International Masters of Science in Environmental Technology and Engineering
Master’s dissertation submitted in partial fulfilment of the requirements for the joint degree of EU Erasmus+ Master course organized by
University of Chemistry and Technology, Prague, the Czech Republic IHE Delft Institute for Water Education, Delft, the Netherlands
Ghent University, Ghent, Belgium
Academic year 2018-2020
Fundamentals and application of sulfate
radical based advanced oxidation
processes for removal of organic
micropollutants in water: focus on the
ozone-PMS process
Host institution: Ghent University, Ghent, Belgium
Ram Prasath Alagappan
ES-IMETE.20-26
Supervisors: Prof. Dr. Ir. Kristof Demeestere & Prof. Dr. Ir. Stijn Van Hulle
International Masters of Science in Environmental Technology and Engineering
Master’s dissertation submitted in partial fulfilment of the requirements for the joint degree of EU Erasmus+ Master course organized by
University of Chemistry and Technology, Prague, the Czech Republic IHE Delft Institute for Water Education, Delft, the Netherlands
Ghent University, Ghent, Belgium
Academic year 2018-2020
Fundamentals and application of sulfate
radical based advanced oxidation
processes for removal of organic
micropollutants in water: focus on the
ozone-PMS process
Host institution: Ghent University, Ghent, Belgium
Ram Prasath Alagappan
ES-IMETE.20-26
Supervisors: Prof. Dr. Ir. Kristof Demeestere & Prof. Dr. Ir. Stijn Van Hulle
III Copyright
The author, mentor and supervisors permit to make this master’s dissertation available for inspection and to copy parts of it for personal use only. All other use is subject to the copyright restrictions, specifically regarding the obligation to explicitly mention the source when citing results of this master’s thesis.
De auteur, tutor en promotoren staan toe om dit proefschrift voor consultatie beschikbaar te stellen en delen ervan uitsluitend voor persoonlijk gebruik te kopiëren. Elk ander gebruik is onderhevig aan de auteursrechtelijke beperkingen, in het bijzonder met betrekking tot de verplichting om de bron expliciet te vermelden wanneer resultaten van deze masterproef worden aangehaald.
Ghent, 21 August 2020
Supervisor, Supervisor,
‘Signature’ ‘Signature’
Prof. Dr. Ir. Kristof Demeestere Prof. Dr. Ir. Stijn Van Hulle
Mentor, Author,
‘Signature’ ‘Signature’
IV Acknowledgement
First and foremost I would like to thank God, to whom I owe my very existence and for all the love and blessings I have received since birth. I would like to express my sincere gratitude to the European Commission for awarding me with “Erasmus + EM partner country scholarship” and providing me all the financial support that was needed to successfully complete this two-year IMETE program. Next, I would like to thank the management of Ghent University (Campus Ghent and Campus Kortrijk) for providing me with the necessary technical support to carry out this research in a successful way.
I take immense pleasure in expressing my gratitude to both my supervisors Prof. Dr. Ir. Kristof Demeestere and Prof. Dr. Ir. Stijn Van Hulle, for suggesting this topic and allowing me to work on it. They have been actively involved in the research and have provided me with a great deal of motivation and continuous guidance to enable the successful completion of this work.
Words would not be enough to thank my beloved mentor Ing. Emma Deniere for all her constant supports, insights, suggestions, guidance, expertise, and revisions, which proved to be monumental for completing this master’s thesis. Additionally, I would also like to thank Em. Prof. Dr. Ir. Herman Van Langenhove, Prof. Dr. Ir. Christophe Walgraeve and other members of my EnVOC research team who provided me with inspiration and guidance during the progress of my research work through monthly meetings.
Finally, I would like to acknowledge my family and friends for their constant support and prayers throughout this entire period of master’s. Last but definitely not the least I would like to thank everyone who contributed to this work for all their constant support and without whose aid this dissertation would not have gone smoothly.
V Preamble
A thesis, in general, is a presentation of original research work carried out by the author, the findings of which may be of significant value to future application and studies. However, due to the unexpected experimental shutdowns over COVID-19 pandemic, issued by the Belgian government, the author was only capable of performing experiments pertaining to micropollutant degradation studies in different real waters and distilled water. The HPLC analysis and the fluorescence analysis of the collected samples were done by the mentor (Emma Deniere). Also, the fluorescence analysis was not performed for all the samples collected (only the samples from the first repetition of experiments were analysed) due to the reduction in fluorescence intensities of these samples that is expected as a result of storing them for more than 3 weeks at 4℃. Hence, only a part of this analysis was carried out, as a trial to verify if any valuable results could be obtained and so the results of this section might be incomplete. Moreover, various water quality parameters were obtained from an experimental study conducted by bachelor’s students of Ghent University on the same waters.
In addition, due to COVID-19 crisis, the objective of this particular study has been modified a bit from being mostly a practical research work into a research study with some experimental work and a more extended literature study (theoretical).
VI Abstract
Wastewater treatment plants using biological techniques have been identified as a major pathway through which anthropogenic (organic) micropollutants enter natural water bodies. Hydroxyl-radical (HO•) based AOPs such as ozonation are currently being considered as tertiary treatment to remove these micropollutants from effluents. However, sulfate-radical (SO4•−) based technologies
are being investigated lately to overcome the challenges of scavenging by various organic and inorganic fractions in water faced by HO•. Especially, the newly developed ozone/peroxymonosulfate (O3/PMS) process which produces both SO4•− and HO• is gaining
attention in recent times due to its various added benefits over conventional ozonation. Although the results of O3/PMS process appear to be positive in lab-scale studies using synthetic wastewater,
very few studies have actually tested this process in real waters. This study was aimed to bridge this missing piece of information by making an extensive comparison on the effectiveness of O3/PMS process in various real waters such as surface water (SW), groundwater (GW) and effluent
(EFF). In addition, the robustness of this new technology was tested using three different O3
-recalcitrant micropollutants (atrazine, chloramphenicol and para-nitrobenzoic acid), which exhibit varying rate constants with SO4•−. In all the real waters tested, O3/PMS oxidant system proved to
be more efficient than the conventional O3 system, mainly because of the generation of both SO4•−
and HO• radicals in O3/PMS system. Less than 50% of the applied PMS dose (64 mg/L) was found
to be utilized for achieving around 60-80% removal of micropollutants (initial dose-5μM) within 30 s in SW and GW. HO• and not SO4•− was found to be the major oxidant species for the removal
of all micropollutants in real waters, possibly due to the conversion of the SO4•− basedprocess into
a HO• based process by Cl− ions present in water. Four different components (3 humic-like and 1 protein-like substance) were identified from PARAFAC analysis, and the average removal of intensities of these fluorophores was found to be equal or higher for the O3/PMS process than for
the O3 process. The possible reasons for this observation could be due to the higher production of
radicals in the O3/PMS process than in the O3 process at neutral pH and the conversion of a more
selective SO4•− basedprocess into a less selective HO• based process. Based on the comparison of
fluorophore removal with micropollutant removal for EFF water matrix, it was found that humic-like substances are better removed than protein-humic-like substance during both O3 and O3/PMS
processes. Although higher removal of fluorophores was seen in O3/PMS process compared to O3
process, it was still more effective in removing the micropollutants.
Keywords: advanced oxidation process, ozone, peroxymonosulfate, micropollutants, sulfate radicals, real waters
VII Table of Contents Copyright ... III Acknowledgement ... IV Preamble ... V Abstract ... VI List of Figures ... X
List of Tables ... XII
List of Abbreviations ... XIII
1. Introduction ... 1
1.1 Background ... 1
1.2 Objectives... 2
2. Literature review ... 4
2.1 Ozonation ... 4
2.2 Interactions of O3 and HO• oxidants with the water matrix ... 7
2.2.1 Interactions of O3 with the water matrix ... 8
2.2.1.1 Organic Compounds ... 8
2.2.1.2 Inorganic Compounds ... 9
2.2.2 Interactions of HO• with the water matrix ... 9
2.2.2.1 Organic Compounds ... 9
2.2.2.2 Inorganic Compounds ... 10
2.3 Sulfate radical based AOPs: an innovative approach to tackle scavenging limitations ... 11
2.3.1 Homogeneous activation using transition metals ... 12
2.3.2 Heterogeneous activation using transition metals ... 13
2.3.3 Heterogeneous activation using metal free catalysts ... 14
2.3.4 Activation through energy (heat, ultrasound and ultraviolet light) ... 15
2.3.5 Other activation methods ... 16
2.4 Interactions of SO4•−, PMS and PDS oxidants with the water matrix ... 16
2.4.1 Reactions of PMS with the water matrix ... 17
2.4.1.1 Organic Compounds ... 17
2.4.1.2 Inorganic Compounds ... 17
2.4.2 Reactions of PDS with the water matrix ... 18
2.4.2.1 Organic Compounds ... 18
2.4.2.2 Inorganic Compounds ... 19
2.4.3 Interactions of SO4•− with the water matrix ... 19
VIII
2.4.3.2 Inorganic Compounds ... 20
2.5 The ozone/PMS process ... 21
2.5.1 Ozone as an activator for SO4•− production ... 21
2.5.2 Production of SO4•− during O3/PMS process ... 21
2.5.3 Application of O3/PMS for micropollutant removal ... 22
2.5.4. Influence of various parameters on the performance of O3/PMS process ... 23
2.5.4.1 pH ... 23
2.5.4.2 PMS dosage ... 24
2.5.4.3 Bulk organic and inorganic water constituents ... 24
2.6 Application of fluorescence monitoring of DOM in hydroxyl and sulfate-radical based AOPs ... 25
3. Materials and Methods ... 28
3.1. Chemicals ... 28
3.2. Preparation of ozone stock solution ... 28
3.3. Collection and storage of water samples ... 28
3.4. Filtration of collected water samples ... 28
3.5. Micropollutant degradation: experimental set-up and procedure ... 29
3.5.1 Degradation experiments using O3 and O3/PMS oxidants ... 29
3.5.2 Scavenging experiments using TBA and methanol ... 30
3.5.3 Sample collection ... 31
3.6. Analytical procedures ... 32
3.6.1 Measurement of water quality parameters ... 32
3.6.1.1 IOD/IOPD ... 32
3.6.1.2 Alkalinity ... 32
3.6.1.3 Nitrite ... 32
3.6.1.4 COD ... 33
3.6.1.5 Chloride and sulfate ... 33
3.6.1.6 pH ... 33
3.6.2 Determination of the O3 concentration ... 33
3.6.3 Determination of the PMS concentration ... 33
3.6.4 Determination of the micropollutants concentration ... 33
3.6.5 Fluorescence analysis – PARAFAC ... 34
3.7. Calculations ... 35
3.7.1 Comparison of ozonation and O3/PMS process ... 35
3.7.2 Contribution of various oxidants towards micropollutants removal ... 35
IX
4.1 Properties of real waters (SW, GW and EFF) ... 36
4.2 Degradation of micropollutants in various water matrices using O3 and O3/PMS systems ... 36
4.2.1 DW matrix ... 36
4.2.2 Real water matrices (SW, GW and EFF) ... 38
4.2.3 Influence of real water constituents on O3 and O3/PMS processes ... 38
4.2.4 Residual PMS concentration ... 40
4.3 Scavenging experiments using TBA and MeOH ... 41
4.3.1 Role of HO• radicals in O3 process ... 41
4.3.2 Role of HO• and SO4•─ radicals in O3/PMS process ... 42
4.4 Fluorescence results – PARAFAC analysis ... 45
4.4.1 Identification of fluorescing components ... 45
4.4.2 Removal of fluorescing components ... 46
4.4.3 Relationship between fluorophore removal and micropollutant removal ... 48
5. Conclusion ... 51 6. Future perspectives ... 52 7. References ... 53 8. Appendix ... 64 8.1 Tables ... 64 8.2 Figures ... 68
X List of Figures
Figure 1. Composition of beakers (for 80% concentration) used for the batch lab-scale degradation (O3 and O3/PMS) and scavenging experiments (O3/TBA, O3/PMS/TBA, and O3/PMS/MeOH) for
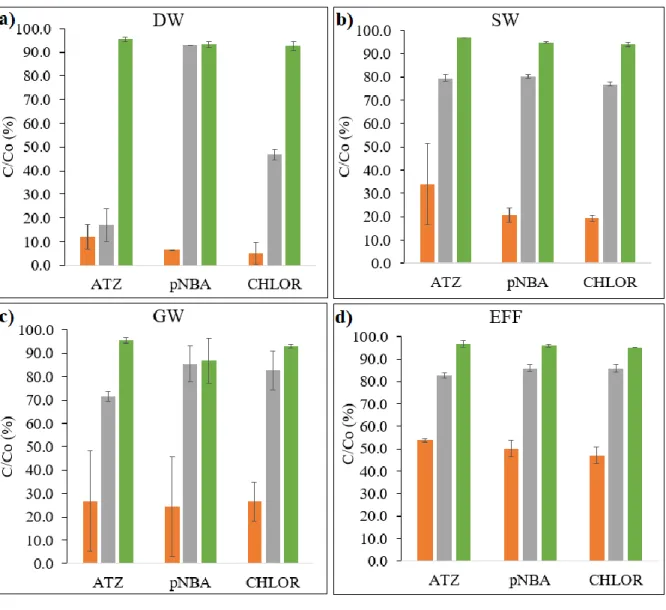
the removal of micropollutants in various real waters (EFF, SW and GW). The ‘Sample’ (light blue) shown in the figure consists of the water sample (EFF/SW/GW: 20 mL), the micropollutant (5 μM), and DW (to make up the total volume as 25 mL)... 30 Figure 2. Residual concentration (expressed as C/Co (%)) of three micropollutants by O3 and
O3/PMS process after a reaction time of 30 s in DW matrix. Experimental conditions: [ATZ]o =
5µM, [pNBA]o = 5µM, [CHLOR]o = 5µM, [O3]o =18 mg/L, [PMS]o = 64 mg/L, [phosphate
buffer]o=10mM and pH= 7.5. The error bars represent the standard deviation of three replicates.
... 37 Figure 3. Residual concentration (expressed as C/Co (%)) of three micropollutants by O3 and
O3/PMS process after a reaction time of 30 s in a) 80% SW, b) 80% GW and c) 80% EFF matrices.
Experimental conditions: [ATZ]o = 5µM, [pNBA]o = 5µM, [CHLOR]o = 5µM, [PMS]o = 64 mg/L
and pH= 7.5. The error bars represent the standard deviation of two replicates... 39 Figure 4. Additional removal of micropollutants from O3/PMS over conventional O3 process in
different diluted EFF matrices after 30 s reaction time. Experimental conditions: [ATZ]o = 5µM,
[pNBA]o = 5µM, [CHLOR]o = 5µM, [O3]o =17.12 mg/L, [PMS]o = 64 mg/L and pH= 7.5. The
error bars represent the standard deviation of two replicates. ... 40 Figure 5. Residual PMS concentration (expressed as C/Co (%)) measured after 30 s of reaction
during the O3/PMS process for the removal of micropollutants from 80% concentrations of SW,
GW and EFF. Experimental condition: [ATZ]o = 5µM, [pNBA]o = 5µM, [CHLOR]o = 5µM,
[PMS]o = 64 mg/L, [O3]o =15-18.7 mg/L and pH= 7.5. The error bars represent the standard
deviation of two replicates. ... 41 Figure 6. Residual concentration (expressed as C/Co (%)) of three micropollutants by O3 ( ) and
O3/TBA ( ) processes after a reaction time of 30 s in a) DW, b) SW, c) GW and d) EFF matrices.
Experimental conditions: [ATZ]o = 5µM, [pNBA]o = 5µM, [CHLOR]o = 5µM, [TBA]o = 74.12
mg/L, [O3]o = 15-18.7 mg/L and pH= 7.5. The error bars represent the standard deviation of two
replicates for SW, GW and EFF and the standard deviation of three replicates for DW. ... 42 Figure 7. Residual concentration (expressed as C/Co (%)) of three micropollutants by O3/PMS (
), O3/PMS/TBA ( ) and O3/PMS/MeOH ( ) processes after a reaction time of 30 s in a) SW, b)
DW, c) GW and d) EFF matrices. Experimental conditions: [ATZ]o = 5µM, [pNBA]o = 5µM,
[CHLOR]o = 5µM, [PMS]o = 64 mg/L, [O3]o =15-18.7 mg/L, [TBA]o = 74.12 mg/L, [MeOH]o =
320.4 mg/L and pH= 7.5. The error bars represent the standard deviation of two replicates for SW, GW and EFF and the standard deviation of three replicates for DW. ... 44 Figure 8. Average removal of fluorescence intensity of the four different fluorescing components identified using PARAFAC analysis during O3 and O3/PMS process after a reaction time of 30 s in
a) 80% SW, b) 80% GW and c) 80% EFF matrices. Experimental conditions: [ATZ]o = 5µM,
[pNBA]o = 5µM, [CHLOR]o = 5µM, [PMS]o = 64 mg/L, [O3]o =15-18.7 mg/L and pH= 7.5. The
error bars represent the standard deviation on the ΔFmax values obtained in three different experiments. ... 48 Figure 9. The removal of (a) ATZ, (b) CHLOR and (c) pNBA in relation to the removal of the four identified PARAFAC components ((E1- ), (E2- ), (E3- ) and (E4- )) during O3/PMS (circle)
and O3 (square) processes for 20%, 40%, 60% and 80% concentrations of EFF water samples. .. 49
Figure A1. Activation of peroxymonosulfate (PMS) and peroxydisulfate (PDS) through electron and energy transfer mechanisms (Lee et al., 2020). ... 68
XI
Figure A2. Degradation of atrazine (ATZ) in synthetic water (1 µM) using O3, O3/PDS, O3/H2O2
and O3/PMS process. Experimental conditions: [O3]o=20 µM, [PDS]o=20 µM, [H2O2]o=10 µM,
[PMS]o=10 µM, [phosphate buffer]=10 mM, temperature= 20±1 ℃ and pH =8 (Yang et al., 2015).
... 68 Figure A3. Degradation of ibuprofen (IBP) in distilled water by different oxidation systems. Experimental conditions: [IBP]o=5 µM, [O3]o=31.3 µM, [PDS]o=20 µM, [H2O2]o=10 µM,
[PMS]o=6.5 µM, temperature= 20 ℃ and pH =7 (Yuan et al., 2017). ... 69 Figure A4. Removal of IBP in distilled water using O3, PMS and O3/PMS at different pH values.
Experimental conditions: [IBP]o=5 µM, [O3]o=31.3 µM, [PMS]o=6.5 µM, temperature= 20 ℃
(Yuan et al., 2017). ... 69 Figure A5. Degradation of atrazine (ATZ) in real water matrices (surface water-SW and ground water-GW) by O3, O3/PMS and O3/H2O2. Experimental conditions: [O3]o=40 µM, [ATZ]o=1 µM,
[H2O2]o=20 µM, [PMS]o=20 µM and temperature= 15 ℃ (Yang et al., 2015). ... 70 Figure A6. Spectral loadings of 4-component after split-half analysis in order to validate the developed PARAFAC model for EFF samples. ... 70 Figure A7. Spectral loadings of 4-component after split-half analysis in order to validate the developed PARAFAC model for SW and GW samples. ... 71 Figure A8. Residual concentration (expressed as C/Co (%)) of three micropollutants by a) O3 and
b) O3/PMS process after a reaction time of 30 s in 20% EFF( ), 40% EFF ( ), 60% EFF ( ) and
80% EFF ( ) matrices. Experimental conditions: [ATZ]o = 5µM, [pNBA]o = 5µM, [CHLOR]o =
5µM, [PMS]o = 64 mg/L, [O3]o =17.12 mg/L and pH= 7.5. The error bars represent the standard
deviation of two replicates. ... 71 Figure A9. Visualized location of the defined PARAFAC components within the EEMs for EFF. ... 72 Figure A10. Visualized location of the defined PARAFAC components within the EEMs for SW and GW. ... 72
XII List of Tables
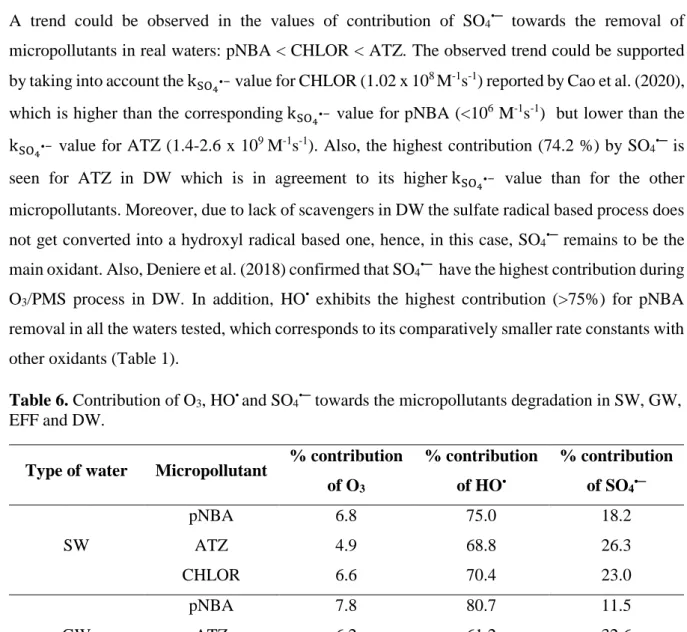
Table 1. Second-order reaction rate constants of micropollutants used in this study with various oxidants ... 2 Table 2. Enhanced ozone-based methods aimed at increasing hydroxyl radical production ... 6 Table 3. Second-order reaction rate constants of O3 and HO• with scavengers present in real water
matrices ... 8 Table 4. Reaction rate constants between scavengers present in real water matrices and SO4•− .. 11 Table 5. Various physical-chemical properties of EFF, SW and GW used in this study ... 36 Table 6. Contribution of O3, HO• and SO4•─ towards the micropollutants degradation in SW, GW,
EFF and DW. ... 45 Table 7. Fluorescing components determined by PARAFAC modelling for EFF, and their wavelengths corresponding to the positions of the excitation and emission maxima. ... 46 Table 8. Fluorescing components determined by PARAFAC modelling for SW and GW, and their wavelengths corresponding to the positions of the excitation and emission maxima. ... 46
Table A1. Characteristics and properties of peroxydisulfate and peroxymonosulfate (Wacławek et al., 2017; Ghanbari & Moradi, 2017)... 64 Table A2. The volumes of the different (stock) solutions added for the degradation study of ATZ (5 µM) in EFF through the O3 process (working volume: 25 mL) ... 64 Table A3. The volumes of the different (stock) solutions added for the degradation study of ATZ (5 µM) in EFF through the O3/PMS process (working volume: 25 mL) ... 65 Table A4. The volumes of the different (stock) solutions added for the degradation study of ATZ (5 µM) in EFF through the O3/TBA process (working volume: 25 mL) ... 65 Table A5. The volumes of the different (stock) solutions added for the degradation study of ATZ (5 µM) in EFF through the O3/PMS/TBA process (working volume: 25 mL) ... 65 Table A6. The volumes of the different (stock) solutions added for the degradation study of ATZ (5 µM) in EFF through the O3/PMS/MeOH process (working volume: 25 mL) ... 66 Table A7. O3 concentrations in both the stock solutions and in the beakers used for the degradation
and scavenging experiments performed with real water matrices (SW, GW and EFF) and distilled water (DW) ... 66 Table A8. Fmax values of all the four components obtained from PARAFAC analysis at time 0 s and 30 s for 80% concentration of the real water matrices during ATZ removal by O3 and O3/PMS
processes ... 67 Table A9. Fmax values of all the four components obtained from PARAFAC analysis at time 0 s and 30 s for 80% concentration of the real water matrices during CHLOR removal by O3 and
O3/PMS processes ... 67 Table A10. Fmax values of all the four components obtained from PARAFAC analysis at time 0 s and 30 s for 80% concentration of the real water matrices during pNBA removal by O3 and O3/PMS
processes ... 67
XIII List of Abbreviations
ACE Acesulfame
AO7 Acid orange 7
AOPs Advanced oxidation processes
AOX Adsorbable organic halides
ATZ Atrazine
CHLOR Chloramphenicol
DOC Dissolved organic carbon
DOM Dissolved organic matter
DW Distilled water
EEM Excitation/emission matrix
EFFs Secondary effluents
EU European Union
FA Fulvic acid
GAC Granular activated carbon
GW Ground water
HA Humic acid
IBP Ibuprofen
IOD Instantaneous ozone demand
IOPD Instantaneous ozone PMS demand
MeOH Methanol
NOM Natural organic matter
OBPs Oxidation by-products
PABA Para-aminobenzoic acid
PARAFAC Parallel factor analysis
PCB28 2,4,4′-trichlorobiphenyl
pCBA Para-chlorobenzoic acid
PDS Peroxydisulfate
PMS Peroxymonosulfate
pNBA Para-nitrobenzoic acid
RO Reverse osmosis
SW Surface water
TBA t-butanol
TBBPA Tetrabromobisphenol A
US Ultrasound
1 1. Introduction
1.1 Background
The eco(toxico)logical effects caused by organic micropollutants on various ecosystems have become one of the growing concerns lately (Stamm, 2020; Feito et al., 2013). These compounds have an anthropogenic origin and belong to product groups such as pharmaceuticals, endocrine disrupting compounds and pesticides that are used in daily life. They enter natural water bodies through diverse pathways and one noticeable pathway is through secondary effluent (EFF) discharged from wastewater treatment plants (WWTPs) (Antakyali et al., 2015). This implies that the currently employed biological techniques for treating waters are not effective towards these recalcitrant micropollutants. Though the presence of these pollutants in the environment is known, there are no stringent laws currently in the European Union (EU) to eliminate these compounds from water bodies. However, Switzerland (a non-EU country in Europe) has imposed local legislation (New Water Conservation Legislation, 2016) according to which 120 WWTPs are to be retrofitted with technologies to remove organic trace substances and micropollutants (Sousa et al., 2018).
One of the primary techniques for dealing with these micropollutants is advanced oxidation processes (AOPs) that employ highly reactive radical species for oxidising micropollutants. Currently, the most widely employed solution in Switzerland is ozonation, wherein ozone (O3) and
hydroxyl radicals (HO•), produced during ozonation, both oxidize these compounds. Although the process has shown promising micropollutant removal from waters, there are still some issues associated with it and, hence, there is still some room for improvement. One such issue is that HO• radicals have a high reactivity not only with the micropollutants but also with other organic and inorganic bulk compounds present in the waters, which are not the target in this case. Consequently, a significant portion of the oxidants (O3 and HO• radicals) is lost due to scavenging, resulting in a
reduction of the micropollutant elimination efficiency (~25-75%) (Katsoyiannis et al., 2011; Choi et al., 2014; Giannakis et al., 2015).
Sulfate radicals (SO4•−) have been reported in literature to be effective in removing micropollutants.
These radicals are more selective in nature than HO•, i.e. in the same water SO4•− are less scavenged
by the bulk organic matter compared to HO• due to smaller rate constants (Mahdi-Ahmed & Chiron, 2014). As a result, SO4•− can more efficiently target and remove the micropollutants that have
comparable rate constants with both (SO4•− and HO•) radicals. The predominant way to produce
SO4•− radicals is through activation of peroxymonosulfate (PMS) and peroxydisulfate (PDS) by
energy and electron transfer mechanisms. Benefit of using PMS over PDS is that the former produces both SO4•− and HO• on activation while the latter only produces SO4•−.
2
Removal of micropollutants using ozone activated PMS is gaining attention lately. Preliminary experimental results on micropollutant removal using SO4•− radicals produced from O3/PMS look
positive (Yang et al., 2015; Deniere et al., 2018). However, most of these studies are based on lab-scale using distilled water spiked with micropollutants (so called synthetic (waste)water), and very few degradation studies have been done with real (waste)waters. Also, no extensive study has been performed in a way to compare the micropollutant degradation in various real water matrices with this technology. Additionally, it is necessary to verify if O3/PMS process is robust enough for
removing diverse O3-recalcitrant compounds, exhibiting varying rate constants with SO4•− radicals.
1.2 Objectives
This study focuses on using O3/PMS technology for the removal of three different O3-recalcitrant
micropollutants, namely chloramphenicol (CHLOR), atrazine (ATZ) and para-nitrobenzoic acid (pNBA), from three different real water samples: surface water (SW), groundwater (GW) and effluent (EFF). These micropollutants have dissimilar rate constants with SO4•−, while they exhibit
a comparable rate constant with HO• and O3 (Table 1).
Table 1. Second-order reaction rate constants of micropollutants used in this study with various oxidants.
Micropollutant O3 (M-1s-1) HO• (x 109 M-1s-1) SO4•─ (x 109 M-1s-1) References
CHLOR 0.1 5.8 0.1; 13.3-61.8 Cao et al., 2020;
Tan et al., 2018
ATZ 6.3 2.5-3.0 1.4-2.6
Azenha et al., 2003; Yang et al., 2015
pNBA ~0.15 2.6 ≤0.001 Nate et al., 1977
The specific objectives of this study are:
1. To compare the micropollutant removal efficiencies between ozonation and O3/PMS
processes in SW, GW and EFF
2. To evaluate the contribution of various oxidants (O3, HO• and SO4•─) in ozonation and
O3/PMS towards the removal of micropollutants
3. To monitor the removal of different dissolved organic matter (DOM) fractions using fluorescence analysis, and to investigate correlations with the removal of micropollutants.
3
The following section of this thesis report consists of four major chapters, starting with a literature review, which covers various topics under different sections that are necessary to get a background knowledge about this research field. Followed by a materials and methodology section that describes in detail how the experiments and the analyses were performed. Then comes the results and discussion section, which elaborates the outcomes of the experiments along with the possible explanations associated with them. Finally, the last chapter describes the conclusions of this thesis as well as gives suggestions for future scientific investigations.
4 2. Literature review
2.1 Ozonation
Ozonation is a well-established AOP that has been widely applied for the production of drinking water since the late 19th century. Ozone acts as an excellent disinfectant, eliminating pathogens that are resistant to conventional disinfectants (Von Gunten, 2003). However, the application of ozonation for wastewater treatment is gaining a lot of attention lately due to the strong oxidising potential of O3 (E0=2.07 V) to degrade both organic and inorganic pollutants (Petrovic et al., 2011).
The removal of organic micropollutants by ozonation occurs through two different pathways, i.e. either through direct reactions between the pollutant and O3 molecules (Equation 1) or through
indirect reactions between the target compound and HO• produced during ozonation(Equation 2) (Von Sonntag & Von Gunten, 2012).
P + O3 → Pox (1)
P + •OH → Pox (2)
Where P denotes the organic micropollutant that needs to be removed and the subscript ‘ox’ represents the oxidised products. HO• are mainly produced when O3 decomposes at neutral or alkaline pH (Equations 3-10) or as a result of reaction between O3 and DOM (Equations 11-12) (Ribeiro et al., 2019). O3 + HO− → HO2− + O2 k = 70 M-1s-1 (3) O3 + HO2− → HO• + O2•− + O2 k = 2.8 x 106 M-1s-1 (4) O3 + O2•− → O3•− + O2 k = 1.6 x 109 M-1s-1 (5) O3•− + H+ ↔ HO3• (pH < 8) kforward = 5 x 1010 M-1 s-1 kbackward = 3.3 x 102 M-1s-1 (6) HO3• → HO• + O2 (pH < 8) k = 1.4 x 105 M-1s-1 (7) O3•− ↔ O•− + O2 (pH > 8) kforward = 2.1 x 103 M-1s -1 kbackward = 3.3 x 109 M-1s-1 (8) O•− + H2O → HO• + HO− (pH > 8) k = 108 M-1s-1 (9) HO• + O3 → HO2• + O2 k = 1 x 108 M-1s-1 – 2 x 109 M-1s-1 (10) O3 + DOMinitiator → HO• (11) HO• + DOMpromotor →… → O2•− (12)
Ozone itself as an oxidant is highly selective and primarily targets electron-rich moieties/functional groups, i.e. double bonds and aromatic rings, in organic molecules. In general, organic compounds having a rate constant (kO3) with O3 below 103 M-1s-1 are referred to as ozone recalcitrant
compounds, and those with kO3above 103 M-1s-1 are referred to as ozone sensitive compounds.
5
constants (kHO•) for all types of micropollutants, compared to their corresponding ozone reaction
rate constants. This was observed in a study conducted by Hollender et al. (2009) aimed at the removal of micropollutants from EFF. It was seen that micropollutants which contained active aromatic moieties or amine functional groups or double bonds (e.g. trimethoprim and carbamazepine) were eliminated (>95% removal) at a lower ozone dosage of 0.36 g O3 g-1 dissolved
organic carbon (DOC). Nevertheless, compounds such as atrazine and iopromide, which lacks these type of functional groups exhibited relatively low degradation, i.e. respectively 49% and 62% elimination, even at a high ozone dosage of 1.16 g O3 g-1 DOC. The micropollutants selected for
this study (ATZ, pNBA and CHLOR) lack these electron-rich functional groups, hence their relative rate constant with O3is extremely smaller than their rate constant with HO• (Table 1).
Moreover, the contribution of oxidants (O3 and HO•) towards the pollutant degradation depends on
several factors, i.e. the exposure of the oxidising agent with the pollutant, pH of the solution, and the second order reaction rate constants (Li et al., 2016; Lee et al., 2013).
Various methods for initiating the formation of HO• during ozonation have been summarised in Table 2. The decomposition of ozone is almost 2-3 times faster during these techniques than during ozonation (Von Gunten, 2003; Nawrocki & Kasprzyk-Hordern, 2010), producing a higher amount of HO•. However, these processes have not been used on full scale, except for the peroxone process, which has been used for disinfection across the globe in drinking water treatment (Lenntech, 2020). The UV/O3 process has been reported to give a very small free HO• quantum yield (ratio of number
of times a reaction occurs per photon absorbed by the system) of 0.1 since only a small quantity of hydrogen peroxide (H2O2)isproduced from oxygen atom that further yields HO• (Reisz et al.,
2003). Furthermore, the decomposition of ozone could also be increased by increasing the pH (Equations 3-10). For instance the percentage of kHO• with respect to the total decomposition rate
constant for 1,5-naphthalene was found to be 15, 36, 59 and 84 % at a pH of 3, 5, 7 and 9 respectively (Calderara et al., 2002). Though heterogeneous and homogeneous catalytic ozonation provide positive results at lab scale, the lack in comprehension of their mechanisms limits the full-scale application of these processes (Wu et al., 2016; Beltrán et al., 2005). On the other hand, the peroxone process has been tested on a pilot scale level for treating wastewaters and has proven to be efficient (Knol et al., 2015). Yet, the application of the peroxone process for the mitigation of micropollutants from water is still questionable, due to various reasons like self-scavenging of HO• by H2O2(Katsoyiannis et al., 2011) and a reduction in O3 exposure along with the shortening of O3
6
Table 2. Enhanced ozone-based methods aimed at increasing hydroxyl radical production.
Method Principle Reactions References
UV/O3
Cleavage of ozone molecules by UV irradiation (λ<300 nm) forms singlet atomic oxygen that reacts with water to form H2O2,
which further decomposes into HO•.
O3 + hν → O(1D) + O2(1Δg) O(1D) + H2O → H2O2 (hot) H2O2 (hot) → 2 HO• Von Sonntag, 2008 Ozonation at an elevated pH
A high pH value (pH ≥ 8) favours the decomposition of ozone into HO•, due to the presence of more HO−.
Reactions (3) - (10) Buffle et al., 2006
Peroxone process (O3/H2O2)
Ozone reacts with a peroxide anion, forming HO2• and O3•− as intermediates,
which subsequently react to yieldHO•.
H2O2 ⇄ HO2− + H+ HO2− + O3 → HO2• + O3•− HO2• ⇄ O2•− + H+ O2•− + O3 → O2 + O3•− O3•− + H+ ⇄ HO3• HO3• → O2 + HO• Merényi et al., 2010 Homogeneous catalytic ozonation Two mechanisms:
(a) Direct decomposition of ozone molecules into HO• by metal ions.
(b) A complex is formed from the reaction of metal ion and the target organic molecule to be removed. Then this formed complex is oxidised by O3 leading to HO• yield.
(a) Fe2+ + O 3 → FeO2+ + O2 FeO2+ + H2O → Fe3+ + HO• + OH− Fe3+ + H2O + hν → Fe2+ + HO• + H+ (b) Fe3+ + C2O4− → FeC2O4+ FeC2O4+ + C2O4− → Fe(C2O4)2− FeC2O4+ + O3 → 2 CO2+ Fe3+ + 2 O3•− Fe(C2O4)2− + 2 O3 → 2 CO2 + FeC2O4+ + 2 O3•− 2 O3•− + H+ →HO3• → HO• + O2
Sauleda & Brillas, 2001; Beltrán et al., 2005
Heterogeneous catalytic ozonation
Different complex reaction pathways especially mediated through metallic oxide (multi-phase transport system) mechanisms
7
Regarding the real time application of ozonation, a full-scale operational WWTP implementing ozonation as a post treatment has already been set up in Switzerland, aiming at an 80% removal of specific micropollutants (Bourgin et al., 2018). Similarly, Hollender et al. (2009) tested the removal efficiency of micropollutants in a municipal WWTP that was upgraded with a full-scale post-ozonation followed by sand filtration. With an ozone dosage of 0.6 g O3 g-1 DOC, only 11
recalcitrant micropollutants out of 220 micropollutants were detected with a concentration higher than 0.1 µg/L in the final ozonated EFF.
Even if ozonation provides positive results towards micropollutant removal in real waters. It is still quite essential to have a knowledge on how these oxidants (O3 and HO•) behave and interact with
the various organic and inorganic compounds present in waters (apart from the targeted micropollutants). Which would aid in a deeper understanding of the technology and help to predict the outcomes when used for real waters. In addition, it would also help in further improvement of this technology.
2.2 Interactions of O3 and HO• oxidants with the water matrix
Numerous studies have been performed on the elimination of micropollutants present in real wastewaters using ozonation techniques (El-Taliawy et al., 2017, Hernández-Leal et al., 2011). However, one of the major issues reported during ozonation is the scavenging of oxidants (O3 and
HO•) by substances present in the bulk water matrix (Buffle et al., 2006). Scavenging refers to the reduction in the number of oxidant species due to the reaction of the oxidant species with unwanted/uninterested substances in the water matrix. Consequently, the number of available oxidants for the removal of target compound decreases. Some of the most common scavengers found in real waters include HCO3−, CO32−, NO3−, NO2−, Cl−, Br− and DOM (i.e. humic acid (HA)
and fulvic acid (FA)) (Gomes et al., 2017). The second order reaction rate constants of O3 and HO•
with the major scavengers present in water matrix are shown in Table 3. O3 is mainly scavenged
by the electron rich functional groups present in waters, whereas HO• are also scavenged by inorganic ions like carbonate and bicarbonate (Petre et al., 2013).
Yet another major issue associated with AOPs is the production of unwanted oxidation by-products (OBPs) that possess potential health risks, at times being more harmful than the parent compound itself. The OBP formation depends on a number of factors like radical exposure, the abundancy of other influential water constituents (i.e. radical scavengers) and the direct reaction of the applied oxidants especially in the case of O3.
8
Table 3. Second-order reaction rate constants of O3 and HO• with scavengers present in real water
matrices.
Scavenger 𝐤𝐎𝟑 (M
-1s-1) 𝐤
𝐇𝐎• (M-1s-1) Reference
Dissolved organic
carbon (DOC) N.A 1.4 – 4.5 x 10
8 Von Sonntag & Von Gunten, 2012;
Chaplin, 2014 Bicarbonate
(HCO3−)
≪ 0.01 8.5 x 106 Hoigné et al., 1985; Buxton et al., 1988
Carbonate (CO32−) < 0.1 3.9 x 108
Hoigné et al., 1985; Buxton et al., 1988
Nitrate (NO3−) < 10-4 < 5 x 105
Hoigné et al., 1985; Ross & Ross, 1977
Nitrite (NO2−) 5.83 x 105 1 x 1010 Liu et al., 2001; Buxton et al., 1988
Chloride (Cl−) < 3 x 10−3 4.3 x 109 Hoigné et al., 1985; Buxton et al., 1988
Bromide (Br−) 258 (at 25 °C) 1.1 x 1010 Liu et al., 2001; Buxton et al., 1988 Hydroxide (OH−) 70 1.2 x 1010 Ribeiro et al., 2019; Poskrebyshev et
al., 2002
N.A – no data available
2.2.1 Interactions of O3 with the water matrix
2.2.1.1 Organic Compounds
Organic matter contributes to a significant portion of the real water bodies and the reactions between organic matter and O3 could result in the production of unwanted OBPs. Generally, the
ozone decomposes rapidly during the initial phase of reaction (t < 30 s) during ozonation of natural waters, with a little production of HO•, this is known as the instantaneous ozone demand (IOD). While during the second phase of reactions (t > 30 s), O3 decomposes following an apparent
first-order rate law (i.e. ozone reaction rate constant values with specific organic moieties and some inorganic ions in waters remain the same) based radical reactions pathway, predominantly generating •OH radicals (Buffle et al., 2006). From a study conducted by Cho et al. (2003), on the investigation of O3 reaction characteristics in river waters, it was found that 40% of the applied
ozone dose (0.65 g O3 g-1 DOC) was consumed during IOD, which was mainly caused by natural
organic matter (NOM) in water. In general, waters with high NOM concentrations tend to have a higher IOD (Shin et al., 2016). When making a comparison between the IODs of real waters it could be seen that the IOD of EFF (4.3 mg/L DOC) (Hasegawa et al., 2008) is ~3 times and ~1.6 times higher than that of GW (0.8 mg/L DOC) (Park et al., 2001) and SW (2.7 mg/L DOC) (Cho et al., 2003) respectively. Therefore, it is clear that NOM poses a significant concern during
9
micropollutant elimination from wastewaters (Rahman et al. 2010) and only those micropollutants that have kO3 > 103 M-1s-1 could be eliminated at a moderate ozone dosage (0.4 g O3 g-1 DOC)
(Nöthe et al., 2009).
2.2.1.2 Inorganic Compounds
Comparing the rate constants of O3 with various inorganic ions from Table 3, it could be clearly
understood that O3 reacts with nitrite (NO2−) very rapidly, yielding nitrate (NO3−) as the main
product. An intermediate is usually formed which further decomposes into nitrate and oxygen in its singlet state (Equation 13) (Naumov et al., 2010).
NO2− + O3 → −OOONO2 → NO3− + 1O2 (13)
In the same study, the formation of peroxynitrite (2.6%) from the adduct intermediate was observed. In addition, during the scavenging of O3 a small percentage of HO• was also noticed to
be formed from the reaction of O3 with nitrite.
Another scavenging reaction of interest is the reaction of O3 with bromide ion (Br−), mainly because
of the formation of the carcinogenic bromate (BrO3−) as the final product. Hypobromite and bromite
are formed as intermediates and the overall reaction is given in Equation 14. However, this reaction is reversible and slow (Naumov & von Sonntag, 2008b). In addition, Br− could be considered as an ozone refractory compound owing to the fact that the kO3 < 103 M-1s-1.
Br− + O3 ↔ BrOOO− (14)
The typical reactions of ozone with other inorganic scavengers can be neglected since they are comparatively slow. Hence, the reactions of ozone with these inorganic scavengers will be largely overrun by competing reactions with other species in water that have higher rate constants.
2.2.2 Interactions of HO• with the water matrix 2.2.2.1 Organic Compounds
The presence of some organic compounds like HA and FA in the waters have found to affect the HO• yield, by terminating the radical chain production reaction and yielding a non-radical product. These organic compounds are referred to as radical inhibitors in general (Von Sonntag & Von Gunten, 2012).
Park et al. (2001) studied the influence of IOD on the production of HO• by conducting an experiment with a series of ozone injections (0.36 mg/L, 0.48 mg/L and 0.64 mg/L). pCBA (kO3 =
0.15 M-1s-1 and kHO• = 5x 109 M-1s-1) was used as the probe compound, a compound which
10
with another species (in this case O3), which in turn enables the indirect measurement of former
specie’s concentration. It was observed that there was no significant removal of pCBA during the first ozone dosage of 0.36 mg/L because this quantity of ozone was utilized for satisfying the IOD of water. While a linear decrease in the concentration of pCBA was observed for the second and third ozone dosages, indicating the production of HO•. Therefore, it could be concluded that the applied ozone dosage should be high enough to satisfy IOD first, else there would be delayed or no production of HO• (Wert et al., 2009). Contrarily, Jin et al. (2017) reported a significant production of HO• during the IOD phase because of reaction between O3 and specific moieties of DOM present
in the EFF. Hence, it can be concluded that depending on the nature of DOM present in the water sample it could act either as a radical initiator or as radical inhibitor.
Besides, with respect to OBP formation, Andrzejewski et al. (2006) conducted an experiment to analyse the formation of N-nitrosodimethylamine (NDMA; carcinogen) during the ozonation of waters containing dimethylamine. It was found that HO• played a major role in production of NDMA, especially at a pH of 10.5 and higher by causing the destruction of dimethylamine forming free amines, thereby leading to NDMA formation.
2.2.2.2 Inorganic Compounds
The main inorganic OBPs formed during O3-based oxidation processes include oxyhalides like
bromate, chlorite, chlorate and perchlorate, which are generated from the interaction of HO• with the various inorganic ions present in water. Bromate is formed from the reaction of HO• with Br− (von Gunten and Oliver, 1998), but this reaction is found to be suppressed by DOM present in waters (Lutze et al., 2014) as it hinders the reaction from HOBr to BrO3− (von Gunten., 2003).
Linden et al. (2005) even postulated that the peroxone process could be considered as an AOP that has no significant OBP formation, i.e. BrO3−, which leads to no or minor increase in genotoxicity
when applied to surface waters. Nevertheless, contradicting to the observations made by Linden et al. (2005), significant OBP formation was recorded when working with peroxone process under the operating conditions of low pH (~ 3) and high Cl− concentrations (> 1000 mg/L) (Baycan et al., 2007). In this study, adsorbable organic halides (AOX) were formed as OBPs (Equations 15-17). HO• + Cl− ↔ •ClOH− kforward = 4.3 x 109 M-1s-1 kbackward = 6.1 x 109 M-1s-1 (15) •ClOH− + H+ ↔ Cl• + H
2O kforward = 2.6 x 1010 M-1s-1 kbackward = 3.6 x 103 M-1s-1 (16)
Cl• + R2C=CR2 → R2ClC•−CR2 → Chlorohydrocarbons (AOX formation) (17)
Apart from Cl−, the scavenging effects displayed by carbonate and bicarbonate are well known and have been reported in many studies (Petre et al., 2013, Gomes et al., 2018). Hence, it can be
11
concluded that most of these anions present in natural waters appear to act as hindrances to wastewater treatment.
2.3 Sulfate radical based AOPs: an innovative approach to tackle scavenging limitations Ozonation has proven to be a promising technology for tackling the problem of micropollutants in waters (El-Taliawy et al., 2017, Hernández -Leal et al., 2011). One major drawback is the scavenging of highly reactive HO• by the components of water matrix causing a reduction in the overall effectiveness of the technology (Nöthe et al., 2009). The solution of increasing the oxidant dosage to tackle this issue comes at a cost of increasing the price of the technology significantly (Wert et al., 2009). Therefore an interest in SO4•− based AOPs have grown. The higher selectivity
of SO4•−, which react mainly through electron transfer mechanisms, is a huge benefit when
compared to HO• (Lee et al., 2020). The reaction rate constants of SO4•− (kSO4•−) with scavengers
present in the water matrix are shown in Table 4. It can be clearly seen that scavengers rate constants for reactions with HO• are ten to a hundred times higher than their reaction rate constants with SO4•−. As such, the SO4•− would be less scavenged. Other advantages of SO4•− based oxidation
include high radical yields, diverse methods for activation of persulfate (general term referring to PMS and PDS), less dependency of degradation efficiency on operational parameters, and cheap transportation and storage costs of persulfate salts (Lee et al., 2020). In addition, the second order reaction rate constants for most of the known micropollutants and SO4•− are of the magnitude 108
-109 M-1s-1, which is very much comparable to those with HO• (Deniere et al., 2018). The above stated advantages make SO4•− based AOPs a convincing technique for treating waters contaminated
with micropollutants. PMS and PDS have proven to be potential raw materials for producing SO4•−
radicals and degrading organics in water (Deniere et al., 2018; Zhang et al., 2016).
Table 4. Reaction rate constants between scavengers present in real water matrices and SO4•−.
Scavenger 𝐤𝐒𝐎𝟒•−(M
-1s-1)
Reference Dissolved organic carbon
(DOC) 2.5 x 10
7
– 8.1 x 107 Lee et al., 2020 Bicarbonate (HCO3−) 1.6 x 106 Zuo et al., 1999
Carbonate (CO32−) 6.1 x 106 Zuo et al., 1999
Nitrate (NO3−) 1.4 x 106 Ross & Neta, 1979
Nitrite (NO2−) 8.8 x 108 Ross & Neta, 1979
Chloride (Cl−) 1.9 x 108 Ross & Neta, 1979
Bromide (Br−) 3.5 x 109 Ross & Neta, 1979
12
The production of SO4•− has been achieved (in-situ) by the cleavage of the peroxide bond (O−O)
in PMS and PDS through energy and electron transfer mechanisms (Wacławek et al., 2017). Though PMS (Eo = 1.8 V) and PDS (Eo = 2.1 V) appear to be thermodynamically a strong oxidant, their direct reaction with the majority of the pollutants is too slow, making their activation a prerequisite (Yen et al., 2011). For instance, in a study conducted by Lou et al. (2014), SO4•−
produced from PMS/phosphate anions system showed higher than 90% degradation of acid orange 7 (AO7) within 15 min while unactivated PMS exhibited almost zero degradation for the same time interval. Some characteristics and properties of PMS and PDS have been listed in Table A1 (Appendix) (Wacławek et al., 2017; Ghanbari & Moradi, 2017).
It could be seen from Table A1 that compared to PDS, PMS has a shorter bond length (1.497 Å vs 1.453 Å), and hence theoretically PMS requires more energy to produce radical species through homolytic cleavage of the peroxide bond. During activation, PDS decays in radical pathways resulting in the formation of SO4•−, while PMS may decay giving both SO4•− and HO• (Luo et al.,
2015). This gives the advantage of degrading the compounds that are more resilient to one radical by the other, e.g. nitrobenzene, can be eliminated by HO• radicals but is almost inert towards SO4•− radicals (Lu et al., 2019). The activation of PMS and PDS through energy and
electron transfer mechanisms is shown in Figure A1 (Appendix) (Lee et al., 2020). The major activators of the peroxo-group in the persulfates include ultraviolet (energy transfer), ultrasound (energy transfer), heat (energy transfer), transition metals (electron transfer), carbon catalysts (electron transfer), and ozone (electron transfer). Various methodologies for effectively activating PMS and PDS for the production of SO4•− are discussed below.
2.3.1 Homogeneous activation using transition metals
In a wide group of transition metal ions (Ag+, Co2+, Fe2+, Cu2+, Ni2+, Ru3+), cobalt was found to be the most efficient catalyst for activating PMS (Anipsitakis & Dionysiou, 2003). The efficiency of Co2+/PMS system was tested against conventional Fenton’s reagent (Fe2+/H2O2) for the degradation
of three structurally different organic contaminants. The overall reaction of Co2+/PMS and
Fe2+/H
2O2 systems are:
Co2+ + HSO5− → Co3+ + SO4•− + OH− (18)
Fe2+ + H2O2 → HO• + Fe3+ + OH− (19)
Co2+/PMS exhibited exceptional performance in removing 2,4-dichlorophenol and ATZ: higher than 90% removal was seen in 60 min at all pH ranges 3-8. While Fenton’s reagent only exhibited a higher degradation of 80% at a lower pH (3.0), the degradation decreased as the pH increased
13
and no removal was observed at neutral pH and higher (Anipsitakis & Dionysiou, 2003). However, one problem faced when working with cobalt is the elimination of excess cobalt from the aqueous system as it is carcinogenic in nature. Therefore, apart from Co2+, Fe2+ is another most commonly
used transition metal for PMS (Zhiyong et al., 2013) and PDS activation (Li et al., 2015). The overall reaction of Fe2+/PMS (Equation (20)) and Fe2+/PDS (Equation (21)) are given below: Fe2+ + HSO5− → Fe3+ + SO4•− + OH− (20)
S2O82− + Fe2+ → SO4•− + SO42− + Fe3+ (21)
Also, it has been observed that transition metal ions with a higher oxidation state produce peroxymonosulfate radicals (SO5•−) (Equations (22) and (23)) instead of SO4•− (Zhiyong et al.,
2013). Co3+ + HSO
5− → Co2+ + SO5•− + H+ (22)
Fe3+ + HSO5− → Fe2+ + SO5•− + H+ (23)
In addition to these two metals, Cu2+ has also been effectively utilized for activating PMS and degrading organic contaminants (Chi et al., 2019). The dosage of the transition metal used for catalysis gains more attention, cause when applied in excess, these metals themselves lead to self-scavenging of the SO4•− radicals (Wacławek et al., 2016). Hence, activation of peroxo-groups
should be slow to ensure long-term formation of radicals for the removal of contaminants. One of the most common ways of achieving this is by using chelated transition metals that significantly decrease the metal concentration available for activation (Zhang et al., 2016) or by using heterogeneous metals, which would be easier to separate.
2.3.2 Heterogeneous activation using transition metals
The mass transfer limitations in homogeneous catalysts are found to be insignificant and PMS reacts with the catalysts freely. Hence, this leads to a great difficulty in recovery of the homogeneous catalysts and further separation has found to be not technically and economically feasible (Pirkanniemi & Sillanpaa, 2002). Naim & Ghauch (2016) found a solution for reducing the self-scavenging reactions caused by metals ions by adopting the use of solid iron particles. These particles cause a smooth release of iron species, which are responsible for activation of PMS and overcome the problem of SO4•− radical quenching (any reaction in which the free radicals form
products that are not free radicals). Therefore, a solution to the above stated problems of separation and radical scavenging is using a catalyst that is in a different state, so that it can be readily separated and would be less harmful to the environment (Yang et al., 2015).
14
In heterogeneous catalysis, the surface of the catalyst plays a vital role because all the reactions take place at the surface, hence mesoporous catalysts are being utilized for the decomposition of PMS and PDS. Since Co2+ has been found to be a very good activator for PMS, the heterogeneous
catalysts also mostly consist of cobalt (especially for PMS activation). Among the five types of cobalt oxide that exists, CoO, Co2O3 and Co3O4 are mostly used for PMS activation, with Co3O4
exhibiting excellent PMS decomposition due to presence of both Co2+ and Co3+ in its molecular structure (Hu & Long, 2016). Nonetheless, a major issue faced when using Co3O4 for activating
the peroxo-group is an excessive quantity of cobalt leaching. In order to decrease leaching and to enhance the catalytic capacity, cobalt could be attached to several supports like other metal oxides (e.g. Al2O3, TiO2, SiO2, MgO), activated carbon or graphene (Yang et al., 2007). Yet another
solution to overcome cobalt leaching and contamination was suggested by Rhadfi et al. (2010), to partially replace the cobalt in Co3O4 catalyst with manganese (Mn), thereby decreasing the total
quantity of harmful Co metal. Although the application of catalysts in a different state aids with its easy recovery and reuse, the issue associated with using harmful metal-based catalytic systems still exists. Hence, to overcome the issue, the application of non-metal based catalytic systems is being investigated lately (Tao et al., 2015).
2.3.3 Heterogeneous activation using metal free catalysts
Recently, heterogeneous catalysts without any metal content are being used for activation due to several advantages like lack of secondary pollution, good environmental compatibility and good chemical stability (Tao et al., 2015). Wang et al. (2016) proved that functional groups of carbonaceous materials containing oxygen (especially carbonyl group) could efficiently contribute to the activation of persulfates. Zhang et al. (2013) used granular activated carbon (GAC) as a catalyst to activate PMS and further degrade AO7 in aqueous solution. It was found that the dye decolourization was almost 10 times faster in a GAC/PMS system than in individual GAC or PMS oxidation systems. In another study, Duan et al. (2015) tested the ability of various nanocarbons to activate PDS for the removal of phenolic compounds, dyes and other intermediates formed during the treatment. Among the various activation compounds tested, single wall carbon nanotubes, mesoporous carbon and reduced graphene oxide exhibited great catalytic performances when compared to fullerenes, nanodiamonds and graphite carbon nitride. Moreover, the carbo-catalysts showed much higher activity towards PDS than activated carbon and other metal oxides like Fe3O4,
Co3O4, MnO2, and CuO. Metal free heterogeneous catalysts exhibit positive results in activating
PMS and PDS but the studies performed so far have been concentrated on removing one specific contaminant. In real waters, however, many organic contaminants coexist simultaneously and
15
hence this information is necessary to verify if this technique could be efficiently used for the treatment of real waters.
2.3.4 Activation through energy (heat, ultrasound and ultraviolet light)
Employing energy for scission of the peroxide bond in PMS and PDS is a second possible method for activation (Zhang et al., 2015). The application of energy could be in the form of heat, ultraviolet or ultrasound (US) irradiation. The reaction equations of UV activation of the three oxidants are given below in Equations (24)-(26), and are also applicable in the case of heat and US activation. S2O82− ℎ𝑣 → 2 SO4•− (24) HSO5− ℎ𝑣 → SO4•− + HO• (25) H2O2 ℎ𝑣 → 2 HO• (26)
Yang et al. (2010) performed a detailed study on the activation of groups containing O−O bonds by UV and heat irradiation. It was observed that the decomposition efficacy of peroxides (general term for compounds containing O−O bond) activated by UV (254 nm) was PDS > H2O2 > PMS,
while the order of absorbance of these peroxides at 254 nm was PDS > H2O2 ≈ PMS. Although the
absorbance of PMS is more or less similar to H2O2, the difference in removal efficiencies could be
explained due to the structural differences in these molecules. As PMS is unsymmetrical, it is found to favour a heterolytic cleavage with a metal catalyser rather than the homolytic cleavage under UV irradiation like the other two symmetrical oxidants (PDS and H2O2) (Aparicio et al., 2003).
On the other hand, the order of pollutant removal efficiency for heat (80oC) activated peroxides was found to be PDS ≫ PMS > H2O2, which could be attributed to the significant difference in
O−O bond lengths (PMS-1.453 Å, H2O2-1.460 Å and PDS-1.497 Å) that corresponds to different
bond dissociation energies (Yang et al., 2010). The presence of (a) SO3 group(s) in both PMS and
PDS structure significantly decreases their bond dissociation energies thereby facilitating their radical production compared to H2O2 that lacks SO3 group. For instance, PDS exhibited a 99%
AO7 degradation in 40 min at 80oC, while PMS only showed 18% of AO7 removal after 3h at 80oC, and H2O2 did not show any significant removal at the same temperature.
US, especially in the range of 20-1000 kHz, is applied for activating persulfates. Cavitation is the principle based on which this activation occurs. The phenomenon occurs with the formation, nucleation and growth of bubbles in water. Finally, when the bubbles collapse they produce a high temperature (4700 oC) and high pressure (10 atm) which favours the production of free radicals in the solution (Riesz et al., 1985). Kurukutla et al. (2015) investigated the sonochemical degradation of rhodamine B using H2O2, PMS and PDS and found out that the removal efficiency of sonolysis
16
in rhodamine B degradation was 1.6 times and 3.6 times higher in case of PMS when compared to PDS and H2O2 systems, respectively.
Overall, the micropollutant removal efficiencies of persulfates activated using various forms of energies (US, UV and heat) do not follow a consistent order, since the activation using each form of energy relies on specific factors like oxidant structure, O-O bond length and bond dissociation energy.
2.3.5 Other activation methods
Lately, various novel techniques like the application of O3, electrochemical systems (Fe2+/Fe3+ ions)
and specific compounds in DOM (i.e. quinone) for the activation of peroxides (PMS and PDS) are gaining more interest and their efficiencies for removing micropollutants are being tested (Pang et al., 2016; Zou et al., 2013). For instance, Cong et al. (2015) investigated the simultaneous application of O3 and PMS for the elimination of pCBA in distilled water. The results confirmed
that the O3/PMS system was almost 50 times better at eliminating the pollutant when compared to
the stand-alone O3 system. A combined effect of both HO• and SO4•− radicals in elimination of the
pollutant and DOC was observed. In another study, Abu et al. (2014) examined the effects of O3
and O3/PDS on the biodegradable and soluble characteristics of a semi-aerobic stabilized solid
waste leachate. In the end, it was concluded that O3/PDS process is an efficient method to treat
stabilized leachate as it enhances the biodegradability and improves the soluble characteristics of the leachate. In yet another study performed by Govindan et al. (2014), it was proven that electrochemically (Fe2+/Fe3+ ions) activated persulfates could be used for degrading pentachlorophenol. A 75% degradation of pentachlorophenol was achieved in 60 min of reaction time at a solution pH of 4.5 when electrochemically activated PMS was used. Although the presence of NOM has been reported to impart an inhibitory effect on the SO4•− production due to
scavenging effects, some typical functional groups present in NOM have been found to activate PMS and PDS aiding SO4•− production as well. For example, quinone compounds like
p-benzoquinone have been found to accelerate the activation of PMS for the degradation of sulfamethoxazole (antibiotic), especially at a higher pH (pH ≥ 10) (Zhou et al., 2015). Similarly, Lou et al. (2014) demonstrated that PMS could be effectively activated by phosphate ions yielding both SO4•− and HO• at neutral pH, which could be used for micropollutant removal.
2.4 Interactions of SO4•−, PMS and PDS oxidants with the water matrix
It is clear from the values of kSO4•− that SO4•− exhibit less affinity towards various scavengers
present in the water compared to HO•. However, the scavenging of this alternative oxidant species still exists to a significant level and is considered as one of the main limiting factors in the
large-17
scale application of sulfate radical based AOPs. Therefore, it is necessary to understand how SO4•−
and its parent raw material (PMS/PDS) react with various organic and inorganic compounds/ions present in water undergoing non-target reactions. This knowledge would not only be useful for an in-depth understanding of these AOPs but will also be helpful in knowing the various harmful OBPs that could possibly be formed and ways to avoid or eliminate them.
2.4.1 Reactions of PMS with the water matrix 2.4.1.1 Organic Compounds
PMS spontaneously decomposes in water at a slightly alkaline pH yielding a singlet oxygen (1O2).
This singlet oxygen has been found to remove organic contaminants from water in few studies (Zhou et al., 2015). The efficiency of unactivated PMS was tested for the degradation of para-aminobenzoic acid (PABA) in EFF (collected from a WWTP in China) (Zhang et al., 2020). A 12% decrease in PABA degradation by unactivated PMS was observed when the organic matter added in the effluent was increased from 0 mg/L to 7.5 mg/L after a reaction time of 80 min, due to the competition for PMS between effluent organic matter and PABA. On the other hand, in a study conducted by Zhou et al. (2020), unactivated PMS was found to effectively remove tetracycline (TTC) antibiotics present in water. No significant reduction in elimination of TTC was seen even when a HA concentration of 10 mg C/L was added to the system, which in turn indicates that the competitive consumption of PMS by HA is weak. Furthermore, it was concluded from the results of sulfonamide removal studies from water using PMS, that unactivated PMS tends to be more selective compared to O3 and preferentially attacks compounds with electron-rich moieties in the
structures (Ji et al., 2018). Hence, only organic compounds that have aromatic rings, double bonds and amines would be selectively oxidized by unactivated PMS.
2.4.1.2 Inorganic Compounds
Spring et al. (2009) examined the influence of reducing agents including halide ions like Cl−, Br− and I− on PMS. The reactivity of PMS towards these halide ions was found to decrease in the order of I− (kPMS = 1410 M-1s-1) > Br− (kPMS = 0.7 M-1s-1) > Cl− (kPMS = 0.00206 M-1s-1). I3− was found
to be the main product formed when I− reacts with PMS at all pH, while Cl2 was the main product
in case of Cl− reaction with PMS. However, the reaction of PMS ion with Br− produced different products depending on the pH, Br2 was the major product in acidic medium, HOBr in neutral, and
BrO− ion in a slightly basic medium. The role of the in-situ formed HOBr towards the oxidation of tetrabromobisphenol A (TBBPA) using PMS was investigated by Wu et al. (2020). Ammonium (14 mg/L) was used as a scavenger of HOBr (k = 2.2 x 105 M−1s−1) to understand its role in TBBPA removal. A reduced removal of TBBPA (50 %) was seen with the addition of ammonium while a solution without any ammonium exhibited 80 % TBBPA removal at pH 7 after 2h. These results