AMMONIACAL-ALCOHOL LEACHING
FOR THE EXTRACTION OF
VALUABLE METALS FROM TAILINGS
Number of words: 19,284Felipe Alonso Guerrero Araya
Student number: 01801152
Academic promotor: Prof. dr. Gijs Du Laing
Non-academic supervisor: dr. Maarten Everaert (VITO)
Tutor: dr. Maarten Everaert
Master’s Dissertation submitted to Ghent University in partial fulfilment of the requirements for the degree of International Master of Science in Sustainable and Innovative Natural Resource Management
PREFACE
This Master’s dissertation is the culmination of my studies of the International Master of Science in Sustainable and Innovative Natural Resource Management (SINReM) at Ghent University, Uppsala Universitet, and Technische Universität Bergakademie Freiberg, with the valuable support of the Erasmus + program through the Erasmus Mundus Joint Master Degree scholarship.
I sincerely thank the Waste & Recycling Technology Research Group of the Flemish Institute for Technological Research (VITO) for giving me the opportunity to write my Master’s dissertation on solvometallurgy. I express my gratitude to my non-academic supervisor dr. Maarten Everaert for his guidance and support throughout my research, especially in overcoming the difficulties caused by the COVID-19 scenario. This appreciation is extended to dr. Jeroen Spooren for his critical input and advice during this process, and also to Nor Kamariah, Wendy Wouters, and Warre Van Dun for their support during my experimental work. Finally, I would like to thank my academic promotor Prof. dr. Gijs Du Laing for his contribution during the crucial stages of my thesis and for managing the partnership between SINReM and VITO.
This Master’s dissertation is dedicated to my parents who encouraged me to pursue graduate studies abroad, and to my fiancée who supported me in moving to Europe. This 2-year journey would not have been possible without them.
I hope this thesis contributes to improving resource efficiency in the metallurgical industry as part of the sustainable management of natural resources.
Sincerely,
PREAMBLE
This thesis was originally planned according to the following stages:
1. The study of the solubility of ammonium acetate, ammonium chloride, ammonium carbonate and ammonium sulfate in methanol and ethanol at ambient conditions. 2. The study of the solubility of copper sulfate, lead sulfate, zinc sulfate and iron sulfate
in ammoniacal-alcohol and ammoniacal-aqueous media at ambient conditions. 3. The study of optimized ammoniacal-alcohol leaching systems in the extraction of
copper, lead and zinc from microwave-roasted tailings.
However, the third stage of this study was suspended due to the COVID-19 pandemic. In an overall perspective, this step was supposed to involve the performance of agitated leaching tests of microwave-roasted tailing samples in ammonium salt-alcohol solutions. The specifics of these media would have been decided based on the outcomes of stages 1 and 2.
This scenario was overcome by focusing on complementing both data and analysis of stages 1 and 2. In this context, the following stages were conducted.
4. The study of the effect of initial alkalinity of selected ammoniacal-alcohol media on the extraction of copper, lead, zinc and iron from sulfates. For this purpose, the experimental procedure and data analysis were performed by the student under consultation of the non-academic supervisor, whereas the experimental work was performed by VITO technical staff.
5. Modeling of speciation at equilibrium of dissolved ammonium salts in methanol, ethanol and water. This process considered data generated in stages 1, 2 and 4. This preamble was drawn up after consultation between the student and the supervisor and is approved by both.
TABLE OF CONTENTS
Preface 2 Preamble 3 List of abbreviations 6 Abstract 7 1 Introduction 81.1 Importance of base metals in the European Union 8
1.2 Sulfidic tailings as source of base metals 9
1.3 Current processes to recover metals from sulfidic tailings 9 1.4 Ammonia and alcohols for the dissolution of base metals 10
1.5 Research question 11
2 Literature review 12
2.1 Ammoniacal aqueous extraction 12
2.2 Ammoniacal alcohol systems 16
2.2.1 Solubility of ammonia in alcohols 16
2.2.2 Alcohol systems for metal extraction 18
2.2.3 The formation of amines in ammoniacal-alcohol media 20
3 Methodology 23
3.1 Materials 23
3.2 Equipment, instruments, and analytical techniques 24
3.2.1 Solubility test 24
3.2.2 Inductively coupled plasma optical emission spectrometry 24
3.3 Experimental procedure 25
3.3.1 Solubility of ammonium salts in alcohols 25
3.3.2 Solubility of metal sulfates in ammoniacal-alcohol and ammoniacal-aqueous
media 25
3.3.3 Speciation of ammonium salts in alcohol and aqueous media. 27
3.4 Measurement errors 28
4 Results and discussion 29
4.1 Speciation of dissolved ammonium salts 29
4.2 Solubility of ammonium salts in methanol, ethanol and water 32
4.2.2 Modeling of equilibrium speciation of dissolved ammonium salts 35 4.3 Dissolution of metal sulfates in ammoniacal-alcohol and ammoniacal-aqueous
media 39
4.4 Influence of initial alkalinity of ammoniacal-alcohol media on the dissolution of
metals 48
4.4.1 Modeling of equilibrium speciation 48
4.4.2 Solubility of metal sulfates 50
5 Conclusions 54
6 Recommendations for future research 56
7 References 59
Appendices 65
Appendix A. Preparation of alcohol and aqueous solutions for ICP-OES analysis 65 Appendix B. Speciation diagram of dissolved ammonium salts in methanol, ethanol and
water 67
Appendix C. Dissolution of ammonium salts in alcohols 72
Appendix D. Solubility of ammonium salts in methanol, ethanol and water 76 Appendix E. Equilibrium speciation of dissolved ammonium salts in alcohols and water 77 Appendix F. Solubility of metal sulfates in ammoniacal-alcohol and ammoniacal-aqueous
media 82
Appendix G. Modeled equilibrium speciation of dissolved ammonium salts in alcohols with
influence of alkalinity 86
Appendix H. Solubility of metal sulfates in ammoniacal-alcohol media with influence of initial
LIST OF ABBREVIATIONS
Abbreviation Meaning
EU European Union
EI Economic Importance
SR Supply Risk
CRMs Critical Raw Materials
IR Import Reliance
EoL End-of-life
AMD Acid Mine Drainage
MW Microwave
EC Equilibrium Cell
TGC Thermostated Gas Cylinder
SEM Scanning Electron Microscope
XRF X-ray Fluorescence
CAS Chemical Abstract Service
ICP-OES Inductively Coupled Plasma Optical Emission Spectrometry ASTM American Society for Testing and Materials
ABSTRACT
In this thesis, novel solvometallurgical leaching systems for base metals from microwave-roasted sulfidic tailings were explored. The possibility to dissolve ammonia (NH3) in alcohols and the
potential to form soluble metal-ammine complexes motivated the study of ammoniacal-alcohol media for the extraction of Cu, Pb and Zn, while limiting Fe dissolution. A practical approach in the preparation of the leaching media at ambient conditions involved ammonium salts as source of NH3, whereas methanol and ethanol were selected as short hydrocarbon-chain alcohols.
In this research, firstly the solubility and speciation of ammonium chloride, ammonium carbonate, ammonium acetate and ammonium sulfate in both alcohols were determined by experimental work and modeling. Then, the dissolution of Cu, Pb, Zn and Fe sulfates was studied by preparation of supersaturated solutions in ammoniacal-alcohol and ammoniacal-aqueous media. Finally, the effect of the initial alkalinity of selected ammoniacal-alcohol media on metal dissolution was explored by the addition of NaOH at molar ratios of 0, 0.3 and 1, with respect to the initial concentration of ammonium ([NH4+]o).
Ranges of solubility of ammonium salts were determined. The minimum values of each interval in methanol were 0.33 M ammonium chloride, 4.95 M ammonium acetate and 0.55 M ammonium carbonate, whereas in ethanol, 0.03 M ammonium chloride and 1.11 M ammonium acetate were determined. Ammonium sulfate and ammonium carbonate were insoluble in methanol and ethanol, respectively. The modeled speciation indicated that the ammonium-to-ammonia conversion ratio was negligible in ammonium chloride, whereas 61% of ammonium dissolved from ammonium carbonate in methanol was deprotonated. The same parameter for ammonium acetate in methanol and ethanol was calculated in 14% and 52%, respectively.
By the dissolution of metal sulfates in different ammoniacal media, the extraction of the four metals was shown to be higher in water, followed by methanol and ethanol. In alcohols, the highest values were 0.32 M Cu in 0.33 M ammonium chloride-methanol, 0.09 M Pb and 0.27 M Zn in 1.11 M ammonium acetate-ethanol, and 0.10 M Pb and 0.43 M Zn in 4.95 M ammonium acetate-methanol, most of them with a molar ratio over Fe higher than 1. In organic solvents, the dissolution of metals was the result of soluble ammine complexes with Cu and Zn, chloride and acetate complexes with the four metals and insoluble metal carbonates.
The modeling of the effect of a higher initial alkalinity of ammoniacal-alcohol media indicated a higher deprotonation of both ammonium and anion ligands. This effect did not always result in an improved leaching of the target metals, both in quantity and in selectivity over Fe. The best results were obtained in 4.95 M ammonium acetate-methanol at a [OH-]
o/[NH4+]o ratio of 1: 0.71 M Cu and
0.71 M Zn, while Fe was reduced to 0.33 M. Moreover, in 0.33 M ammonium chloride-methanol at a [OH-]
o/[NH4+]o ratio of 1, the lowest concentration of Fe was measured. Regarding Pb, the highest
concentration of 0.10 M was measured in 4.95 M ammonium acetate-methanol at a [OH-]
o/[NH4+]o
ratio of 0. It was shown that metal-hydroxide precipitation could compromise soluble complex formation, and metal dissolution as a whole. In order to improve these results, further research on speciation and solubility of metal-ligand-alcohol systems will be required.
1 INTRODUCTION
1.1 Importance of base metals in the European Union
Base metals refer to industrial, non-ferrous, and more reactive metals (excluding precious metals1) such as copper (Cu), lead (Pb) and zinc (Zn) (Blowes et al., 2003). As seen in Table
1.1, in the European Union (EU) these elements are important components of alloys (as base structure or alloying elements), electrical and technology applications, and products derived from mechanical-thermal treatments.
Table 1.1. End-uses of base metals in the EU (European Commission, 2017b, 2017c).
Metal Major end-uses breakdown
Cu Electrical equipment (22%), metallic products (21%), machinery (15%) Zn Steel products (51%), Zn-alloys (34%), electrical appliances (10%) Pb Batteries (85%), Pb-compounds (6%), rolled and extruded products (4%)
Several indicators leverage the importance of these metals in the development of the EU. Figure 1.1a depicts the Economic Importance (EI) and Supply Risk (SR) indexes of Cu, Pb and Zn, which are compared to the same evaluation but for some precious metals and some Critical Raw Materials2 (CRMs). Even though the SR of base (and precious) metals is lower
than for CRMs, they present a larger EI that motivates the exploration of resources, extraction and processing. Moreover, as presented in Figure 1.1b, the Import Reliance (IR) and the End-of-life (EoL) recycling rate of the studied metals indicate that importing is the main component to meet the EU demand for almost each element per category. However, base metals present higher EoL recycling rate compared to the other groups. Overall, diminishing the IR, maintaining both the low SR and the high EI are strong reasons to optimize the extraction and recovery of base metals from either primary, recycled, or waste streams.
(a) (b)
Figure 1.1. Key indicators of the importance of base, precious and Critical Raw Materials for the EU, a) Economic Importance and Supply Risk indexes, b) Import Reliance and End-of-life recycling rate (European Commission, 2017b, 2017c).
1Precious metals refer to less reactive metals with higher economic value, e.g. gold (Au) and silver (Ag).
2Critical Raw Materials combine raw materials of high importance to the EU economy and of high risk associated with their supply, e.g. indium (In), germanium (Ge), and gallium (Ga) (European Commission, 2017a).
Cu Zn Pb Au Ag Ge Ga In 0.0 0.5 1.0 1.5 2.0 2.0 3.0 4.0 5.0
Supply Risk Index
Economic Importance Index Base Metals Precious Metals Critical Raw Materials
Cu Zn Pb Au Ag Ge Ga In 0 20 40 60 80 100 0 20 40 60 80 100 End -of
-life recycling rate, %
Import reliance, %
1.2 Sulfidic tailings as source of base metals
Regarding the primary processing of base metals-containing sources, sulfide ores are a category of minerals containing sulfide or presulfide anions and represent considerable reserves and resources of Cu, Pb and Zn (Saunders and Miodownik, 1998). The treatment of sulfidic ores to obtain these metals has been commercially driven by flotation, which targets the need to process complex or low-grade ores when gravity-based separation approaches are inefficient. In a general perspective, flotation involves a crushed-and-milled solid (ore), liquid (water) and a gas (bubbled air) phase and relies on increasing the hydrophobicity of the valuable minerals for being attached and collected in air bubbles. The hydrophobic material ends up in a concentrate stream, which is finally processed via pyrometallurgy to obtain high-grade raw materials. The hydrophilic gangue minerals, however, are discharged as tailings. Flotation reagents, equipment circuits and operating parameters are configured according to the ore characterization, but in general, inefficiencies of this technique are seen in the presence of the targeted minerals in the tails, even though in lower amounts through the years due to improvements in flotation techniques. Additionally, this waste has been historically stored mainly for water recovery (Gupta and Yan, 2016).
The EU scenario of sulfidic tailings states a potential reserve of base metals. In this context, geological mapping and geometallurgical studies highlight 10 key operational and closed mines with their placed sulfide tailings in Europe. Chemical analyses of these deposits indicate the presence of up to 5 wt.% of selenium (Se) and base (Cu, Zn, Pb), precious (Au, Ag) and CRM-listed metals (In, Ge, Ga). Moreover, hazardous metals such as arsenic (As) and cadmium (Cd) have also been found in grades up to 5 wt. % (EU-funded SULTAN Project, 2019).
An additional advantage on processing tailings consists on diminishing acid mine drainage (AMD) formation. AMD relies on microbially-catalyzed oxidative weathering of metal sulfides governed by the reductive-oxidative action of ferric and ferrous ions with the net effect of releasing protons. This process lowers the pH of the surroundings, which can lead to environmental hazards as result of the leaching of heavy metals (Blodau, 2006). Even though this process could occur in natural environments, the main impacts have been observed in areas where mining activity of sulfidic ores has been developed (Schwertmann, Bigham and Murad, 1995). Therefore, avoiding AMD formation also motivates the treatment of sulfidic tailing deposits.
1.3 Current processes to recover metals from sulfidic tailings
Tailings deposits have been targeted for metals recovery by chemical/biological leaching or flotation methods. Regarding the first approach, mixed nitric-sulfuric acid solutions at ambient temperature and pressure were able to leach low-grade nickel (Ni)-Cu sulfide tailings in up to 91.5, 85.0 and 54.6% of Ni, Cu and cobalt (Co), respectively (Xie et al., 2005). However, iron (Fe) was also leached up to 41.8%, after which the Fe concentration in the leachate had to be decreased by the addition of sodium (Na) for Na-jarosite precipitation. In the same scope, tailings containing quartz, pyrite and silicate minerals were treated by acid leaching, which
optimal conditions achieved extractions of 98.45%, 21.41%, 17.25% and 56.13% for Cu, Zn, Fe and manganese (Mn), respectively (Chen et al., 2014) and further fractional precipitation steps were studied to separate and recover these metals as individual streams. Finally, bioleaching of Cu from tailings was also performed with the addition of elemental sulfur while maintaining pH 1 at 45 °C (Falagán, Grail and Johnson, 2017). This process achieved more than 80% of extraction and the recovery was followed by the addition of hydrogen sulfide to precipitate Cu-sulfides. However, the selectivity of this step is questionable since Ag also co-precipitated.
On the other hand, flotation with alkaline sodium hypochlorite was studied to recover Cu and Zn from cyanidation tailings produced in gold plants (Lv et al., 2015). This agent oxidizes cyanite to less aggressive cyanate and depresses sphalerite and pyrite at pH over 10 to concentrate chalcopyrite. Even though this process was able to concentrate Cu up to 13.72 wt.% and Zn up to 34.72 wt.% by treating the output Cu-Pb tailings, additional pyrometallurgical and hydrometallurgical processes are also needed to obtain these metals in higher grade.
To conclude, even though current flotation or leaching-based methods achieve high extraction of metals from tailings, downstream stages are crucial for the recovery of specific components; hence, this approach could not be cost-effective. In this context, defining a process focused on the selective extraction of valuable base metals could improve the overall efficiency.
1.4 Ammonia and alcohols for the dissolution of base metals
In the search of a selective extraction process, the role of ammonia (NH3) in the dissolution of
base metals has been widely investigated and applied industrially. Ammoniacal extraction mainly relies on the formation of stable metal-ammine complexes in the form of Metal(NH3)xy+;
NH3 binds to metals by their nitrogen-containing groups, improving metal solubility in most
cases (Forward, 1953). Additional advantages are i) minimizing the dissolution of iron and other undesired species as a result of their stability as insoluble oxy-/hydroxide compounds and ii) limited corrosion problems compared to acid-based extraction processes, which is beneficial for industrial application (Park et al., 2007).
On the other hand, the solvent properties of alcohols present advantages for being used as leaching media, even though their dielectric constant is lower compared to that of water (Mohsen-Nia, Amiri and Jazi, 2010), hence, their ability to separate and stabilize ions is reduced. In general, the solubility of inorganic compounds in alcohols is variable; inorganic salts are soluble when they are capable of forming neutral molecules in solution whereas salts formed by the first three groups of the periodic table mostly are not soluble, since their coordinative power is low and their solid lattice energies are high (Katzin, 1957). Therefore, this selective behavior suggests the use of alcohols as alternative leaching media when dissolving specific base metals.
1.5 Research question
Given the possibility to dissolve ammonia in alcohols and the potential of ammonia to form soluble complexes with base metals but not Fe, a new solvometallurgical route could be proposed to selectively leach Cu, Pb and Zn from Fe-rich waste materials. Analyzing these potential combined benefits implied the following research question:
Is it possible to design an ammoniacal-alcohol system to extract Cu, Pb and Zn while limiting Fe dissolution?
In order to provide an answer, this research aimed at exploring the suitability of ammoniacal leaching of target metals in alcohols and gaining new knowledge on metal behavior in these systems. This was developed under the following stages.
1. Studying the solubility and speciation of ammonium chloride, ammonium carbonate, ammonium acetate and ammonium sulfate in methanol and ethanol.
2. Studying the dissolution and complexation of Cu, Pb, Zn and Fe as sulfates in ammoniacal-alcohol media.
3. Studying the effect of initial alkalinity of ammoniacal-alcohol media on i) the speciation of ammonium salts and ii) the complexation and dissolution of Cu, Pb, Zn and Fe.
2 LITERATURE REVIEW
2.1 Ammoniacal aqueous extraction
Ammoniacal leaching of sulfidic ores in aqueous media drives selectivity towards certain metals. Under oxidative conditions, sulfide components are oxidized to soluble species (e.g. sulfate ions) and to some components with lower solubilization, such as thiosulfate, thionate and sulfamate. Fe is oxidized to its +III oxidation state and is precipitated as ferric oxides under weakly-alkaline conditions. Additionally, Cu, Ni and Co-species are released in their +II oxidation state and are stabilized as complexes with NH3 (Deng, 1994; Nabizadeh and
Aghazadeh, 2015).
Several parameters of this process have been studied to determine their influence on ammoniacal leaching under either oxidative, non-oxidative or reductive conditions. Oxidant agent, temperature, pH, total concentration and ratio of NH3 and ammonium ions (NH4+) (i.e.
[NH3]/[NH4+]) are crucial factors that determine the stability of metal-ammine complexes,
dissolution rates and maximum efficiency of leaching. With respect to pH, equilibrium equations 2.1 and 2.2. indicate that in acid conditions, NH4+ is predominant, and consequently,
not enough free NH3 would be available to form complexes. In fact, increasing pH from 8.98
to 10.59 in Cu leaching from chalcopyrite improved Cu recovery to approximately 75%, followed by a drop in the performance at higher pH values (Nabizadeh and Aghazadeh, 2015). The authors explained this behavior by the Eh-pH diagram of the chalcopyrite-ammonia system, giving stable copper oxides or hydroxides over pH 10.59. Therefore, optimal ammoniacal leaching implies slightly-alkaline media in a narrow pH range which depends on each studied system.
NH4+ = NH3 + H+, K25 °C = 10-9.27 (2.1)
[NH3] / [NH4+] = 10-9.27 / [H+] (2.2)
On the other hand, the addition of an oxidant in the leaching medium is recommended to enhance sulfide dissolution. Bromates, chlorates, peroxide and oxygen have been studied, being the latter widely selected in industrial processes due to its cost-efficiency and non-corrosive properties (Park et al., 2007). In this scenario, the partial pressure of oxygen (!!!) over the solution is an additional parameter in ammoniacal leaching since the direct relation between !!! and dissolved oxygen in the medium. Research on this factor considered varying !!!in treating a Cu-Ni-Co-Fe-rich matte with 2 M NH4OH-(NH4)2SO4 at 150 °C and 1 h;
increasing !!! from 71 to 355 psi improved dissolution of Cu, Ni and Co from 63 to 95%, 39
The influence of an oxidative agent in the leaching solution could be replaced by a treatment of tailings prior to leaching, such as microwave (MW)-assisted roasting. This step has demonstrated that it is capable of improving the performance of leaching. The recovery of valuable metals is increased, as well as operation time is reduced. Although the influence of microwaves on particular materials and leaching systems still need to be better understood (which is a crucial step for achieving industrial applications), several studies already highlighted that the oxidation or sulfur removal of metal sulfides result in the formation of species more amenable to leaching (Kingman and Al-Harahsheh, 2004). In the MW-assisted roasting of a chalcopyrite (CuFeS2) concentrate, for instance, sulfur and sulfur dioxide were
liberated from the sample exposed at 650 W for 7 min (Worner, 1990). In a similar research, the pretreatment of the same primary sulfide at 2.6 kW, 2.45 GHz and 10-120 s partially removed the content of sulfur, since bornite (Cu5FeS4) was present in the output sample. This
process improved the leaching of Cu (Kingman, Vorster and Rowson, 2000). Similarly, MW-assisted roasting at 2.45 GHz, 650 W and 180 s oxidized this ore to copper sulfate (Harrison, 1997).
In terms of the temperature in the leaching process in ammoniacal media, values below 100 °C have not been widely accepted due to slow extraction efficiencies and longer residence times (Park et al., 2007). Nevertheless, extractions over 60% of Cu, Co and Ni from a Co-rich ferromanganese crust have been reported at 80 °C (Niinae et al., 1996). Moreover, 70% of Cu was extracted from a chalcopyrite concentrate leached at 60 °C (Nabizadeh and Aghazadeh, 2015). On the other hand, high-temperature (> 200 °C) pressure leaching processes imply higher oxygen demand and associated operating costs, to name a few disadvantages. Finally, medium-temperature (120-190 °C) leaching processes have been investigated, such as the dissolution of Cu, Co and Ni contained in a matte sample improved by raising the temperature from 100 to 200 °C (Park et al., 2007).
Table 2.1 summarizes studies on ammoniacal-aqueous leaching targeting base and precious metals contained in ores, concentrates or tailings, with their respective operating parameters for the highest extractions. Depending on each metal-containing source, optimal parameters presented a wide range of both working temperatures (45-200 °C) and solid-to-liquid ratios (0.01-0.1 g/mL) whereas narrow ranges of leaching time and stirring speed were applied. Moreover, leaching media also presented differences; oxidative conditions for treating a concentrate, matte and by-products of flotation were applied with aqueous ammonia-ammonium salts mixtures, in [NH3]/[NH4+] ratios above and below 1. Moreover, in the
treatment of crust and malachite, non-oxidative conditions performed efficiently with either ammonium salts or gaseous ammonia dissolved in water. Finally, most of the authors determined that ammoniacal leaching processes are kinetically controlled by diffusion of the lixiviant through passive layers formed while reaction proceeds.
To conclude, defining a suitable ammoniacal-alcohol leaching system supposes the involvement of the same parameters considered in an aqueous solution. Therefore, the concentration and ratio of NH4+ and NH3, the latter dictated by the alkalinity of the solution, are
primary objects of study. Furthermore, the use of MW-assisted roasting prior to metal leaching eliminates the need of oxidative conditions during the leaching step. This is probably a suitable approach for ammoniacal-alcohol leaching systems, as this way possible interactions between oxidizing agents and alcohols can be avoided. The potential of base metals leaching from roasted sulfidic tailings in ammoniacal-alcohol solutions can be explored via solubility experiments in ammoniacal-alcohol media using synthetic metal sulfates, since this is a common metal form in roasted sulfidic tailings. Next to that, a deeper understanding of the possible interactions between metals, NH3 and alcohols is required to define promising
Table 2.1. Metals extraction in ammoniacal-aqueous leaching media. Source Metals grade Process
Optimal conditions Extraction Reference Temp, °C Speed, rpm Solid-to-liquid ratio, g/mL Particle size, microns Reagents Time, min Chalcopyrite concentrate 25% Cu, 24% Fe, 1% ZnO Oxidative ammonia/ammonium carbonate leaching 60 1000 1/20 g/mL 80% - 42 5 M NH3(aq), 0.3 M (NH4)2CO3, O2-rate 1 L/min 240 Cu: 70% (Nabizadeh and Aghazadeh, 2015) Co-rich ferromanganese crust 13.06% Mn 15.81% Fe 0.59% Co 0.38% Ni 0.11% Cu Reductive ammonium sulfide leaching 70 400 5/500 g/mL 100% - 74 0.074 M (NH4)2SO3 pH 8.5-9.5 120 Cu: 60% Ni: 70% Co: 53% (Niinae et al., 1996) Cu-Ni-Co-Fe matte 24.9% Cu 35.1% Ni 4.05% Co 11.5% Fe 24.5% S Oxidative ammonia/ammonium sulfate leaching 200 600 5% w/v 100% - 100 2 M NH3(aq), 2 M (NH4)2SO4, O2-pressure 213 psi 60 Cu: 94% Ni: 85% Co: 77% (Park et al., 2007) Low grade
complex ore 1.15% Cu Ammonia/ammonium chloride leaching 70 500 2.5/250 g/mL -62 + 48 100%
1.5 M NH3(aq), 2.0 M NH4Cl 60 Cu: 70-80% (Wei et al., 2010)
Malachite 15.94% CuO Ammonia-saturated water leaching 45 400 10/100 g/mL 100% - 90 + 75 7.58 M NH3 (water saturated with ammonia gas) 60 90-95% Cu: (Arzutug, Kocakerim and Çopur, 2004) Shale-enriched flotation by-product 2.7% Cu 1.7% Fe 374 g/Mg Ni 1200 g/Mg Zn 190 g/ Mg Ag 572 g/Mg Co Oxidative ammonia/ammonium sulfate leaching 120-160 400-500 1/10 g/dm3 100% - 80 + 70 Over 1.5 M NH3(aq), 0.37 M (NH4)2SO4, O2-pressure 12.5 atm. 90-120 Cu: 95%, Co: 30%, Ni: 70%, Zn: 95%, Ag: 60% (Chmielewski, Wodka and Iwachów, 2009)
2.2 Ammoniacal alcohol systems
2.2.1 Solubility of ammonia in alcohols
One of the main advantages of NH3 as solute relies on presenting higher solubility in alcohols
(or hydroxyl-containing compounds) compared to other solvents at the same temperature and partial pressure (Pierotti, 1963). This is possibly due to the effect of the hydrogen bonding between hydroxyl groups and nitrogen atoms of NH3 molecules.
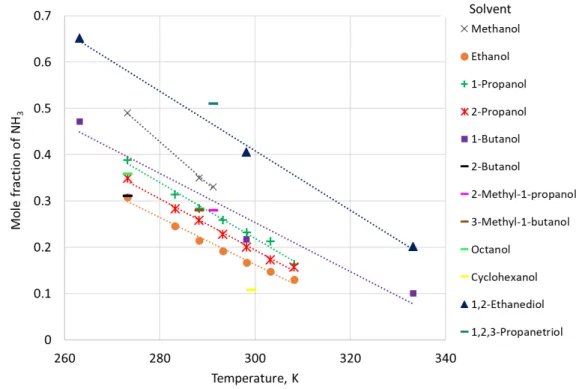
In order to establish tendencies in dissolving gaseous ammonia in different classes of alcohols, previous studies were collected and their experimental procedures were critically reviewed (Young and Fogg, 1985). After selecting reliable data, which is presented in Figure 2.1, the authors highlighted some general trends: when dissolving NH3 in straight-chain
aliphatic alcohols, the final mole fraction of NH3 decreased with an increasing length of the
hydrocarbon chain. Moreover, NH3 solubility significantly increased if two or more hydroxyl
groups are present in the solvent, while it seems to diminish for aromatic alcohols, even though the later just presented one value in the study.
Figure 2.1. Mole fraction of ammonia in alcohols. (Young and Fogg, 1985)
The narrow range of pressures studied in the vapor-liquid equilibrium of ammonia-alcohol mixtures drove the research of the influence of both pressure and temperature on the solubility of gaseous ammonia in ethanol (Huang, Xue and Zeng, 2011). The findings indicated that increasing temperature from 277.35 to 328.15 K decreased the fraction of ammonia in the liquid phase. However, increasing the pressure in the studied range of 0.013 of 0.679 MPa contributed to improve the fraction of this gas. As a consequence, the maximum mole fraction of ammonia in ethanol was 0.56 at 277.35 K and 0.388 MPa and overall the results confirmed the validity of Henry’s law in the given solubility range.
Ammonium salts constitute a valuable source of NH3 to dissolve and then perform ammoniacal
leaching. Table 2.2 indicates the solubility of several ammonium salts in alcohols, ranging from insoluble to very soluble. Although these labels could be useful when stating which solutes are potential high sources of dissolved NH3, their information is limited since i) in most
references only one type of alcohol was tested, and ii) some studies did not specify which alcohol solvent was considered. Additionally, the values of the solubility of some solutes have not been found in literature.
Table 2.2. Solubility of ammonium salts in alcohols.
Term Ammonium salt Reference
Very
soluble Ammonium Cyanide (Patnaik, 2003)
Freely soluble
Ammonium Nitrate (17.1 g in 100 mL methanol at 20 °C), Ammonium Bromide (10 g in 100 mL alcohol at 78 °C), Ammonium Bisulfide
(Lide, 1992) (Weast, 1979) (Patnaik, 2003)
Soluble
Ammonium Acetate (7.89 g in 100 mL methanol at 15 °C), Ammonium Nitrate (3.8 g in 100 mL ethanol at 20 °C), Ammonium Sulfide, Ammonium Thiocyanate, Ammonium Formate, Ammonium Dichromate
(Weast, 1989) (Lide, 1992) (Patnaik, 2003) (Patnaik, 2003) Sparingly Ammonium Chloride (0.6 g in 100 mL methanol at 19 °C) (Weast, 1989)
Slightly soluble
Ammonium Bifluoride, Ammonium Carbamate, Ammonium
Fluoride Ammonium Phosphate (Patnaik, 2003)
Insoluble
Ammonium Phosphate (Dibasic),
Ammonium Sulfate, Ammonium Thiosulfate, Ammonium Molybdate, Ammonium Carbonate, Ammonium Bicarbonate
(Patnaik, 2003)
With respect to measuring the solubility of NH3 or ammonium salts, either direct or indirect
techniques have been applied. For instance, in order to determine the solubility of NH3 fed as
gas in ethanol (Huang, Xue and Zeng, 2011), firstly the degassed solvent was transferred into a vacuumed equilibrium cell (EC). Subsequently, the cell was heated up and ethanol was agitated. Gaseous ammonia was transferred to a thermostated gas cylinder (TGC) and then both EC and TGC were connected and their pressures monitored. After reaching equilibrium, online vapor samples were taken from the EC to be later sent to a gas chromatograph. The composition of the phase was determined, which was used to calculate NH3(aq) by mass
balance.
On the other hand, the technique applied to measure the solubility of ammonium chloride in water involved a high-pressure piston-cylinder type vessel containing a valve (Sawamura et al., 1999). Both solute and water were placed in the reactor and the system was pressurized and shaken under certain temperatures and pressures for 1-2 days. At time intervals, samples of the solution were extracted from the valve while pushing the piston for preventing pressure dropping. Samples were titrated to finally determine the solubility.
Finally, the determination of NH4+ in aqueous solution was performed by their complexation
with salicylic acid (in a 1/2 mol ratio) in the presence of sodium hypochlorite and sodium nitroprusside. This stable complex was measured by spectrophotometry and presented a good selectivity towards ammonia compared to other metals present in the solution (Qiu, Liu and Zhu, 1987).
Overall, in the search for suitable ammoniacal-alcohol leaching media, short hydrocarbon-chain alcohols are interesting to be studied as solvents, whereas ammonium salts constitute a practical source of NH3 added at ambient pressure and temperature. In this context, the
solubility of several ammonium salts in these alcohols needs to be determined experimentally, for instance, by fast-and-easy solubility tests with visual inspection. Moreover, even though the mentioned techniques for determining NH3 and NH4+ in solution are accurate, a practical
approach for the same aim could be modeling of the dissolved ammonium salts considering their respective acid-base equilibrium reactions in alcohols at ambient conditions.
2.2.2 Alcohol systems for metal extraction
The performance of alcohol systems in the extraction of metals needs to be understood, and specifically which metals species could benefit from such leaching media. In this context, preliminary studies indicate that alcohols dissolve alkali metals that are present as hydroxides, but do not dissolve metal carbonates (Simmonds, 1919). Moreover, most of chlorides and some alkali-halides and metallic nitrates are also dissociated by alcohols. The solvents also form crystalline compounds with certain salts, e.g. with calcium chloride or magnesium chloride. On the contrary, metal sulfate or metal phosphate salts are generally insoluble or are only dissolved to a small extent in alcohols (Simmonds, 1919).
The interactions between metals and alcohols have been extensively documented for the synthesis of metal alkoxides (Turova et al., 2006). A direct interaction involves “active metals” such as magnesium (Mg), alkaline and alkaline-earth metals, for instance lithium (Li), potassium (K), calcium (Ca), sodium (Na), and barium (Ba), with ethanol, methanol and phenol. Generally, the process consists on contacting metals with alcohols to produce primary alkoxides, which are re-dissolved by excess of alcohol and finally the solution is heated up to complete desolvation. Alternative techniques such as alkali-metal amalgam and binding polymeric alkoxides with soluble complex have also been explored as mechanisms. Additionally, “less active” metals, e.g. silicon (Si), titanium (Ti), Ge, Fe, Co, and Ni form alkoxides with methanol and ethanol by electrochemical synthesis, which was defined as a more efficient process in terms of simplicity and solvent consumption. A third perspective brings the reaction of metal hydroxides or metal oxides with alcohols to form alkaline and alkaline-earth alkoxides, as the case of Ti in ethanol forming TiO mainly present in the ethanol medium. Finally, applications of alkali-metals alkoxides for organic synthesis account for reduction of carbonyl compounds by firstly dissolving Na in primary alcohols, K in tertiary alcohols and Li in ethanol, respectively (Huffman, 1992).
With respect to the behavior of metal sulfates in pure alcohols, solubilities of Li, Na and K sulfates in absolute methyl and ethyl alcohols were determined in the range of 17-50 °C approximately (Lenox, 1922). Trends were set in terms of descending solubility curve of sodium sulfate in ethyl alcohol, a peak of dissolved potassium sulfate at 35 °C as well as a minimum point in lithium sulfate (Li2SO4) dissolution at 27 °C in the same type of solvent.
Regarding methyl alcohol, both sodium and potassium sulfate presented the same tendency with minimum solubility values at 39 °C approximately, being the former salt more soluble. Even though the studied salts were more soluble in ethyl alcohol, solubility values of 0.005-0.05 gsalt/100 gsolvent consider these targets as insoluble in both media.
Applications of alcohols in aqueous-driven processes have been seen in K leaching from a P2O5/K2O ore performed with HCl(aq) at low temperature and by adding monohydric alcohols
in a 1/80 alcohol/solution volumetric ratio (Zhou et al., 2018). Varying the hydrocarbon chain from C4 to C16 resulted in a peak of K dissolution in 96,8% by using lauryl alcohol (C12) compared to the base case (89,5%). However, adding C4-7 alcohols diminished the leaching performance to less than 84%. Scanning Electron Microscope (SEM) and X-ray Fluorescence (XRF) analyses indicated a small-and-uniform particle size in the residue, suggesting lauryl alcohol decomposes ores more fully; hence, leaching was enhanced.
Another perspective considers alcohols as “selective precipitating agents” for purifying metal sulfates previously dissolved in aqueous media. Studies were developed with respect to precipitation of copper sulfate using ethanol, achieving efficiencies around 90% when increasing dissolved copper concentration, solution pH and ethanol/solution volume ratio (Aktas, 2011). This behavior was explained in terms of lowering the thermodynamic activity of water by the presence of ethanol and therefore, both metal and sulfate ions are more able to form bonds and then precipitate (Aktas et al., 2006). These properties were also tested in recovering valuable metals from spent Li-ion batteries. After leaching both cathodic and anodic active materials in 4 M H2SO4(aq), Co precipitated in a first stage as cobalt sulfate (CoSO4) by
adding ethanol in 1-4 alcohol/solution volume ratio, and in a second stage as cobalt hydroxide (Co(OH)2) by increasing pH to 10. These steps precipitated around 90% of the dissolved Co.
Since CoSO4 has a lower solubility compared to Li2SO4, the latter metal was later targeted
achieving 90% recovery at pH 5 and 3/1 ethanol/solution ratio. Dissolved Cu was also recovered by dosing ethanol, and after 15 min, a similar performance was achieved (Aktas et al., 2006). CoSO4 precipitation was further investigated and the solubility product constant
(Ksp) of this salt in pure ethanol of 1.15·10-5 M2 was determined experimentally (Aktas et al.,
2013). Finally, removal of undesirable sulfate ions has been achieved by mixing the sulfate-containing aqueous solution with ethanol and barium salts to later precipitate barium sulfate, which solubility is decreased while increasing alcohol/solution volume ratio (A. Gomaa, 2012).
To conclude, considering that both mineralogy of sulfide tailings and operational conditions of MW-roasting influence the composition in roasted tailings (e.g. metal oxides, metal sulfates, secondary sulfides and unreacted primary sulfides), the extraction of Cu, Pb or Zn imposes a challenge since most of these metal-containing species have been reported to be insoluble or slightly soluble in alcohols with short hydrocarbon chain. However, this scenario could yet remain interesting for the leaching of valuable metals, as result of the selective complexation of these targets with NH3 ligands added to the solvent. On the other hand, the pH in
alcohol-aqueous solutions has been used to control the solubility or precipitation of metals due to complexation. Therefore, also the acidity of ammoniacal-alcohol media is expected to influence the dissolution of valuable metals.
2.2.3 The formation of amines in ammoniacal-alcohol media
When it comes to understanding the ammonia-alcohol system, another crucial factor relies on the formation of amines, of which the synthesis is mainly based on alcohol amination. The presence of amines in the solution implies toxicity, with a medium lethal toxicity dose for rats in the range of 100-1,890 mgamines/kgbodyweight for primary, secondary and tertiary amines (Greim
et al., 1998). Additionally, the presence of amines could possibly affect the leaching performance when extracting metals from tailings, since the lower availability of ammonia would decrease the stable metal-ammine complexation. Therefore, these forces drive the understanding of the parameters used for the synthesis of amines in order to avoid them when setting leaching conditions in ammonia-alcohol media.
In the amination of alcohols, applied temperature and pressure vary meaningfully according to the substrates and catalysts mostly based on transition metals such as Ru, Ir, Rh, Pt, Pd, Au, Ag, Co, Mn, Ni, Cu, Cr; W and Fe (Shi and Cui, 2018), as well as Al (Bähn et al., 2011). Their presence overcome the poor electrophilicity when combining NH3-containing sources
and alcohols. Therefore, catalyzed reactions will encourage the borrowing hydrogen strategy, which involves two sequential steps: oxidation of alcohols to aldehydes or ketones, followed by reductive amination (Kim, Kim and Chang, 2013), as schemed in Figure 2.2. Even though catalyst-free formation of amines has been reported (Nef, 1901), these reactions typically involve temperatures above 200 °C, high pressures, long (not specified) reaction times and the addition of excess base (Guillena, Ramón and Yus, 2010; Shi and Cui, 2018).
With respect to the application of ammonium salts in the formation of amines under the presence of homogenous catalysts, the first study considered the synthesis of tribenzylamine from 5 equivalents of benzyl alcohol and 1 equivalent of ammonium acetate, catalyzed by [Ru(p-cymene)Cl2]2. The process achieved 68% yield in 24 h; other applied parameters were
not indicated (Williams and Hamid, 2007). Moreover, synthesis of alkylamines has been studied by means of (pentamethylcyclopentadienyl)iridium (Cp*Ir), which catalyzes the N-alkylation of ammonium salts with alcohols (Yamaguchi et al., 2009). In this matter, the catalyzed formation of tertiary amines from ammonium acetate with several primary alcohols was performed under an Ar-atmosphere at 130 °C with vigorous stirring for 17 h. A first approach achieved 83% yield by reacting ammonium acetate and benzyl alcohol in a 1/3.6 mol ratio in the presence of 1 mol% [Cp*IrCl2]2 and 30% mol of sodium bicarbonate. Moreover,
different kind of benzylic alcohols with functional groups such as Cl and Br achieved 77-87% of yield in the same mol ratio. However, tertiary amines formation from this ammonium salt implied a 1/5 molar ratio with aliphatic alcohols at 140 °C and with 5 mol% of Ir-based catalyst to reach 55-73% yield.
The same authors also studied ammonium tetrafluoroborate in forming secondary amines under the same catalyst. Reacting this salt with primary alcohols in a 1/2.2 mol ratio achieved 50-98% of yield at 140 °C and 17 h with under 2-3 mol% of catalyst. In terms of pressure, a sealed reaction flask or a pressure bottle were recommended to be used for obtaining good performance. On the other hand, the performance of this ammonium salt with secondary alcohols in a 1/3 molar ratio was also tested under the same operating parameters as for primary substrates; cyclic alcohols reached 78-84% yield whereas in aliphatic substrates the conversion was lower (54-77 %).
Different ammonium salts were later studied in the amination of alcohols catalyzed by Ru(OH)x/TiO2 (Yamaguchi et al., 2010). At 141 °C, 12 h in Ar-atmosphere; reacting 2.5 mmol
of benzyl alcohol with 0.5 mmol of N as ammonium bicarbonate or ammonium carbonate gave tertiary amines in yields of 61 and 62%, respectively. In contrast, ammonium phosphate, ammonium acetate, ammonium nitrate, ammonium formate, ammonium bisulfate and ammonium chloride presented low and even negligible performances. Given explanations considered that these salts hardly decompose to ammonia under these conditions, which agrees with preliminary studies presented in Table 2.1. Finally, the influence of aliphatic alcohols as substrates was not covered.
Further research was developed in terms of reacting aqueous ammonia with either primary or secondary alcohols to produce tertiary or secondary amines, respectively. An Ir-based catalyst in the form of [Cp*Ir(NH3)]3-[X]2 (with X = Cl, Br; I) was used when combining NH3(aq) (28 %)
with benzyl alcohol in a 1/3 mol ratio at 120-140 °C and 20-24 h. This resulted in yields of 70-100% in contrast to 0% efficiency in the absence of catalyst. By means of an Ir-and-I-based catalyst under similar operating conditions, amines formation from ammonia and primary or secondary alcohols achieved efficiencies over 80% in most cases (Kawahara, Fujita and Yamaguchi, 2010). Finally, the same ammonia concentration was studied in a Ru-catalyzed
reaction at 141 °C and 6-12 h; tertiary amines were formed in 68-87% yield if aromatic (primary benzylic) alcohols were present, and 88% with aliphatic substrates (Yamaguchi et al., 2010). The formation of amines by reacting alcohols and ammonia has also been achieved by heterogeneous (two or more transition metals) catalyst systems. For instance, the reaction between 1 mmol of several primary alcohols and 1 MPa of NH3 has been catalyzed by 50 mg
of NiCuFeOx in a 12-h process achieving an amine formation yield of 59-77% (Cui et al., 2013).
Moreover, primary amines have also been formed by the catalyzing effect of 1 mol % Ni(Al2O3
-supported) (prepared by the in-situ H2-reduction of NiO(Al2O3-supported)) in the reaction of 3
mmol of secondary alcohols and 0.4 MPa of ammonia. These processes achieved yields of 68-96% at 140-160 °C and 13-72 h (Shimizu et al., 2012). Additional research under the same scope considered the application of a 95 wt.%Co-5 wt%Fe alloy as catalyst. However, a low yield of 32% was reached when reacting 0.7 mol/h of biomass-based 1,3-propanediol with 43 mol/h of ammonia at 195 °C and 15 MPa, to name a few extreme conditions (Fischer et al., 1999).
As seen, the formation of amines from alcohols and either ammonium salts or dissolved ammonia has been highly dependent on the presence of either the noble metals Ru or Ir as homogenous catalysts, or the non-noble-based catalysts NiCuFeOx, metallic Ni, or the Co-Fe
alloy. In this context, several approaches emerge in order to avoid the synthesis of toxic amines when using an ammoniacal-alcohol leaching solution; i) not targeting Ni-containing sludges or tailings since the Ni phases that are present could possibly act as catalysts, ii) not targeting sources containing NiCuFeOx or compounds with similar stoichiometry, iii)
performing leaching tests in both low (50-100 °C) and medium (100-200 °C) temperature ranges regardless the non-noble metals in the sources, or iv) limiting the reaction time of the leaching step.
3 METHODOLOGY
3.1 Materials
Reagents used in this research are classified in three main groups: solvents, ammonium salts, metal salts, and others. The details of each component are presented in Table 3.1, which indicates their analytical grade.
Table 3.1. Reagents characterization. Name Supplier CAS3
registry number Molecular formula Molecular weight, g/mol Assay % Main Trace Impurities So lv en ts Methanol VWR LLC 67-56-1 CH3OH 32.04 99.5 Water ≤ 0.1% Ethanol VWR LLC 64-17-5 CH3CH2OH 46.07 99.5 Water ≤ 0.1% Water Milli-QUltra-Pure Water ® Reference H2O 18.00 Deionized water
Am m on iu m s al ts Ammonium acetate VWR LLC 631-61-8 NH4CH3CO2 77.08 98 Pb ≤ 2 ppm SO4-2 ≤ 0.01% Fe ≤ 2 ppm Ammonium
carbonate Aldrich Co. 506-87-6 (NH4)2CO3 96.09 304
Cl- ≤ 5 ppm SO4-2 ≤ 0.002% Fe ≤ 5 ppm Heavy metals ≤ 5 ppm Ammonium sulfate VWR LLC 7783-20-2 (NH4)2SO4 132.14 99.1 NO3- ≤ 0.0005% Cl- ≤ 1 ppm PO4-2 ≤ 1 ppm As ≤ 0.05 ppm Fe ≤ 1 ppm Pb ≤ 2 ppm Ammonium
chloride Merck KGaA 12125-02-9 NH4Cl 53.49 99.8
NO3- ≤ 0.0005% SO4-2 ≤ 0.002% Fe ≤ 0.0002% Cu ≤ 0.0002% Me ta l sal ts Copper Sulfate VWR LLC 7758-99-8 CuSO4∙5H2O 249.68 99.0 Cl ≤ 0.001% N ≤ 0.001% Fe ≤ 0.003% Lead Sulfate Aldrich Co. 7446-14-2 PbSO4 303.26 98 - Zinc
sulfate Merck KGaA 7446-20-0 ZnSO4∙7H2O 287.54 99 Cl ≤ 0.0005% Iron Sulfate VWR LLC 7782-63-0 FeSO4∙7H2O 278.02 99 Fe3+ ≤ 0.1% Na ≤ 0.02% Cu ≤ 0.005% Ca ≤ 0.005% Ot he rs Sodium
Hydroxide Merck KGaA 1310-73-2 NaOH 39.9 97
Na₂CO₃ ≤ 1.0 % Cl ≤ 0.012% SO4 ≤ 0.01%
3 Chemical Abstracts Service. 4 NH
3.2 Equipment, instruments, and analytical techniques
3.2.1 Solubility test
Solubility tests were performed in conical base graduated centrifuge tubes, whereas solid and liquid reagents were manipulated with the proper laboratory utensils and measured by analytical balance and mechanical pipettes, respectively. Moreover, the tested mixtures were agitated in a vortex mixer or in an overhead shaker. Resulting solutions were filtered by syringe filters Chromafil® RC-45/25 – 0.45 µm.
3.2.2 Inductively coupled plasma optical emission spectrometry
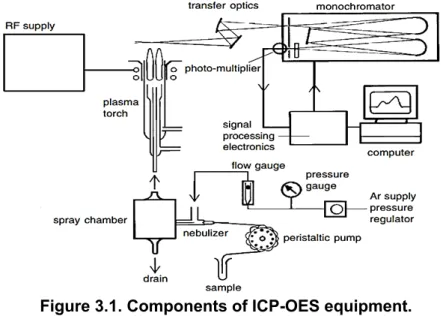
The concentration of metals in the obtained solutions was analyzed by inductively coupled plasma optical emission spectrometry (ICP-OES), which main components in the equipment are represented in Figure 3.1. This analytical technique relies on the formation on an ICP by ionizing an incoming gas flow, e.g. Ar to Ar+. Then, a circular magnetic field is applied to the
free electrons, which resist the induced movement. As a consequence, intense heat is generated.
After the ICP is formed, the aqueous samples containing metals can be treated. In the plasma torch, the solvent of the nebulized aqueous solution is evaporated, forming salt particles between metals and anions. These salts are vaporized to form a gaseous phase, which is later dissociated in atoms. The metallic atoms are either excited or ionized-and-excited and leave the plasma torch. When the targets return to their ground state, they release photons with wavelengths associated with a specific element. These wavelengths are captured in an axial or radial detector to later quantify their intensity. Finally, this intensity measurement is interpreted as the concentration of the metal in the initial sample by an intensity-concentration correlation line (Agilent Technologies, 1999).
Figure 3.1. Components of ICP-OES equipment.
The ICP-OES equipment used was Perkin Elmer AVIO 500®, which operation was managed
by the software Syngistix®. The minimum detection limits for each metal were Cu 10, Pb 25,
Zn 3 and Fe 5 µg/L, whereas the maximum limit was 5000 µg/L for the four metals. Moreover, the performance of this equipment was complemented with a PrepFAST Autodilution System. This tool was configured with dilution factors for the samples of 1, 10 and 50 and operated by the software ESI SC®. Finally, data processing after ICP-OES measurements was performed
with the software Syngistix®.
3.3 Experimental procedure
3.3.1 Solubility of ammonium salts in alcohols
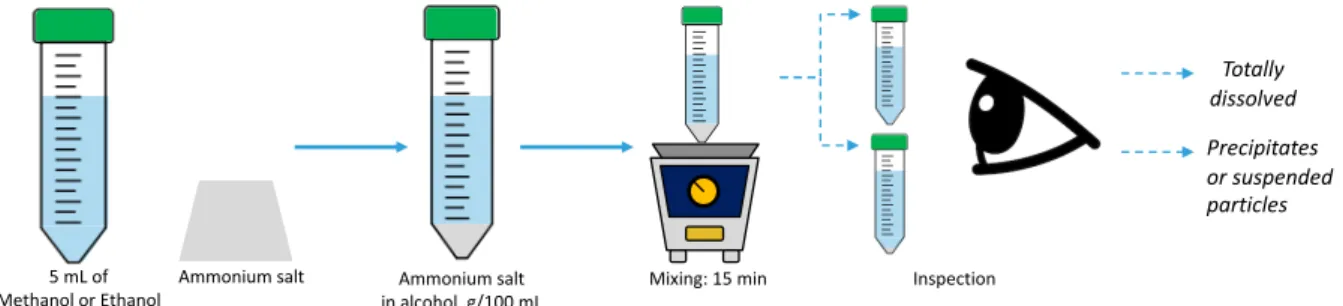
The solubility of the four ammonium salts presented in Table 3.1 was studied in methanol and ethanol at ambient conditions of 20 °C and 1 atm. Several dosages of these solutes were added to 5-mL samples of each solvent and the solutions were agitated in the vortex mixer for 15 min. After agitation, the solutions were visually inspected to determine if the solute was totally dissolved, or if precipitates or suspended particles were present. This stage is represented in Figure 3.2.
Figure 3.2. Dissolution of ammonium salts in alcohols.
3.3.2 Solubility of metal sulfates in ammoniacal-alcohol and ammoniacal-aqueous media
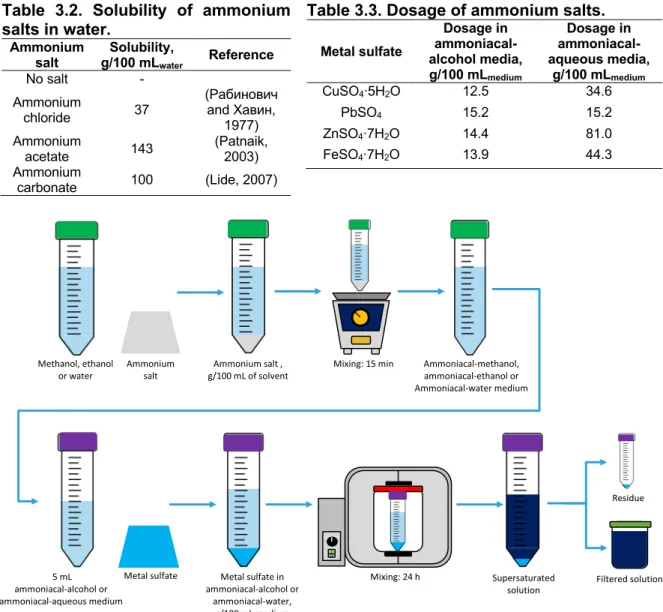
The solubility of the four metal sulfates indicated in Table 3.1 was studied in ammoniacal solvents based on methanol, ethanol and water at measured ambient conditions of 20 °C and 1 atm. The experimental procedure was organized in two sub-stages: i) the effect of ammoniacal-alcohol and ammoniacal-aqueous media on the dissolution of metal sulfates and ii) the effect of the initial alkalinity of selected ammoniacal-alcohol media on the dissolution of metal sulfates.
The first sub-stage considered the dissolution of ammonium salts in methanol, ethanol and water. The dosage of ammonium salts in alcohols was based on the solubility values determined in the previous stage indicated in section 3.3.1, whereas literature values for the solubility of ammonium salts in water (Table 3.2) were considered. Solutions were agitated in a vortex mixer until complete dissolution was reached.
5 mL of
Methanol or Ethanol Ammonium salt in alcohol, g/100 mLAmmonium salt Mixing: 15 min
Totally dissolved Precipitates or suspended particles Inspection
Later, supersaturated solutions of metals sulfates in alcohol and ammoniacal-aqueous media were prepared. 5-mL samples of each medium were contacted with the solutes according to the dosages presented in Table 3.3. The mixtures were agitated in an overhead shaker at 5 rpm for 24 h to later filter the resulting solutions. The experimental procedure of this sub-step is represented in Figure 3.3.
Table 3.2. Solubility of ammonium salts in water. Ammonium salt Solubility, g/100 mLwater Reference No salt - Ammonium chloride 37 (Рабинович and Хавин, 1977) Ammonium acetate 143 (Patnaik, 2003) Ammonium carbonate 100 (Lide, 2007)
Table 3.3. Dosage of ammonium salts. Metal sulfate Dosage in ammoniacal-alcohol media, g/100 mLmedium Dosage in ammoniacal-aqueous media, g/100 mLmedium CuSO4∙5H2O 12.5 34.6 PbSO4 15.2 15.2 ZnSO4∙7H2O 14.4 81.0 FeSO4∙7H2O 13.9 44.3
Figure 3.3. Dissolution of metal sulfates in alcohol and ammoniacal-aqueous media.
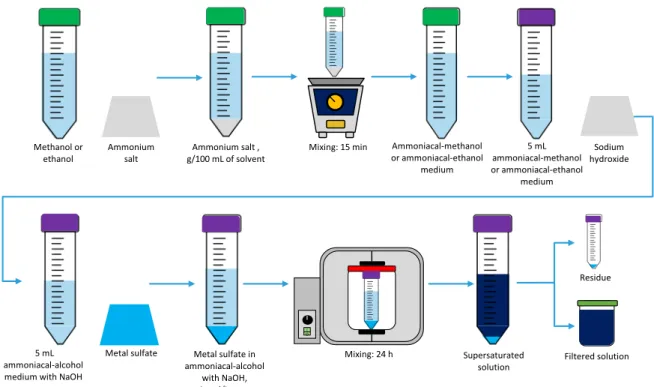
In the second sub-stage, ammonium chloride in methanol, ammonium acetate in ethanol and ammonium acetate in methanol were selected and prepared based on the outcomes of the first sub-stage. Later, 5-mL samples of each medium were separated and NaOH(s) was added
considering [OH-]
o/[NH4+]o 5 molar ratios of 0, 0.3 and 1. Finally, supersaturated solutions of
each metal sulfate were prepared by dosages of 1 molmetal/Lmedium and 24-h mixing. Resulting
solutions were filtered. This sub-stage is depicted in Figure 3.4.
5 [NH
4+]o is the initial concentration of NH4+ dissolved from ammonium salts.
5 mL ammoniacal-alcohol or ammoniacal-aqueous medium Metal sulfate I O Mixing: 24 h Metal sulfate in ammoniacal-alcohol or ammoniacal-water, g/100 mL medium Supersaturated solution Residue Filtered solution Methanol, ethanol or water Ammonium salt Ammonium salt , g/100 mL of solvent
Mixing: 15 min Ammoniacal-methanol, ammoniacal-ethanol or Ammoniacal-water medium
Figure 3.4. Dissolution of sodium hydroxide and metal sulfates in ammoniacal-alcohol media.
The concentration of Cu, Pb, Zn or Fe in the resulting solutions were determined by ICP-OES. The preparation of the alcohol-containing samples involved additional preparation stages such as evaporation of the solvent and digestion of the precipitates to finally transfer them into aqueous solvent to be analyzed by the ICP-OES equipment. The steps and parameters to prepare both aqueous and alcohol-containing samples are described in Appendix A.
3.3.3 Speciation of ammonium salts in alcohol and aqueous media.
The speciation of dissolved ammonium salts was modeled. This step was firstly developed by stating the dissolution reaction of ammonium salts and the acid-base equilibrium reactions of NH4+ and anions. These are listed in Table 3.4.
Table 3.4. Dissolution and acid-base equilibrium reactions of ammonium salts in water, methanol and ethanol.
Ammonium
salt Dissolution reaction
Acid-base equilibrium reactions Acidic dissociation constant (Ka) NH4Cl NH4Cl(s) → NH4+ + Cl- NH4 + ↔ NH 3 + H+ HCl ↔ Cl- + H+ Ka(NH4 +) Ka(HCl)
NH₄CH₃CO₂ NH₄CH₃CO₂(s) → NH4+ + CH₃CO2- NH4
+ ↔ NH 3 + H+ CH₃CO2H ↔ CH₃CO2- + H+ Ka(NH4+) Ka(CH3CO2H) (NH4)2CO3 (NH4)2CO3(s)→ 2NH4+ + CO3 2-NH4+ ↔ NH3 + H+ H2CO3↔ HCO3- + H+ HCO3-↔ CO32- + H+ Ka(NH4+) Ka1(H2CO3) Ka2(H2CO3) (NH4)2SO4 (NH4)2SO4(s)→ 2NH4+ + SO4 2-NH4+ ↔ NH3 + H+ H2SO4↔ HSO4- + H+ HSO4- ↔ SO42- + H+ Ka(NH4+) Ka1(H2SO4) Ka2(H2SO4) 5 mL ammoniacal-alcohol medium with NaOH
Metal sulfate I O Mixing: 24 h Metal sulfate in ammoniacal-alcohol with NaOH, 1 mol/Lmedium Supersaturated solution Residue Filtered solution Methanol or
ethanol Ammonium salt g/100 mL of solventAmmonium salt , Mixing: 15 min
Ammoniacal-methanol or ammoniacal-ethanol medium Sodium hydroxide 5 mL ammoniacal-methanol or ammoniacal-ethanol medium
Then, the acidic dissociation constant (Ka) of dissolved NH4+ and anions at standard states in
methanol and ethanol were determined. According to equation 3.1 (Sager, Robinson and Bates, 1964), Ka values for aqueous systems (wKa) can be converted to values suitable for
other solvents (sKa), by considering the dielectric constants of water (εw) and the new solvent
(εs), charge of the acid (zA) and its respective base (zB), as well as the radius of the acid (rA),
base (rB) and proton (rH). Additional parameters are the elementary charge (e), gas constant
(R), Avogadro number (N) and temperature (T). Values considered for each acid-base reaction are listed in Table 3.5. Finally, calculated Ka values and equilibrium reactions were
considered to plot their respective speciation diagram in the three studied solvents.
!(!#") − !(##") = '($ 4.6052/01 1 3!− 1 3#4 5 6%$ 7% − 6&$ 7& + 1 7'9 (3.1)
Table 3.5. Parameters considered for the determination of sKa in methanol and ethanol. Acid (A) Base (B) p(wKa) εMeOH εEtOH εw ZA ZB rA, Å rB, Å rH+, Å
(VanderWerf, 1961) (Gregory and Clarke, 2005) (Simoes et al., 2017)
NH4+ NH3 9.3 33.6 25.2 80.2 1 0 1.36 -0.86 HCO3- CO32- 10.3 -1 -2 2.07 1.78 H2CO3 HCO3- 6.4 0 -1 - 2.07 CH3CO2H CH3CO2- 4.8 0 -1 - 1.62 HSO4- SO42- 2.0 -1 -2 2.21 2.18 H2SO4 HSO4- -2.0 0 -1 - 2.21 HCl Cl- -8.0 0 -1 - 1.81
3.4 Measurement errors
The experimental procedure conducted sources of error that could affect the obtained results. In terms of the dissolution of ammonium salts in alcohols, the biggest error factor was accredited to the human observation to determine the solubility. This error was aimed to be minimized by taking pictures of the samples, re-evaluating their solubility status during the following four days, and by asking for third-party visualization.
Next to that, two main errors were inferred in the dissolution of metal sulfates. The first source considered the preparation of alcohol-based solutions for ICP-OES analysis, whereas the second factor can be assigned to interferences in this analytical technique. For instance, physical properties of the solution could affect the amount of sample treated in the ICP torch, the emission of excited atoms or ions out of the ICP torch could be suppressed due to higher electron density from easily-ionized elements, e.g. Na, or unwanted light in the optical analysis could affect the interpretation (Agilent Technologies, 1999). In order to minimize their influences, samples were both manually and auto diluted (which also aimed at measuring the concentration of metals within the calibration line). Additional approaches included the online addition of an internal standard to both samples and standard solutions, the selection of the radial selector of emitted light and background corrections in the wavelength quantification. Finally, the experiments of this stage were developed in duplicates and the standard deviation of the concentration of metals was calculated and reported.
4 RESULTS AND DISCUSSION
4.1 Speciation of dissolved ammonium salts
The speciation of NH4+ and the different anions from ammonium salts dissolved in water,
methanol and ethanol was modeled. Since the extent of the pH scale of solvents depends on their autoprotolysis constant, pH ranges of 14, 16.7 and 16.8 were considered for water, methanol and ethanol, respectively (Głąb and Maj-Żurawska, 2013). Calculations of this process are indicated in Appendix B.
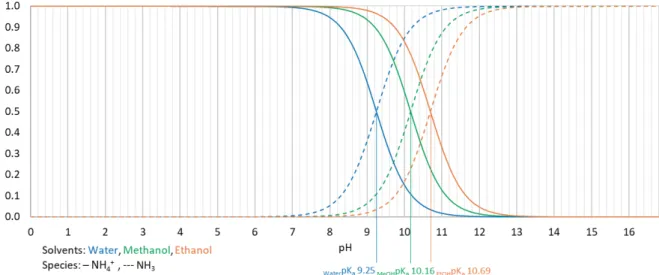
Regarding the NH4+/NH3 equilibrium, of which the speciation diagram is represented in Figure
4.1, pKa values increased from 9.25 in water, to 10.16 in methanol and to 10.69 in ethanol.
Calculated values indicate that deprotonation of NH4+ is weakened in alcohols compared to
the base scenario in water. Therefore, more alkaline conditions in methanol and ethanol are required to obtain a certain fraction of NH3.
Figure 4.1. Speciation diagram of NH3 in water, methanol and ethanol at 20 °C.
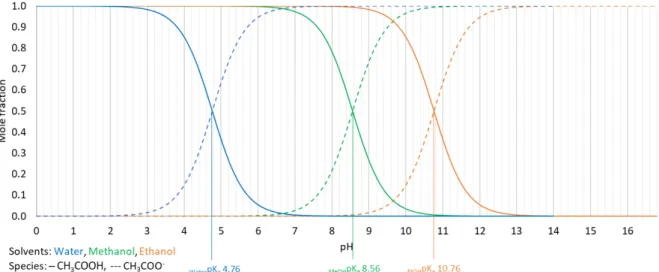
The acid-base equilibrium of dissolved CH3CO2- (Figure 4.2) from ammonium acetate
presents a similar trend than in the NH4+/NH3 reaction. Alkaline ethanol and slightly alkaline
methanol favor the protonation of CH3CO2- and, consequently, the increment of CH3COOH in
the solution is preferred. On the contrary, acidic aqueous media are required to achieve the same objective. Regarding alcohols, a pH lower than 8.56 and 10.76 enhances a higher fraction of CH3COOH in methanol and ethanol, respectively.
In terms of dissolved ammonium carbonate and ammonium sulfate, both CO32- and SO4
2-involve diprotic acid-base equilibria. As depicted in Figure 4.3, the first protonation of CO32-
takes place in more alkaline conditions in alcohols compared to in water. In fact, in ethanol calculated pKa2 was 20.20; hence, no CO32- is present in the stated pH range for this solvent.
In methanol, a pH between 9.86 and 16.58 induces a higher fraction of HCO3-, whereas this
range is moved to 6.35 – 10.33 in water. Consequently, the second protonation of CO32- is
Figure 4.2. Speciation diagram of CH3CO2- in water, methanol and ethanol at 20 °C.
Figure 4.3. Speciation diagram of CO32- in water, methanol and ethanol at 20 °C.
The behavior of SO42- in alcohols is similar to that of CO32-. As seen in Figure 4.4, the pH range
needed for the predominance of protonated species HSO4- and H2SO4 is wider in ethanol (pH
0 – 10.58), followed by methanol (pH 0 – 7.42) and in both solvents is possible to maintain a significant fraction of H2SO4 in relatively mild acid conditions. In contrast, the pH range to
enhance higher fraction of HSO4- in water is extremely acidic (pH 0 – 1.99) and H2SO4is not
stable.
Finally, the speciation diagram of Cl- is represented in Figure 4.5. Calculated pK
a values in
methanol and ethanol of -4.34 and –2.21 respectively are higher compared to pKa -8.0 in
water, hence, in the three solvents a negative pH would induce predominantly a HCl speciation. Therefore, in the studied pH range Cl- can be considered as negligible base.
Figure 4.4. Speciation diagram of SO42- in water, methanol and ethanol at 20 °C.
Figure 4.5. Speciation diagram of Cl- in water, methanol and ethanol at 20 °C.
To conclude, alcohols as solvents for ammonium salts importantly influence the acid-base equilibria of dissolved ions. Weak NH4+ dissociation in aqueous system becomes slightly
weaker in ethanol and methanol; therefore, higher alkalinity is required to enhance deprotonation. Additionally, the effect of organic solvents is more pronounced in the acid-base equilibria of dissolved anions. In alcohols, these species become stronger bases than in water. In this perspective, CH3CO2-, CO32- and even SO42- could act as proton acceptors. In the scope
of supporting NH4+/NH3 equilibrium in alcohols towards higher concentration of NH3 for future
metal-ammine complexation, the presence of the mentioned anions could favor the deprotonation of NH4+ by the formation of weak acids.
4.2 Solubility of ammonium salts in methanol, ethanol and water
4.2.1 Dissolution of ammonium salts
The aim was to determine the solubility of ammonium salts in organic solvents. In this context, Figure 4.6 displays the dissolution of four ammonium salts in methanol and ethanol at ambient conditions. Each mixture was evaluated to determine whether the solute was completely dissolved, or precipitates were observed. The dissolution test of ammonium sulfate in ethanol was not performed since all tested dosages of this salt in methanol precipitated. Detailed results are indicated in Appendix C.
Ammonium chloride Ammonium carbonate Ammonium acetate Ammonium sulfate
Figure 4.6. Dissolution of ammonium salts in methanol and ethanol at 20 °C and 1 atm.
For each tested system, a range of solubility was established considering the maximum dosage of ammonium salt that was completely dissolved in alcohols, together with the minimum dosage which precipitated (Table 4.1). These ranges are plotted in Figure 4.7, to which also literature values for ammonium salt solubility in water were added.
Table 4.1. Solubility range of ammonium salts in methanol and ethanol at 20 °C and 1 atm. Values in gammonium salt/100 mLsolvent.
Solvent Methanol Ethanol Ammonium chloride [ 1.8 ; 2.7 [ [ 0.15 ; 0.34 [ Ammonium acetate [ 55.0 ; 56.9 [ [ 9.5 ; 11.2 [ Ammonium carbonate [ 5.5 ; 7.4 [ [ - ; 0.04 [ Ammonium sulfate [ - ; 0.02 [