International Masters of Science in Environmental Technology and Engineering
Master’s dissertation submitted in partial fulfilment of the requirements for the joint degree of EU Erasmus+ Master course organized by
University of Chemistry and Technology, Prague, the Czech Republic IHE Delft Institute for Water Education, Delft, the Netherlands
Ghent University, Ghent, Belgium
Academic year 2019-2020
Competition between anaerobic ammonia
oxidising bacteria and heterotrophic
denitrifiers under thermophilic conditions
for nitrogen removal from wastewater
Ghent University
Badri Narayan Ravikumar
(ID-identifier: ES-IMETE.20-19)Promotor: Dr. Ramon Ganigué
International Masters of Science in Environmental Technology and Engineering
Master’s dissertation submitted in partial fulfilment of the requirements for the joint degree of EU Erasmus+ Master course organized by
University of Chemistry and Technology, Prague, the Czech Republic IHE Delft Institute for Water Education, Delft, the Netherlands
Ghent University, Ghent, Belgium
Academic year 2019-2020
Competition between anaerobic ammonia
oxidising bacteria and heterotrophic
denitrifiers under thermophilic conditions
for nitrogen removal from wastewater
Ghent University
Badri Narayan Ravikumar
Promotor: Dr. Ramon GaniguéDeze pagina is niet beschikbaar omdat ze persoonsgegevens bevat.
Universiteitsbibliotheek Gent, 2021.
This page is not available because it contains personal information.
Ghent University, Library, 2021.
i
ACKNOWLEDGEMENTS
I would like to take this opportunity to thank a few amazing people who have been pivotal in shaping my journey as a graduate student for the last 2 years and especially as a researcher for the last 7 months. The journey has been full of peaks and valleys filled with fantastic emotions, making it a memorable one to remember forever.
I wish to specifically acknowledge my co-promotor Dr. Jose and my promotor Prof. Dr. Ramon for their tremendous contributions in formulating my thesis. Without their efforts, this thesis wouldn’t have attained this quality. Jose has been a great tutor from whom I gained immense amount of knowledge about my research field and relevant practical experience. He was always willing to help me anytime selflessly, even when he was miles away. Prof. Ramon, thank you very much for providing me with great insights, invaluable suggestions and guidance to execute research in a professional manner. Your constant encouragement and trust in my skills meant a lot to me. I would also like to extend my sincere gratitude to all the staff members of CMET who made sure my thesis was carried out in a smooth way.
I express my heartfelt obligations to all my classmates of IMETE program with whom I shared a lot of exhilarating memories and also to all the professors of IMETE program from UCT Prague, IHE Delft Institute for Water Education and Ghent University, from whom I learned the most fruitful life lessons in these 2 years. I thank the European commission for providing me with a partner country scholarship (project number: 2017-1957/001-001-EMJMD) to pursue the Erasmus Mundus IMETE program.
From a personal view, I would like to express my true devotion to my mother for her unconditional love and blessings, which has been my pillar of strength to overcome all the challenges I face throughout my life and career. I also thank my whole family for their moral support, wherever I go and in whatever I decide. Last but never the least, I thank the almighty for allowing me to meet all these fabulous people and offering me everything else in life.
ii
ABSTRACT
Thermophilic nitrogen removal via anammox could be a major breakthrough for treating nitrogen rich warm wastewaters. A part of this study was focussed to determine the maximum specific growth rate (µmax) of thermophilic anammox bacteria growing in the form of suspended cells in a membrane
biore-actor (MBR) at 49°C. The steady state operation of the MBR at an SRT of 2 days yielded a specific growth rate of 0.5 d-1 but was uncertain to be the µmax of the thermophilic anammox species. Still, the
growth rate observed in this study is higher than all the mesophilic anammox µmax ever reported. A
novel steady state chemostat model (SSCM) and a biokinetic model was developed to simulate the simultaneous anammox and denitrification (SAD) process to understand the nitrite competition between anammox and denitrifiers at thermophilic condition. The SSCM estimated a high anammox enrichment (95.6±0.2%) under COD limitation. The biokinetic model predicted that the denitrifiers were never able to outcompete anammox bacteria for nitrite as an electron acceptor for heterotrophic nitrogen produc-tion under a broad range of COD/NO2--N ratios. The lower anammox nitrite affinity constant than that
of denitrifiers played a crucial role to outcompete denitrifiers for nitrite. A comparative study between mesophilic and thermophilic SNAD process simulated using a biofilm model was reported for the first time. Broader optimal DO range (0.4-1 mgO2/L) for peak total nitrogen (TN) was required for
meso-philic condition when compared to thermomeso-philic condition (0.7-1 mgO2/L). Similarly, the mesophilic
SNAD reactor was observed to perform better in terms of TN removal under higher COD/NH4+-N ratios
than thermophilic SNAD reactor. Anammox was the major pathway for nitrogen removal irrespective of the temperature. These simulations justified the importance of maintaining optimal conditions to steer the dynamics of substrate competition towards achieving high nitrogen removal from warm wastewaters.
iii
TABLE OF CONTENTS
ACKNOWLEDGEMENTS ... i
ABSTRACT ... ii
TABLE OF CONTENTS ... iii
LIST OF TABLES ... v
LIST OF FIGURES ... vi
LIST OF SYMBOLS & ABBREVIATIONS ... viii
CHAPTER 1. INTRODUCTION ... 1
1.1 The need for wastewater treatment and nutrient removal in the 21st century ... 1
1.2 Global nitrogen cycle and its key players in wastewater treatment ... 2
1.2.1 Nitrification ... 3
1.2.1.1 Aerobic ammonia oxidation ... 4
1.2.1.2 Nitrite oxidation ... 5
1.2.2 Denitrification ... 5
1.2.3 Anammox ... 5
1.2.3.1 History and discovery of anammox ... 6
1.2.3.2 Unique cell biology of anammox bacteria ... 6
1.2.3.2.1 Anatomy ... 6
1.2.3.2.2 Metabolism ... 7
1.2.3.3 Stoichiometry and growth of anammox bacteria ... 8
1.3 Commercial application of mesophilic anammox in wastewater treatment ... 9
1.3.1 The need for thermophilic nitrogen removal ... 10
1.3.2 Thermophilic anammox as a novel tool ... 11
1.4 Ecological interactions between different nitrogen transforming microorganisms ... 12
1.5 Mathematic modelling of biological nitrogen removal (BNR) ... 13
1.5.1 Insight into BNR modelling ... 13
1.5.2 Modelling of simultaneous nitrogen conversion processes in biofilms ... 14
1.5.3 Modelling of substrate competition for the coexistence of autotrophic and heterotrophic bacteria for BNR ... 15
iv
CHAPTER 3. MATERIALS & METHODS ... 18
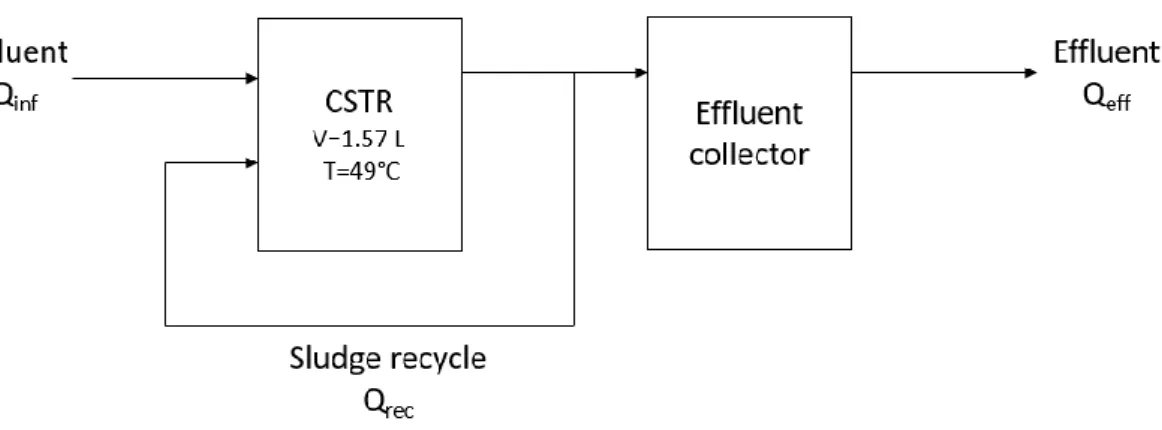
3.1 Reactor operation ... 18
3.2 Chemical analyses of effluent ... 20
3.3 Determination of maximum specific growth rate of thermophilic anammox bacteria ... 20
3.4 Steady state modelling of SAD process using mass balances ... 21
3.4.1 Model development ... 21
3.4.2 Simulation ... 22
3.4.3 Scenario tested using the SSCM ... 23
3.5 Biokinetic modelling of SAD ... 23
3.5.1 Model development ... 23
3.5.2 Simulation ... 23
3.5.3 Scenarios tested using the biokinetic model ... 24
3.6 Biofilm modelling of a granular SNAD reactor ... 25
3.6.1 Model development ... 25
3.6.2 Simulation ... 26
3.6.3 Scenarios tested using the biofilm model ... 26
CHAPTER 4. RESULTS & DISCUSSION ... 28
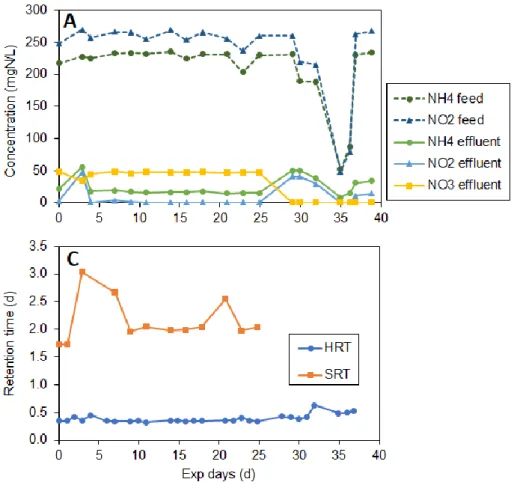
4.1 MBR performance ... 28
4.2 Estimation of maximum specific growth rate of thermophilic anammox bacteria ... 30
4.3 Simulation results of steady state modelling of SAD process ... 31
4.4 Simulation results of biokinetic modelling of SAD process ... 33
4.4.1 Effect of SRT ... 33
4.4.2 Effect of change in influent ammonium concentration ... 34
4.4.3 Effect of influent COD addition ... 36
4.5 Simulation results of biofilm modelling of SNAD process ... 40
4.5.1 Effect of bulk DO concentration ... 41
4.5.2 Effect of influent bulk COD concentration ... 45
REFERENCES ... 51
v
LIST OF TABLES
Table 1.1 Various oxidation states of nitrogen and nitrogen species found in the environment ... 2
Table 3.1. Feed composition for cultivating thermophilic anammox bacteria in an MBR ... 19
Table 3.2. Steady state experimental data of MBR of operated at 6 different SRT periods with operational conditions: Volume, V=1.57 L; Temperature, T=49°C; pH=7.6 ... 22
Table 4.1. Illumina DNA analysis results ... 31
Table A1. List of some modelling studies on mesophilic BNR with different specifications ... 61
Table A2. Composition of trace elements solution ... 62
Table A3. Stoichiometric matrix of the SSCM ... 63
Table A4. Kinetics equations of the components derived based on mass balances and the metabolic processes of Table A3 ... 64
Table A5. Kinetic and stoichiometric parameter values of the SSCM at 49°C... 65
Table A6. Stoichiometric matrix of the biokinetic model ... 66
Table A7. Kinetic expressions of the processes mentioned in Table A6 ... 67
Table A8. Kinetic and stoichiometric parameter values of the biokinetic model at 49°C ... 68
Table A9. Stoichiometric matrix of the biofilm SNAD model ... 69
Table A10. Kinetic expressions of the processes mentioned in Table A9 ... 70
Table A11. Kinetic and stoichiometric parameter values of the biofilm SNAD model at mesophilic and thermophilic conditions ... 72
Table A12. Maximum specific growth rate and minimum doubling time values of anammox bacteria reported at different temperatures ... 76
vi
LIST OF FIGURES
Figure 1.1. Urban wastewater system in Netherlands before and after the beginning of 21st century
(European Environmental Agency, 2017) ... 2 Figure 1.2. Classic biogeochemical nitrogen cycle updated with the recently discovered comammox process (Dang & Chen, 2017) ... 3 Figure 1.3. Cell anatomy of anammox bacterium showing different components (van Teeseling et al., 2014) ... 7 Figure 1.4. Enzymatic pathway of anammox metabolism based on Oshiki et al. (2016b). NXR: Nitrite/nitrate oxidoreductase; NirK: Nitrite reductase containing copper; NirS: cytochrome cd1-type
nitrite reductase; HAO: Hydroxylamine oxidoreductase; HZS: Hydrazine synthase; HDH: Hydrazine dehydrogenase; ACS: CO dehydrogenase/ acetyl coA synthase ... 8 Figure 3.1. Continuously operated membrane bioreactor containing thermophilic anammox
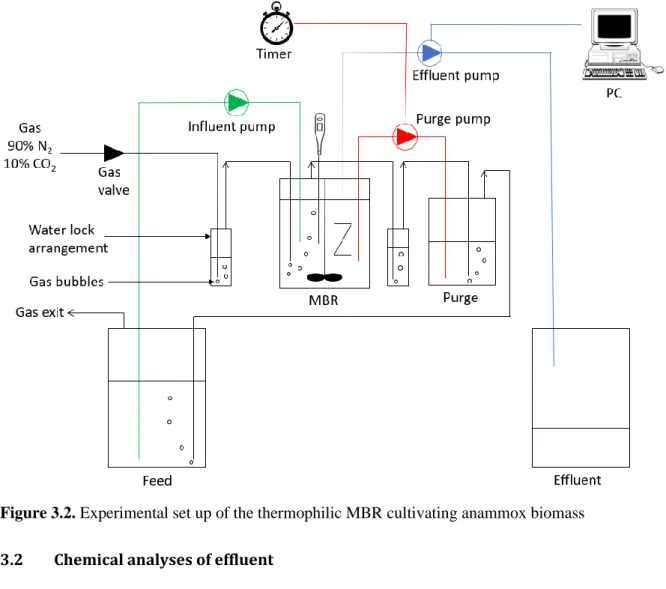
enrichment culture (Vandekerckhove et al., 2020) ... 18 Figure 3.2. Experimental set up of the thermophilic MBR cultivating anammox biomass ... 20 Figure 3.3. Schematic flow diagram of the thermophilic SAD process model implemented on
Aquasim ... 24 Figure 4.1. Performance of the MBR shown as, A: Concentration profile of the N species in the influent and effluent; B: Total N loading and removal rates observed during the period of operation; C: Calculated HRT and SRT values; D: Stoichiometric molar ratio between nitrite and ammonium removal and the ratio between nitrate production and ammonium removal, respectively... 29 Figure 4.2. Degree of enrichment of anammox bacteria and denitrifiers predicted by the SSCM at steady state with respect to SRT ... 31 Figure 4.3. Effect of anammox decay rate constant on steady state anammox and denitrifiers fraction predicted by the SSCM ... 32 Figure 4.4. Steady state biomass production measured in gram COD biomass produced per gram nitrogen removed per day observed from short to long SRT periods in a mixed reactor simulating SAD process... 33
Figure 4.5. Effect of influent NO2-/NH4+ ratio on the steady state behaviour of SAD process taking
place in a mixed reactor operated at an SRT of 10 d. A: Effluent concentration profile, B: Total nitrogen removal efficiency trend, C: Biomass concentration profile, D: Fraction of anammox bacteria and denitrifiers coexisting in the sludge ... 35 Figure 4.6. Effect of influent COD addition on the steady state behaviour of SAD process at SRT=10 d; influent NH4+=230 mgN/L, NO2-=265 mgN/L. A: Biomass concentration profile; B: Effluent N
species profile; C: Volumetric COD loading rate (LR) and removal rate (RR); D: Contribution of anammox and heterotrophic denitrification to N2 gas production; E: Degree of enrichment of
vii
Figure 4.7. Sensitivity analysis of KHETNO2 on the nitrite competition between anammox bacteria
and denitrifiers observed at steady state. A: Contribution of anammox and heterotrophic
denitrification to N2 production; B: Degree of enrichment of anammox bacteria and heterotrophic
denitrifiers ... 39 Figure 4.8. Effect of bulk DO concentration on the steady state behaviour of granular mesophilic and thermophilic SNAD reactor for a fixed influent ammonium concentration=300 mgN/L and COD concentration=300 mgCOD/L. Plots on the left (A,C,E) represent mesophilic condition, plots on the right (B,D,F) represent thermophilic condition. A and B: Effluent bulk concentrations profile; C and D: Contribution of anammox and heterotrophic denitrification to production of N2 gas; E and F:
Biomass fractions in the granule ... 42 Figure 4.9. Simulation results of mesophilic and thermophilic granular SNAD process represented by, Top (A,B,C,D): Steady state biomass distribution within a granule corresponding to 10,000 d; Centre (E,F,G,H): Steady state oxygen profile within a granule corresponding to 10,000 days; Bottom (I,J,K,L): Dynamic effluent bulk concentration profile; Left (A,B,E,F,I,J): Mesophilic condition; Right (C,D,G,H,K,L): Thermophilic condition ... 43 Figure 4.10. Effect of bulk COD concentration on the steady state behaviour of granular mesophilic and thermophilic SNAD reactor for a fixed influent ammonium concentration=300 mgN/L and DO concentration=1 mgO2/L. Plots on the left (A,C,E,G) represent mesophilic conditions, plots on the
right (B,D,F,H) represent thermophilic conditions. A and B: Effluent bulk concentrations profile; C and D: Contribution of anammox and heterotrophic denitrification to production of N2 gas; E and F:
TN removal efficiency trend; G and H: Biomass fractions in the granule ... 47
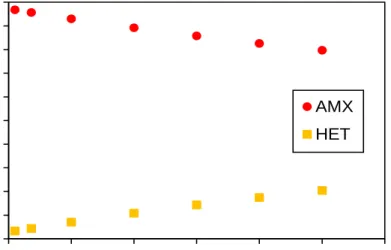
Figure A1. Sensitivity analysis of bAMX simulated by the SSCM. A: Fraction of anammox bacteria at
different bAMX values with respect to SRT, B: Fraction of denitrifiers at different bAMX values with
viii
LIST OF SYMBOLS & ABBREVIATIONS
µ𝐴𝑀𝑋𝑚𝑎𝑥 Maximum specific growth rate of AMX
µ𝐴𝑂𝐴𝑚𝑎𝑥 Maximum specific growth rate of AOA
µ𝐴𝑂𝐵𝑚𝑎𝑥 Maximum specific growth rate of AOB
µ𝐻𝐸𝑇𝑚𝑎𝑥 Maximum specific growth rate of HET
µ𝑁𝑂𝐵𝑚𝑎𝑥 Maximum specific growth rate of NOB
𝐾𝐴𝑀𝑋𝑁𝐻 Ammonium affinity constant for AMX
𝐾𝐴𝑀𝑋𝑁𝑂2 Nitrite affinity constant for AMX
𝐾𝐴𝑀𝑋𝑂2 Oxygen inhibition constant for AMX
𝐾𝐴𝑂𝐴𝑁𝐻 Ammonium affinity constant for AOA
𝐾𝐴𝑂𝐴𝑂2 Oxygen affinity constant for AOA
𝐾𝐴𝑂𝐵𝑁𝐻 Ammonium affinity constant for AOB
𝐾𝐴𝑂𝐵𝑂2 Oxygen affinity constant for AOB
𝐾𝐻𝐸𝑇𝑁𝑂2 Nitrite affinity constant for HET 𝐾𝐻𝐸𝑇𝑁𝑂3 Nitrate affinity constant for HET
𝐾𝐻𝐸𝑇𝑂2 Oxygen affinity constant for HET
𝐾𝐻𝐸𝑇𝑆 Oxygen affinity constant for HET
𝐾𝑁𝑂𝐵𝑁𝑂2 Nitrite affinity constant for NOB
𝐾𝑁𝑂𝐵𝑂2 Oxygen affinity constant for NOB
𝑌𝐴𝑀𝑋 Yield factor of AMX
𝑌𝐴𝑂𝐴 Yield factor of AOA
𝑌𝐴𝑂𝐵 Yield factor of AOB
𝑌𝐻𝐸𝑇𝐴𝐸𝑅 Aerobic yield factor of HET
𝑌𝐻𝐸𝑇𝐴𝑁 Anoxic yield factor of HET
𝑌𝑁𝑂𝐵 Yield factor of NOB
𝑏𝐴𝑀𝑋 Decay rate constant of AMX
𝑏𝐴𝑂𝐴 Decay rate constant of AOA
𝑏𝐴𝑂𝐵 Decay rate constant of AOB
𝑏𝐻𝐸𝑇 Decay rate constant of HET
𝑏𝑁𝑂𝐵 Decay rate constant of NOB
𝑡𝑑𝑚𝑖𝑛 Minimum doubling time
𝜀𝐴𝑀𝑋𝑖𝑛𝑖 Initial fraction of AMX in a granule 𝜀𝐴𝑂𝐴𝑖𝑛𝑖 Initial fraction of AOA in a granule 𝜀𝐴𝑂𝐵𝑖𝑛𝑖 Initial fraction of AOB in a granule 𝜀𝐻𝐸𝑇𝑖𝑛𝑖 Initial fraction of AOB in a granule 𝜀𝑁𝑂𝐵𝑖𝑛𝑖 Initial fraction of NOB in a granule
𝜀𝑤 Porosity of a granule
ix
µmax Maximum specific growth rate
ACS CO dehydrogenase/Acetyl coA synthase
AMO Ammonium monooxygenase
AMX Anammox bacteria
Anammox Anaerobic/Anoxic ammonia oxidation
AOA Ammonia oxidising archaea
AOB Ammonia oxidising bacteria
ASM Activated sludge model
ATP Adenosine triphosphate
bCOD Biodegradable chemical oxygen demand
BNR Biological nitrogen removal
CANON Complete autotrophic nitrogen removal over nitrite
COD Chemical oxygen demand
CSTR Continuous stirred tank reactor
D Dilution rate
DEAMOX Denitrifying ammonium oxidation
DEMON Deammonification
DN2 Diffusion coefficient of dinitrogen gas
DNA Deoxyribonucleic acid
DNH Diffusion coefficient of ammonium
DNO2 Diffusion coefficient of nitrite
DNO3 Diffusion coefficient of nitrate
DNRA Dissimilatory nitrate reduction to ammonium
DO Dissolved oxygen
DO2 Diffusion coefficient of oxygen
DS Diffusion coefficient of soluble COD
EPS Extracellular polymeric substances
fI Fraction of inert particulate COD due to biomass decay
HAO Hydroxylamine oxidoreductase
HDH Hydrazine dehydrogenase
HET Heterotrophic bacteria
HZS Hydrazine synthase
iNSS Nitrogen content in soluble COD
iNXB Nitrogen content in biomass
iNXI Nitrogen content in inert particulate COD fraction
MABR Membrane aerated biofilm reactor
MBR Membrane bioreactor
NirK Copper containing nitrite reductase
x
NOB Nitrite oxidising bacteria
NRBC Nonwoven rotating biological contactor
NXR Nitrite/nitrate oxidoreductase
OLAND Oxygen limited autotrophic nitrification/denitrification
PN/A Partial nitritation/anammox
Qeff Effluent flow rate
Qin Influent flow rate
Qp Flow rate of purge stream
Qrec Sludge recycle flow rate
RBC Rotating biological contactor
ri Conversion rate of components
SAD Simultaneous anammox and denitrification
SBR Sequencing batch reactor
SHARON Sustainable high rate ammonium removal over nitrite
SN2 Dinitrogen gas concentration
SNAD Simultaneous partial nitrification, anammox and denitrification
SNH Ammonium concentration
SNO2 Nitrite concentration
SNO3 Nitrate concentration
SO2 Dissolved oxygen concentration
SRT Sludge retention time
SS Soluble COD concentration
SSCM Steady state chemostat model
T Temperature
td Doubling time
TN Total nitrogen
TOT Total biomass
UASB Upflow anaerobic sludge bed
V Reactor volume
VSS Volatile suspended solids
WWTP Wastewater treatment plant
XAMX Concentration of anammox bacteria
XAOA Concentration of ammonia oxidising archaea
XAOB Concentration of ammonia oxidising bacteria
XHET Concentration of heterotrophs
XI Inert particulate COD fraction due to biomass decay
XNOB Concentration of nitrite oxidising bacteria
ρj Process rate
1
CHAPTER 1. INTRODUCTION
1.1 The need for wastewater treatment and nutrient removal in the 21st century
For the first time in human history, in 2008 the number of people living in urban centres was more than the number of people thriving in the rural communities (UN-HABITAT, 2008). Urbanization along with the expected population growth of 9.7 billion people by 2050 (United Nations, 2019), would result in the growth of smart cities and mega urban civilisations. This directly implies a huge burden on the natural resources such as clean drinking water, fresh air, unpolluted soil etc. (Nasrollahi et al., 2020). Another consequence of the above-mentioned global population explosion and urbanization is the in-crease in urban wastewater production. Wastewater is defined as the superfluous polluted water from domestic circuits, roads (run-off), industries that has served its purpose (Singhal and Perez-Garcia, 2016), and end up in the physical infrastructure to remediate the polluted wastewater, termed as wastewater treatment plant (WWTP) (Li, 2018). This wastewater is generally rich in organic carbon and nutrients like nitrogen and phosphorous.
Until mid-20th century, wastewater in most parts of the world was discharged into the surface water
bodies without any treatment (Christoffersen and Kaas, 2000). This led to the awareness of a harmful aquatic condition called eutrophication. Eutrophication is the phenomenon of algal or phytoplanktonic bloom in fresh and marine waters as a result of accumulation of excess nutrients (C, N, P) as a result of discharging insufficiently treated wastewater. It poses detrimental consequences such as deterioration of water quality, toxicity due to cyanobacterial growth, depletion of dissolved oxygen etc., which affects the biotic ecosystem as a whole (Malovanyy, 2017). Apart from eutrophication, reactive forms of ni-trogen such as nitrate/nitrite ions also contributed to significant health issues in humans. Excessive use of fertilizers on agricultural fields resulted in leaching of nitrate ions into the groundwater table and surface water bodies, which are potential sources of drinking water (Woodley et al., 2018; Huang et al., 2017). If the concentration of nitrate ions in the drinking water exceeds certain level, it causes a fatal condition in infants known as methemoglobinemia, where the blood cells lose their capacity to bind oxygen causing tissue hypoxia. Thus, it can be clearly seen that issues related to untreated wastewater has always been a problem in the past and ironically it has been a consistent problem in the present, even in 21st century. It would definitely remain as a problem in the future as well due to the expected
increase in the consequences of global issues such as climate change, population rise, extensive urban-ization and industrialurban-ization. Hence, the trend in 21st century has become more than obvious for many
nations worldwide, to remove not only organic carbon and suspended solids, but also nutrients (mainly nitrogen and phosphorous) from the wastewater and also more stringent effluent discharge limits for the treatment plants are being enforced as a measure to protect the water bodies (Kraemer et al., 2012).
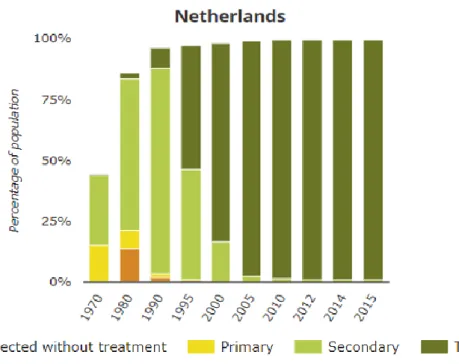
Figure 1.1 shows a perfect example of how the wastewater treatment approach has changed since the end of 20th century in Netherlands owing to the strict European Union (EU) regulations set for the
parameters of effluent water discharge quality. From Figure 1.1 it can be clearly seen that there has been a marked distinction between the treatment methodology followed before the start of 21st century
where much focus was on removing COD, and after the start of 21st century, the focus was diverted
towards nutrients (N and P) removal as well, from the same wastewaters in the same treatment plants. The most conventional process of biological wastewater treatment for nutrient removal has been the activated sludge process. Several innovations in the biological nutrient removal processes were devel-oped since then and one such innovation in anaerobic nitrogen removal was the anaerobic ammonia
2
oxidation (anammox). From here on, nutrient removal refers to only nitrogen removal as the scope of the thesis is oriented only towards nitrogen removal from wastewater.
Figure 1.1. Urban wastewater system in Netherlands before and after the beginning of 21st century
(European Environmental Agency, 2017)*
* Initially before wastewater treatment facilities, sewage collection systems were installed (orange bars). Wastewater was subjected to primary treatment (yellow bars) such as clarification to remove suspended matter, then to secondary biological treatment (light green bars) to remove organic carbon/chemical oxygen demand (COD), followed by more stringent tertiary treatment methods (dark green bars) to remove nutrients
1.2 Global nitrogen cycle and its key players in wastewater treatment
Nitrogen is the most abundant element in the atmosphere which composes of 78% by volume of air in the form of inert dinitrogen gas (N2). Nitrogen exists in various oxidation states ranging from -3 to +5.
Table 1.1 shows different forms of nitrogen present in the environment with respect to their oxidation state. Nitrogen is also the fourth most abundant element in the cells of living creatures as it is the build-ing block for proteins and nucleic acids.
Table 1.1 Various oxidation states of nitrogen and nitrogen species found in the environment
Oxidation state N species Molecular formula
+5 Nitrate ion NO3 -+4 Nitrogen dioxide NO2 +3 Nitrite ion NO2- +2 Nitric oxide NO +1 Nitrous oxide N2O 0 Dinitrogen gas N2 -1 Hydroxylamine NH2OH -2 Hydrazine N2H4 -3 Ammonia/Ammonium ion NH3/NH4+
3
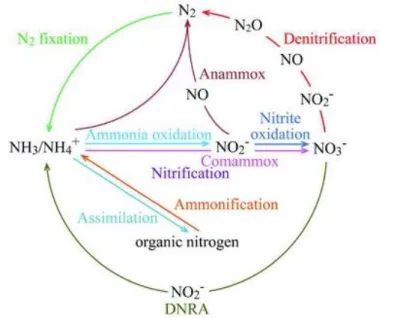
The biogeochemical cycle by which nitrogen undergoes biological and physio-chemical conversions into various chemical forms, where it circulates among different ecosystems is termed as the nitrogen cycle (Stein and Klotz, 2016). The nitrogen cycle demands particular scrutiny from ecologists since nitrogen availability affects the progress of essential processes of various ecosystems, such as biomass production and decomposition (Stein and Klotz, 2016; Kuypers et al., 2018). There are various stages in the global nitrogen cycle, each playing crucial roles in maintaining nitrogen balance on Earth (Figure 1.2).
Figure 1.2. Classic biogeochemical nitrogen cycle updated with the recently discovered comammox process (Dang & Chen, 2017)
With respect to conventional municipal wastewater treatment by the activated sludge process, dissolved nitrogen is removed by two different routes: (1) nitrification coupled to denitrification, and (2) assimi-lation of ammonium during microbial growth. However, to remove dissolve nitrogen from various kinds of N-rich wastewaters, anammox has been found to be a more economical and eco-friendlier alternative route (Hauck et al., 2016). In this thesis, only the main contributors of nitrogen cycle associated with the biological nitrogen removal process in wastewater treatment are discussed below. They are,
• Nitrification • Denitrification • Anammox 1.2.1 Nitrification
Nitrification is the biological conversion of ammonia to nitrate via nitrite. Warington in 1878 discovered the microbial proof of nitrification interestingly only 20 years after denitrification had been discovered (Warington, 1878). Later, Winogradsky justified that specific groups of bacteria were responsible for nitrification process, especially that the task of nitrification was divided between two distinct groups of aerobic chemolithoautotrophic bacteria named ammonium oxidising bacteria (AOB) and nitrite oxidis-ing bacteria (NOB) feedoxidis-ing on inorganic nitrogen species. Hence it was known that nitrification was carried out in two steps. The first step is the oxidation of ammonium (NH4+) to nitrite (NO2-) by AOB
and the second step is the oxidation of nitrite to nitrate (NO3-) by NOB. Oxygen is the electron acceptor
4
above a dissolved oxygen (DO) concentration of 1 mg O2/L (van der Star, 2008). The overall
nitrifica-tion reacnitrifica-tion is given by (1),
NH4++ 2O
2 → NO3−+ 2H++ H2O (1)
However, recently some species of nitrifying bacteria belonging to the genus Nitrospira were found to oxidise ammonia via nitrite to nitrate on their own without division of labour between two bacterial groups as seen above with another organism (van Kessel et al., 2015). This complete oxidation of am-monia to nitrate performed by a single group of bacteria, was termed as comammox process and has been reported to exist in several nitrification reactors of wastewater treatment plants (M. Wang et al., 2018; Roots et al., 2019). The biodiversity and metabolism of comammox bacteria are still under early stages of research and falls out of the scope of this thesis.
1.2.1.1 Aerobic ammonia oxidation
Ammonia oxidation, otherwise called as nitritation, is the oxidation of ammonia to nitrite and is the first step of nitrification. Four genera of AOB namely, Nitrosomonas, Nitrosospira, Nitrosococcus,
Nitro-sovibrio have been commonly reported to carry out ammonia oxidation at mesophilic conditions, out
of which Nitrosomonas europaea is the most frequently studied and usually found in activated sludge based municipal wastewater treatment units with an optimum pH range of 6-8 and temperature range of 20-30°C (Arp et al., 2002; Purkhold et al., 2003). The mechanism of ammonia oxidation studied in AOB like Nitrosomonas europaea proved that it involved two reactions. Initially, ammonia, the electron donor, is oxidised to hydroxylamine (NH2OH) in the presence of oxygen as the electron acceptor which
has been shown in (2). A key enzyme named ammonium monooxygenase (AMO) catalyses the reaction. Then hydroxylamine is further converted to nitrite with the aid of another enzyme termed as hydroxyl-amine oxidoreductase (HAO) and the chemical reaction has been shown in (2). Two of the four elec-trons released in (3) are consumed in (2) and the remaining two elecelec-trons go through the electron transport chain to generate a proton motive force and thereby reduce oxygen as shown in (4) (Arp et al., 2002).
NH3+ O2+ 2H++ 2e−→ NH2OH + H2O (2) NH2OH + H2O → NO2−+ 5H++ 4e− (3)
0.5O2+ 2H++ 2e−→ H2O (4)
Interestingly, there have been lab scale thermophilic nitrification studies at 50°C which have reported that the dominant community carrying out ammonium oxidation under thermophilic conditions were ammonium oxidising archaea (AOA) (Courtens et al., 2016; Vandekerckhove et al., 2019). Five im-portant clusters of AOA such as Nitrosocosmicus, Nitrososphaera, Nitrosotaela, Nitrosocaldus, and
Nitrosopumilus have been identified in the literature so far (Lehtovirta-Morley, 2018). These
microor-ganisms not only exist in natural habitats along with AOB, but have also been found to thrive in extreme ecological conditions such as deep marine sediments, hot springs etc (Dang et al., 2009; De La Torre et al., 2008), where other ammonia oxidisers cannot thrive. The enzymatic pathway for ammonium oxi-dation in AOA has been reported to be slightly different from AOB. AOA have been identified to con-tain diverse subunits of AMO enzyme for carrying out ammonia oxidation to hydroxylamine, but have been speculated not to contain the enzyme HAO for hydroxylamine oxidation, implying the existence of a novel enzyme for hydroxylamine oxidation in AOA (Lehtovirta-Morley, 2018; Vajrala et al., 2013).
5
Another study has proposed a different pathway which involves nitroxyl (HNO) instead of hydroxyla-mine as an intermediate, where nitroxyl oxidoreductase was responsible for carrying out nitroxyl oxi-dation to nitrite (Walker et al., 2010). However, the exact enzymatic pathway of ammonia oxioxi-dation in AOA is still under debate.
1.2.1.2 Nitrite oxidation
Oxidation of nitrite formed in the previous step to nitrate is the second step of nitrification, commonly termed as nitratation. Nitrite oxidation is performed by a specific group of NOB namely Nitrobacter,
Nitrospira, Nitrococcus, Nitritroga, Nitrospina and Nitrolancea, out of which genera like Nitrobacter
and Nitrospira are the most typically studied species and usually spotted in municipal activated sludge-based wastewater treatment systems (Daims et al., 2016). The primary enzyme of NOB responsible for catalysing the nitrite oxidation is nitrite oxidoreductase. Nitrite acts as the electron donor and it flows through the electron transport chain generating a proton motive force, with oxygen as the terminal elec-tron acceptor, subsequently reduced to water as given in (5) and (6) (Bartosch et al., 1999).
NO2−+ H
2O → NO3−+ 2H++ 2e− (5) 0.5O2+ 2H++ 2e−→ H2O (6) 1.2.2 Denitrification
The biological reduction of the produced nitrate from nitrification takes place under anoxic conditions and is termed as denitrification. It is performed mainly by the chemoorganoheterotrophic bacteria be-longing to the alpha and beta classes of Proteobacteria (Malovanyy, 2007). Nitrate is used as the ter-minal electron acceptor in the anaerobic respiration chain, which is a four-step mechanism in which nitrate is reduced in subsequent steps to NO2-, NO, N2O, N2 with the help of the enzymes nitrate
reduc-tase, nitrite reducreduc-tase, nitric oxide reductase and nitrous oxide reducreduc-tase, respectively. The end product is the evolution of dinitrogen gas as shown in (7) (Ding, 2015).
4NO3+ 5[CH2O] + 4H+ → 2N2+ 5CO2+ 6H2O (7)
Since many of these bacteria are chemoorganoheterotrophic, they require organic carbon as electron source for catabolism and as carbon source for anabolism. Normally, readily biodegradable organic substances present in the wastewater are consumed by the denitrifiers as their carbon source but in case of deficiency, external carbon rich substrates such as methanol is added to substitute for the organic carbon lacking in the wastewater (Peng et al., 2007; Ahn, 2006)
1.2.3 Anammox
Anammox refers to anaerobic ammonia oxidation, sometimes also referred to as anoxic ammonia oxi-dation, since the process takes place in anoxic conditions. Anammox bacteria use ammonium as the inorganic electron source, nitrite as the electron acceptor and catabolize them to dinitrogen gas. Anam-mox is a key process in global nitrogen cycle as they are found ubiquitously in anoxic ecosystems, especially in marine sediments (Lam et al., 2009), WWTPs (Jetten et al., 2003), lakes (Schubert et al., 2006) etc. It has been reported that anammox contributes to 24-67% of global N loss in marine sedi-ments (Zhang and Okabe, 2020). Since these bacteria have autotrophic mode of nutrition, they fixate
6
CO2 as source of carbon for anabolism. Anaerobic ammonia oxidation is performed by a group of
ob-ligate anaerobic chemolithoautotrophic bacteria belonging to the phylum Planctomycetes. Till date, 19 species belonging to 6 genera namely, Kuenenia, Brocadia, Anammoxoglobus, Jettenia, Scalindua and
Anammoximicrobium have been identified from various synthetic and natural ecosystems (Khramenkov
et al., 2013; Oshiki et al., 2016). In spite of having a relatively large phylogenetic distance, all the anammox bacteria are placed under the same order, Brocadiales.
1.2.3.1 History and discovery of anammox
Broda anticipated in 1977 based on thermodynamic and evolutionary aspects, that oxidation of ammo-nia under anoxic environment in the presence of nitrate or nitrite should have existed/exist (Broda, 1997). However, anoxic ammonium conversion was found for the first time in a pilot level denitrifica-tion reactor operating at a yeast producdenitrifica-tion facility in Delft, The Netherlands in 1985. This novel process was then termed as anammox, which stood for anoxic/anaerobic ammonium oxidation (Mulder et al., 1995). The pilot level reactor in which the anammox process was observed for the first time was scaled down to laboratory for the enrichment of anammox culture, where nitrite was found to be the actual electron acceptor for the biochemical catabolic process (van de Graaf et al., 1996).
1.2.3.2 Unique cell biology of anammox bacteria
Anammox bacteria have complicated cell characteristics with unusual structural and metabolic features when compared to typical bacterial ones, but they do possess features that conform to all the three domains namely, Bacteria, Archaea and Eukarya (van Niftrik and Jetten, 2012). All anammox bacteria fall under the phylum Planctomycetes, but are anatomically and physiologically different from other
Planctomycetes. One of them is, they are anaerobic chemolithoautotrophic in nature, whereas almost
all the other Planctomycetes are aerobic chemoorganoheterotrophic in nature (van Niftrik and Jetten, 2012). Planctomycetes in general are different from other groups of bacteria by their cellular structure. They have more complex compartmentalization inside their cells because of the presence of intracyto-plasmic membranes.
1.2.3.2.1 Anatomy
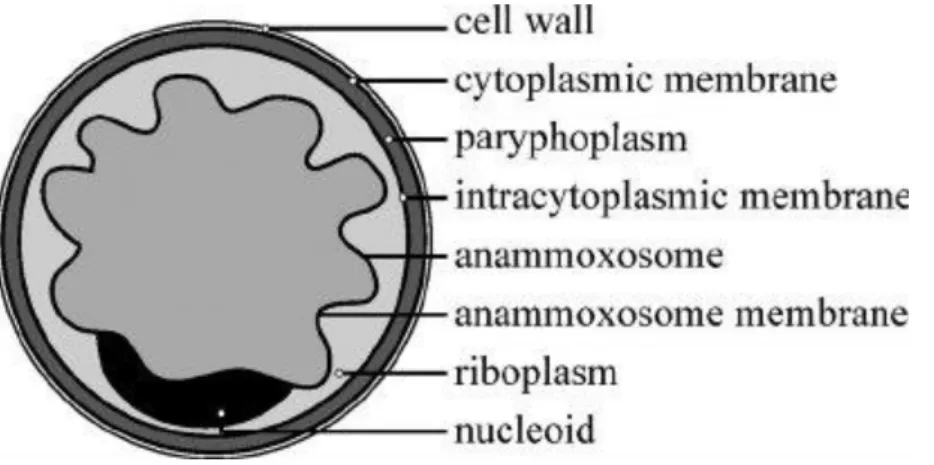
Anammox bacteria have an average cell diameter between 800 and 1,100 nm and are coccoid in shape (van Niftrik et al., 2008). As seen above, anammox bacteria are proposed to have internal compartments. The anammox bacterial cell structure given in van Teeseling et al. (2014) has been shown in Figure 1.3. There are totally 3 cytoplasmic compartments found in anammox bacteria that are covered by single bilayer lipid membranes. The main and innermost compartment is called as the anammoxosome and this compartment acts as the power house of the cell, as it is the place where the catabolic reactions are hypothesized to occur. Anammoxosome is surrounded by the anammoxosome membrane which plays a crucial role in generating a proton motive force to synthesise adenosine triphosphate (ATP). Hence this membrane could be figured out as relatively less permeable for protons in order to hamper the breakdown of proton gradient across the membrane during ATP synthesis and this could be attributed to the presence of unique ladderane phospholipids (Moss et al., 2018). These dense ladderane lipids are a special feature of anammox bacteria and their structure is unique in such a way that it gives more density and rigidness to the membranes than usual straight chain lipids would give. The ladderane lipids not only make the anammoxosome membrane less permeable for proton diffusion but at the same time render the membrane more permeable for other metabolites such as hydrazine to diffuse to the other compartments inside the cell (Moss et al., 2018).
7
Figure 1.3. Cell anatomy of anammox bacterium showing different components (van Teeseling et al., 2014)
1.2.3.2.2 Metabolism
The metabolism of anammox bacteria is different from other Planctomycetes and chemolithotrophic organisms. The main substrates for anammox catabolism are ammonium and nitrite. The anammoxo-some is the site where catabolic reactions take place. This was first hypothesized based on observing a hydroxylamine oxidoreductase (HAO)-like enzyme in the anammoxosome of Ca. Brocadia anammox-idans and Ca. Kuenenia stuttgartiensis (Jetten et al., 2009), justifying that anammoxosome is the cata-bolic centre. Apart from ammonium and nitrite, other substances such as formate, acetate and propio-nate have been used as electron donors for energy conservation by oxidising them to CO2 with
ni-trate/nitrite as electron acceptor in Ca. Kuenenia stuttgartiensis, Ca. Brocadia fulgida, Ca. Annamoxoglobus propionicus, (Lawson et al., 2019; Kartal et al., 2008; 2007a). Ca. Kuenenia stuttgratiensis has also been reported to carry out dissimilatory nitrate reduction to ammonium (DNRA) under ammonium limiting conditions (Kartal et al., 2007b).
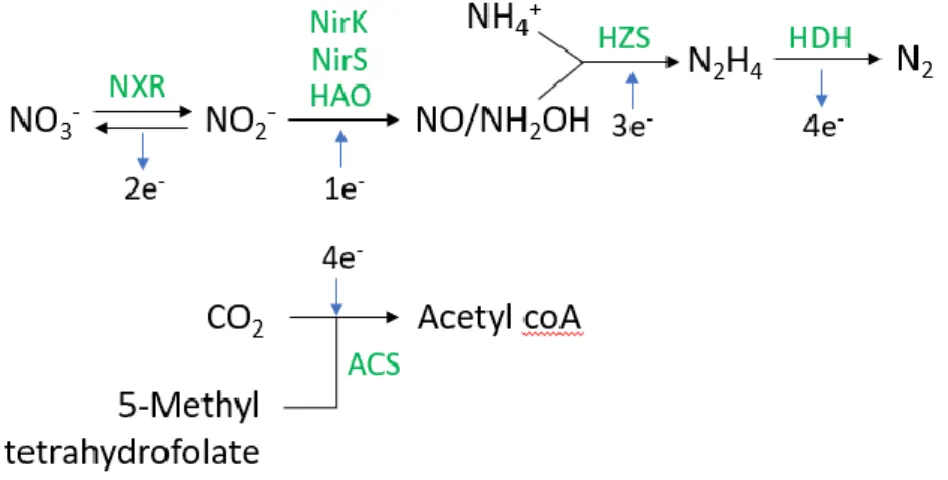
The detailed enzymatic pathway of conventional anammox metabolism similar to the one proposed by Oshiki et al. (2016a) has been shown in Figure 1.4. The anammox catabolic reaction involves the re-duction of nitrite to nitric oxide (NO) by nitrite reductase enzyme as the first step, where cytochrome cd1-type nitrite reductase (NirS) has been expressed in Ca. Kuenenia stuttgartiensis (Strous et al., 2006)
and Ca. Scalindua profunda (van de Vossenberg et al., 2013), copper containing nitrite reductase (NirK) has been expressed in Ca. Jettenia caeni (Hira et al., 2012) and Ca. Brocadia caroliniensis (Park et al., 2017). Some other Ca. Brocadia sp. such as Ca. Brocadia sinica have been hypothesised to lack the canonical Nir genes but express other HAO-like proteins that catalyze the reduction of nitrite to hy-droxylamine (NH2OH), instead of nitric oxide (Oshiki et al., 2016b). Nitric oxide combined with
am-monium to produce another intermediate hydrazine (N2H4) in the presence of the enzyme hydrazine
synthase (HZS). Hydrazine is further oxidised to dinitrogen (N2) gas by the enzyme hydrazine
dehy-drogenase (HDH). As a result of these reactions, a proton motive force is generated which results in passive diffusion of protons from the anammoxosome to riboplasm through the membrane bound ATPases, thereby catalysing the synthesis of ATP (van Niftrik and Jetten, 2012). Anammox anabolic reaction involves CO2 fixation via the Wood-Ljungdahl pathway, catalysed by the key enzyme CO
dehydrogenase/acetyl coA synthase (ACS). Anabolic reaction also involves the oxidation of nitrite to nitrate to obtain electrons for CO2 reduction, catalysed by the enzyme nitrite/nitrate oxidoreductase
(NXR), which also helps in the reduction of nitrate to nitrite for oxidising organic compounds as seen above (Oshiki et al., 2016a).
8
Figure 1.4. Enzymatic pathway of anammox metabolism based on Oshiki et al. (2016b). NXR: Ni-trite/nitrate oxidoreductase; NirK: Nitrite reductase containing copper; NirS: cytochrome cd1-type
ni-trite reductase; HAO: Hydroxylamine oxidoreductase; HZS: Hydrazine synthase; HDH: Hydrazine de-hydrogenase; ACS: CO dehydrogenase/ acetyl coA synthase
1.2.3.3 Stoichiometry and growth of anammox bacteria
As discussed earlier, the anammox process involves ammonium and nitrite as the main substrates for energy generation. The mode of nutrition is autotrophic, so anammox bacteria can fix CO2 instead of
releasing it. Therefore, bicarbonate (HCO3-) is the carbon source for growth and it has been proposed
that ammonium is the nitrogen source for assimilation (Strous et al., 1998). The biomass yield factor on ammonium reported by many studies has remained fairly constant between 0.05-0.15 mol-C/mol-NH4+ in most of the studies (Tang et al., 2010; Puyol et al., 2013). The overall anammox stoichiometric
equation provided by Strous et al. (1998) has been shown in (8),
NH4++ 1.32NO 2 −+ 0.066HCO 3 − + 0.13H+→ 1.02N 2+ 2.03H2O + 0.066CH2O1.5N0.15+ 0.26NO3− (8)
Later, Lotti et al. (2014) used a highly pure enrichment culture (98%) growing in free-cell suspension in a membrane bioreactor (MBR) to study the anammox stoichiometry and kinetic parameters. As ex-pected, the anammox stoichiometry was revised as shown in (9),
NH4++ 1.146NO2−+ 0.071HCO3−+ 0.75H+
→ 0.986N2+ 2.002H2O + 0.071CH2O1.5N0.15+ 0.161NO3− (9)
The inaccuracy in the work of Strous et al. (1998) was a result of the aggregate nature of the biomass enriched in the sequencing batch reactor (SBR) and also the enrichment level was only 74%. In order to evaluate the exact stoichiometry and kinetic parameters, it is highly essential to have a relatively pure enrichment culture and suspended cell growth of the biomass, since the mass transfer limitations within the system would be the least (Lotti et al., 2014).
Similarly, anammox bacteria are generally considered to be slow growing microorganisms when com-pared to other chemolithoautotrophic microorganisms. Maximum specific growth rates (µmax) as low as
0.065 d-1 (Strous et al., 1998), with doubling times (T
d) in the range of 10-30 days (Wett et al., 2010)
9
Brocadia sp.40 (0.334 d-1), with a corresponding doubling time of around 2 days. The reason was
at-tributed to the higher biomass specific electron transfer rates achieved due to minimum mass transfer limitations experienced in a suspended cell culture operated at very low solids/sludge retention time (SRT). Moreover, in the previous studies, the systems were operated under substrate limited conditions in order to avoid potential nitrite inhibition (Carvajal-Arroyo et al., 2013) and at high SRT values using aggregated biomass, which led to inaccurate estimation of maximum growth rate values (Lotti et al., 2012). Recently, Zhang et al. (2017) also achieved an equally high µmax of immobilized planktonic cells
of Ca. Brocadia sinica (0.33 d-1) by tracking its population increase using newly developed quantitative
polymeric chain reaction (qPCR) assays. Therefore, anammox bacteria cannot be abruptly branded as slow growing microorganisms, as in the end it depends on the way the microorganisms are cultured (Lotti et al., 2015).
1.3 Commercial application of mesophilic anammox in wastewater treatment
There have been a lot of advances in the nitrogen removal technologies over the last 50 years. However, conventional nitrification-denitrification remains as the dominant approach in activated sludge (CAS) process. But this process has some drawbacks especially when considering that the aeration required for COD and N removal is energy intensive and the addition of excess organic carbon source such as methanol for pre-denitrification is required in case of low COD/N in the influent. This in turn implies that the conventional biological nitrogen removal process would become expensive and the overall footprint of WWTPs could be compromised (van Loosdrecht et al., 2004).
A possible alternative way of removing nitrogen from wastewaters deficient of biodegradable organic carbon in a more sustainable and economical way is the anammox process. But its application is not straightforward. Wastewaters generally do not contain much nitrite as most of the total nitrogen would be in the form of organic N or ammonium. Hence, the supply of nitrite for anammox bacteria determines the implementation of the process. The most commonly used configuration to overcome the limitation of nitrite availability in wastewater is the combined partial nitritation and anammox (PN/A) system. In this approach, half of the ammonium present in the influent is oxidised to nitrite by AOB and the re-maining half of the ammonium and the produced nitrite ions could be used as substrates by the anam-mox consortium, suppressing the NOB growth (van Dongen et al., 2001). It is important to suppress NOB in PN/A systems since NOB can compete with anammox bacteria for nitrite, thereby reducing the anammox activity and hence the overall nitrogen removal efficiency. The ways to suppress NOB and select for AOB and anammox bacteria in PN/A process have been discussed in detail in sub-section 1.4.3. The PN/A technique can be applied in one stage (singe reactor) or in two stages (two reactors), depending upon the requirements. Several anammox technologies and configurations such as SHARON (Sustainable high rate ammonium removal over nitrite)-Anammox (van Dongen et al., 2001), CANON (Complete autotrophic nitrogen removal over nitrite) (Sliekers et al., 2003), OLAND (Oxygen limited autotrophic nitrification/denitrification) (Kuai and Verstraete, 1998), DEAMOX (Denitrifying ammo-nium oxidation) (Kalyuzhnyi et al., 2006), DEMON (Deammonification) (Wett, 2006) etc. have been developed over the years and are still under research in order to engineer their robustness and improve their economic viability.
The anaerobic chemolithoautotrophic nature and low biomass yield of anammox bacteria offers several advantages for economical application of anammox process via PN/A as an alternative to treat wastewaters having low COD/N ratio over CAS (Jetten et al., 2005; van Loosdrecht et al., 2004). How-ever, there are some more challenges in applying anammox process for mainstream municipal wastewater treatment. Anammox process has been found to work optimally in wastewaters having low
10
quantities of biodegradable organic substrates (bCOD), preferably bCOD/N<3 under mesophilic con-ditions (30-40°C) (Lackner et al., 2014). Since, the influent sewage is mostly rich in bCOD and often the temperature of the wastewaters drops below 15°C, mainstream mesophilic treatment by anammox has been challenging (Ali and Okabe, 2015). The higher bCOD load in the mainstream line enhances the growth of heterotrophic denitrifiers which can also compete for nitrite as a substrate, disrupting the anammox growth. As a result, anammox process has been successfully implemented for treating side stream wastewater rich in ammonium, scarce in biodegradable organics and also at mesophilic temper-atures, for example, reject water from dewatering anaerobically digested sludge which could signifi-cantly add to the total N load of the mainstream treatment line if recycled untreated. Recently, there have been some studies about the various operational strategies that would be required for achieving the application of PN/A for mainstream sewage treatment at cold temperatures (<15°C) (Laureni et al., 2016; Agrawal et al., 2018). The application of mainstream anammox process in WWTPs could change their status from energy intensive to energy neutral or generating units (Verstraete and Vlaeminck, 2011). Apart from municipal wastewater, anammox process has also been implemented to treat indus-trial and other wastewaters such as landfill leachate (Ganigué et al., 2008), pig manure (Deng et al., 2019), fertilizer (Du et al., 2019), etc. These wastewaters are characterized by high total nitrogen (TN) when compared to low biodegradable organic substances, facilitating the application of anammox pro-cess to treat them.
1.3.1 The need for thermophilic nitrogen removal
Biological treatment of wastewater at high temperature, especially at thermophilic temperature range (40-60°C) has been studied extensively in the recent years due to its potential opportunities and practi-calities (Lopez-Vazquez et al., 2014). Biological nitrogen removal by CAS process in cold and temper-ate regions is normally carried out at temperatures between 10-20°C, but the same process has also been proven viable for treating relatively warmer wastewater of industries, with temperatures as high as 35-40°C (Pinzón Pardo et al., 2007). However, there are several sources of hot wastewaters such as, (i) Reject water from thermophilic anaerobic digesters, (ii) Specific industrial effluents and (iii)Factories where waste heat could be recuperated for heating process water (Courtens et al., 2014). Typically, in order to treat warm wastewaters biologically, a pre-treatment is required for cooling down the wastewater to mesophilic temperatures using heat exchangers or cooling towers. However, this step demands high water consumption and pumping energy which increases the operational costs. Therefore, the development of thermophilic biological nitrogen removal technologies would be a more sustainable and economical option for treating warm wastewaters (>40°C), since it would make the pre-cooling step redundant (Lopez-Vazquez et al., 2014).
Thermophilic treatment methods have been broadly explored in wastewater treatment mainly for COD removal and also in anaerobic digestion, due to its potential benefits over mesophilic treatment such as higher activity of microorganisms, higher removal rates, lower sludge yield, higher biogas production and better disinfection (Layden et al., 2007; De Vrieze et al., 2016). But research on thermophilic ni-trogen removal is quite limited and has been emerging only over the past few years. There are recent studies on thermophilic nitrifying community in lab scale bioreactors with synthetic wastewater at 50°C, which showed the dominance of thermophilic ammonium oxidising archaea (AOA) in contrary to the AOB in mesophilic ammonia oxidation, carrying out ammonium oxidation under thermophilic condi-tion and NOB carrying out nitrite oxidacondi-tion (Courtens et al., 2016; Vandekerckhove et al., 2019). The kinetic parameters of the thermophilic AOA and NOB showed that the nitrite oxidation could be inhib-ited achieving the selection of thermophilic AOA for partial nitritation. Similarly, thermophilic hetero-trophic denitrification and denitritation at 55°C were also studied recently in lab scale with synthetic
11
medium and the results showed decreased sludge production, better sludge settleability and lower or-ganic carbon requirement compared to mesophilic (Courtens et al., 2014; Vandekerckhove et al., 2018). These recent lab scale experiments have opened up the possibility of using thermophilic nitrification/de-nitrification processes to treat warm wastewater characterized by the presence of high organic load (bCOD/N>3) without economic constraints. However, extensive research should be carried out with real wastewater to test the inhibitory effects of wastewater metabolites on the activity of thermophilic biomass. Hence the full-scale application of thermophilic CAS is presently very limited but has an optimistic scope in near future. The development of thermophilic nitrifying and denitrifying communi-ties became a motivation factor for exploring the possibility of thermophilic anammox as a part of the thermophilic nitrogen removal processes.
1.3.2 Thermophilic anammox as a novel tool
Thermophilic nitrogen removal by PN/A process could be a major breakthrough for treating carbon deficient warm wastewaters but are quite rich in nitrogen, especially when the bCOD/N value drops below 2 or 3 (Vlaeminck et al., 2012). Unsurprisingly, the exploration of thermophilic anammox culture has hardly ventured out till date. Yet, the ubiquitous nature of anammox bacteria has been a testimony of its presence in extreme environments, which justifies the possibility of some uncultured thermophilic anammox species. There have been previous literature reports based on 16S rRNA genes providing evidences of anammox bacteria in hydrothermal vents in Atlantic Ocean (Byrne et al., 2009). Few years later, the existence of anammox bacteria in oil reservoirs at 55-75°C in China was reported by Li et al. (2010). Quite recently a study observed the thermotolerance and adaptation of mesophilic anammox bacteria to thermophilic temperature in a UASB reactor operated for just 2 weeks at 50 °C and recorded a nitrogen removal rate of 0.53±0.23 gN/L/d (Zhang et al., 2018). This was attained by steadily increas-ing the temperature in the reactor by 0.18°C/d. However, in all the above works, the physiological and kinetic characterization of the anammox bacteria responsible for thermophilic nitrogen removal was not carried out.
The most recent work reported by Vandekerckhove et al. (2020) involved the characterization of a thermophilic anammox community. First, a fixed bed biofilm reactor (FBBR) was used to enrich a thermophilic anammox culture by employing a step wise increase in the temperature from 38°C to over 48°C, operating the reactor for more than 200 days at 48°C. Then the thermophilic anammox enrich-ment culture was inoculated in an MBR at 50°C to obtain suspended biomass growth for characteriza-tion. Sanger sequencing of the 16S rRNA genes showed 94-95 % similarity to Ca. Brocadia related species which accounted for 45% of the community (Vandekerckhove et al., 2020). The study also hypothesized the presence of a novel thermophilic anammox species but further research on phyloge-netic analysis should be performed to validate it. The net growth rate of the thermophilic anammox community was found to be in the range of 0.075-0.19 d-1 an was higher than the growth rates of
mes-ophilic anammox species grown in reactors with high SRT (Vandekerckhove et al., 2020). But the study lacks information about the kinetic and stoichiometric parameters such as affinity constants, maximum specific growth rate or true yield of the thermophilic anammox culture.
Moreover, from the above discussed studies on thermophilic nitrification (Courtens et al., 2016; Vandekerckhove et al., 2019), the possibility for the implementation of novel single stage thermophilic PN/A process for treating warm wastewaters such as reject water of thermophilic digesters has been put to interest. However, there could be a risk of higher sludge production because of the higher growth yield of thermophilic AOA, which might complicate the sludge handling (Vandekerckhove et al., 2019).
12
But the intrinsic kinetic parameters of both thermophilic nitrifiers and anammox have not been esti-mated accurately so far and hence still bound to modifications. Further research with highly en-riched/pure cultures cultivated in the form of free planktonic cells is needed to obtain the accurate knowledge on the stoichiometry, growth and physiological characteristics of these bacteria.
1.4 Ecological interactions between different nitrogen transforming microorganisms
The main concept behind the simultaneous nitrogen transformation processes is that the different mi-crobial groups collaborate as well as compete for similar substrates and space, so that the efficiency of substrate competition as well as the nitrogen removal efficiency depends on the process parameters and operational conditions. Initially, the partial nitritation process (SHARON) was coupled with anammox process in two stages for example in the WWTP of Dokhaven (van Loosdrecht et al., 2004). However, to reduce the footprint and CAPEX of the treatment systems, there have been various developments to combine PN/A in a single reactor. In single-stage PN/A (CANON) process, NOB growth has to be suppressed to maintain high nitrogen removal as NOB could compete for substrates such as oxygen with AOB and nitrite with anammox bacteria (when operated as single stage), thereby hampering the autotrophic nitrogen removal efficiency. But, AOB can outcompete NOB for oxygen at low DO con-centrations since AOB has a higher affinity for oxygen than NOB (𝐾𝐴𝑂𝐵𝑂2 <𝐾𝑁𝑂𝐵𝑂2 ). Even though there are different ways to suppress NOB growth with different operational strategies at different operational conditions as explained in Agrawal et al. (2018), from modelling point of view in this study, by main-taining low DO concentrations in the wastewater nitrite oxidation can be prevented (Joss et al., 2009). Maintaining low DO concentrations is also very essential as the anammox bacteria are sensitive to ox-ygen and are inhibited reversibly even by the presence of even small amount of oxox-ygen of about 0.5-2% of air saturation (Strous et al., 1997; Egli et al., 2001). For this reason, the single stage OLAND process is normally carried out with biofilms such as granular sludge, so that the anammox bacteria could grow well inside the granules where they have very less exposure to oxygen (Liu et al., 2008). Several studies have reported the distribution of the bacterial community within the CANON/OLAND biofilms which show that the nitrifiers tend to occupy the outer aerobic layers as the oxygen diffusion is relatively good and the anammox bacteria are located in the inner anaerobic layers where oxygen cannot diffuse as it is almost completely consumed by the over-lying nitrifiers (Zhang et al., 2014; Gonzalez-Martinez et al., 2016).
Most of the above-mentioned studies were focussed on removing nitrogen from wastewaters devoid of organic carbon in an exclusively autotrophic way. However, in reality, most of the high-strength nitrog-enous wastewaters contain some amount of biodegradable COD with low carbon to nitrogen ratios (C/N), such as pre-treated municipal sewage (Leal et al., 2016), urine (Jenni et al., 2014), digester su-pernatant (Joss et al., 2009) etc. which might promote the growth of heterotrophic bacteria. There have also been reports of the coexistence of heterotrophic bacteria in autotrophic biofilms, consuming the biomass decay matter and extracellular polymeric substances (EPS) present in the biofilms (Okabe et al., 2005; Kindaichi et al., 2004). Nevertheless, heterotrophic denitrifying bacteria can utilize nitrate, produced by anammox bacteria as a by-product of anabolic reaction to oxidise the COD present in the influent wastewater. Heterotrophic denitrifiers can also compete with anammox for nitrite as electron acceptor to oxidise COD (Ruscalleda et al., 2008; 2010). Hence high amount of organic matter might favour the growth of denitrifiers as anammox bacteria could be outcompeted for nitrite due to the higher growth rate of denitrifiers (Vlaeminck et al., 2012; Nogueira et al., 2005; Lackner et al., 2008). The most important parameter which could affect this competition between anammox and denitrifiers for nitrite would be the COD/N ratio (Han et al., 2016; De Clippeleir et al., 2013). Typical mainstream municipal wastewater line has a very high COD/N ratio around 10-20 which would promote excessive
13
growth of denitrifiers and anammox bacteria would not be able to compete for nitrite under such cir-cumstances (Ali and Okabe, 2015; Zhang et al., 2019).
1.5 Mathematic modelling of biological nitrogen removal (BNR)
1.5.1 Insight into BNR modelling
A model can be stated as a meaningful and simplified description of a process/system of interest (Henze et al., 2008). With comprehensive mathematical models, it has been possible to design nutrient removal processes with high precision when compared to the traditional black-box approach of design based on influent and effluent composition. The diverse and complicated set of information fed to the models makes the transition from black-box approach to grey- or white-box approach smooth, depending upon the requirement (Gernaey et al., 2004). Modelling offers opportunities for process development when compared to mere experimental work, since various process conditions can be tested in a short period of time which makes the overall process optimization easier, especially during the scale up of wastewater treatment processes. It also helps to achieve multiple goals of sustainable nutrient removal from wastewater by giving a better insight about the unit processes involved and simultaneously meet-ing the economic demands as well (Hao et al., 2005). Modellmeet-ing studies on wastewater treatment to remove nutrients have been gaining a lot of attention over the past decade since the formulation of activated sludge models (ASM) (Henze et al., 2000). Several models such as ASM1, ASM2, ASM2d, ASM3 and ASMN have been applied for biological nitrogen removal at different configurations and conditions by various authors (Hiatt and Grady, 2008; Hao et al., 2002). Table A1 gives an idea about the different models used by various authors for BNR modelling. Models are normally calibrated/vali-dated using experimental data and simulated using a simulation software. Different simulation packages used for modelling nitrogen removal by various authors have been also listed in Table A1.
Modelling BNR can be carried out in two ways, steady state modelling and dynamic modelling. Steady state modelling involves deriving steady state rate equations of the biochemical conversion processes taking place in the system using mass balances of the components involved in the conversion (Jia et al., 2018). The model simulations are not performed with respect to time as the mass balance data used for model calibration are already under steady state. On the contrary, dynamic modelling aims to establish the changes taking place in the biochemical conversion processes with respect to time (Henze et al., 2008). Dynamic models are usually calibrated with the real time reactor performance data and are sim-ulated using a simulation program such as MATLAB, Aquasim etc. Moreover, dynamic models can also be built without calibrating on reactor performance data, just to predict and visualize the trend of particular transformation processes mathematically, with respect to some changes in the inputs given to the model (Henze et al., 2008).
Generally, the different model components which represent the measurable wastewater should be ex-pressed as influent vectors, in terms of concentrations. The treatment unit has to be hydraulically mod-elled, with various compartments as the required reactors of the process flow scheme. Each reactor has to be modelled separately according to the specific mass balance equations, mixing, mass transfer char-acteristics etc. and the reactors are linked hydraulically to complete the process flow (Meijer and Brdja-novic, 2012). The mass balance equations used to describe the processes taking place in a reactor correspond to a biokinetic model. These equations measure the concentration changes in a system, re-sulting out of biochemical transformations as a function of time. At steady state the concentration change with respect to time is zero. The simple representation of a mass balance equation to measure
14
the rate of conversion of a particular component (i) in the reactor can be expressed in terms of the difference in its influent and effluent concentrations with time as shown in 10,
𝑟𝑖= (𝑆𝑒𝑓𝑓− 𝑆𝑖𝑛𝑓) ∗ 𝑄
𝑉 (10)
Sinf, Seff are the influent and effluent concentrations of component i (gN/m3 or gCOD/m3), Q is the
influent flow rate (m3/d), V is the volume of reactor (m3), r
i is the rate of conversion of i (gN/m3/d or
gCOD/m3/d).
The stoichiometry of the reactions can be expressed based on the components involved in the model and balances are employed to assign the respective coefficients. In biomass growth based kinetic mod-els, the biomass stoichiometric coefficient is set as 1 and the stoichiometric coefficients of other com-ponents are expressed as a function of biomass yield coefficient (Y), which are then translated into a Gujer matrix (Henze et al., 2000). The matrix format is preferred as it helps in formulating the model into a simulation program. The matrix columns represent the components and rows represent the pro-cesses involved in the model. The sign conventions followed for the coefficients is, negative sign is used to represent consumption and positive sign is used to represent production. The stoichiometric coefficients of the components that influence the particular process have to be listed along the respective process row. The biochemical conversion processes are listed along the left-most column and their pro-cess rate equations are listed along the right-most column of the matrix, respectively. In ASM, Monod kinetic expression is employed as the process rate equations to express microbial growth as shown in 11,
𝜌𝑗= 𝜇𝑚𝑎𝑥∗ 𝑆
𝑆+𝐾𝑠∗ 𝑋 (11)
ρj represents the rate equation of process j (gCOD/m3/d), µmax denotes the maximum specific growth
rate of bacteria (d-1), S denotes the substrate concentration (g/m3), K
s represents the substrate affinity
constant (g/m3) and X represents the biomass concentration.
These process rate equations are multiplied with the respective stoichiometric coefficients to obtain the total conversion rate expression for each component (12).
𝑟
𝑖= ∑ 𝐴
𝑗 𝑖𝑗∗ 𝜌
𝑗(12)
where ri is the rate of conversion of i (gN/m3/d or gCOD/m3/d), Aij is the stoichiometric coefficients, ρj
represents the process rate equations (gCOD/m3/d).
1.5.2 Modelling of simultaneous nitrogen conversion processes in biofilms
Even though the activated sludge models were designed based on conventional nitrification and deni-trification for nitrogen removal, innovative nitrogen removal processes such as anammox have been included in the models based on its respective stoichiometry and parameters. Anammox process has been commonly modelled together with nitrification as partial nitritation/anammox (PN/A) process (Volcke et al., 2010), with heterotrophic denitrification as simultaneous anammox and denitrification (SAD) process (Bi et al., 2015), with both nitrification and heterotrophic denitrification as simultaneous partial nitrification, anammox and denitrification (SNAD) process (Liu et al., 2017). The most frequent configuration opted for modelling these simultaneous nitrogen removal processes in the literature has