig te etsen, al dan niet volledig uniform, en zo de WCA te vergroten. [19]
Zuurstofhoudende plasma’s hebben een duidelijk etsend effect op (polymeer) substraten, leidende tot een verhoogde ruwheid en dus een positief effect op de bevochtigbaarheid – indien het vermogen niet té groot is. [18]
Tabel III Overzicht van de atomaire oppervlaktecomposities van