Ghent University – Department of Plant Biotechnology and Bioinformatics VIB – Center for Plant Systems Biology
Research Group: Root Development
Dissection of CPK-based signalling in root
growth regulation
Anse Warmoes
Student number: 01508931Promoters: Prof. Dr. Tom Beeckman and Prof. Dr. Ir. Steffen Vanneste Scientific supervisor: Ren Wang
Master’s dissertation submitted to Ghent University to obtain the degree of Master of Science in Biochemistry and Biotechnology. Major Plant Biotechnology
Acknowledgements
Acknowledgements
First of all, I would like to thank my promotors of the University of Ghent, Prof. Tom Beeckman and Prof. Steffen Vanneste, for the continuous support, motivation and critical reading. Their guidance helped me during the research and writing of this thesis.
Furthermore, I would like to thank my supervisor Ren Wang for the useful comments, remarks and engagement through the learning process of this master thesis.
My sincere thanks also goes to the Root Development Group of Prof. Tom Beeckman (Ghent VIB), who welcomed me with open arms and helped me when needed. You are all wonderful and kind-hearted people.
I would also like to take this opportunity to express gratitude to all of the Department faculty members for the five years of education which shaped me to become the person I am today. Last but not the least, I would like to thank my family and friends for providing me with unfailing support and continuous encouragement throughout my years of study. This accomplishment would not have been possible without them. Thank you.
Table of contents
Table of contents
Acknowledgements ... i Table of contents ... ii List of abbreviations ... iv Preamble ... viiEnglish summary ... viii
Part 1: Introduction ... 1
1.1 Root development ... 1
1.1.1 The root apical meristem regulates root growth ... 1
1.1.2 Developmental zones of the root ... 3
1.2 Auxin is a regulator of root development ... 4
1.2.1 Auxin signalling and transport ... 4
1.2.2 Auxin-regulated root growth ... 4
1.2.3 Environmental cues alter root growth ... 6
1.3 Nitrate-regulated root growth ... 7
1.3.1 Nitrate signalling ... 7
1.4 Ca2+ signalling in plant development ... 8
1.4.1 Ca2+ signatures ... 9
1.4.2 CPKs as calcium sensors ... 9
1.4.3 Biological functions of CPKs in plant immunity ... 11
1.4.4 Biological functions of CPKs in stress signalling ... 11
1.4.5 Biological functions of CPKs in development ... 12
1.4.6 Biological functions of CPKs in nutrient sensing ... 13
Part 2: Aim of Research Project ... 14
Part 3: Results ... 15
3.1 Characterization of CPK30 (master 1 project) ... 15
3.2 Forward genetics approach: phenotypic suppressor screen ... 18
3.2.1 Suppressor screen (Master 1 project) ... 18
3.2.2 Phenotypic characterization of the 13 candidates ... 18
3.2.3 Nitrate response ... 20
3.2.4 Immunoscreening ... 21
3.2.5 Backcross ... 23
3.2.6 Summary of the forward genetics screen ... 24
3.3 Reverse genetics approach: promoter studies ... 25
3.3.1 Promoter reporters (proCPK::NLS-GFP-GUS) ... 25
3.3.2 Translational fusions (proCPK::CPKCDS-YFP) ... 31
Part 4: Discussion ... 35
Table of contents
4.2 Forward genetics screening suggests CPK30 acts through ethylene and auxin transport ... 36
4.3 Characterization of the expression domain and subcellular localization of CPKs ... 37
Part 5: Materials and Methods ... 40
Plant materials and growth conditions ... 40
EMS screening ... 40
DNA extraction ... 41
Immunostaining ... 41
Backcross ... 41
Statistical Analysis and visualization ... 41
Floral dip ... 41
GUS-staining ... 41
PI staining ... 42
Gibson cloning ... 42
Salt stress response ... 42
Microscopy and image analysis ... 42
References ... 43 Attachments ... a Figures ... a Tables ... s Protocols ... t
List of abbreviations
List of abbreviations
ABA abscisic acid
ACC 1-aminocyclopropane-1-carboxylic acid
AGL21 AGAMOUS-LIKE 21
ANR1 ARABIDOPSIS NITRATE REGULATED 1
ARF AUXIN RESPONSE FACTOR
AUX 1 AUXIN RESISTANT 1
BFA brefeldin A
BSA bovine serum albumin
CA constitutively active
Ca2+ calcium
[Ca2+]cyt cytosolic free calcium
CaM calmodulin
CaMIN medium without Ca2+
CaPLUS medium with Ca2+
CBL calcineurin B-like protein
CDS coding sequence
CEI cortex/endodermal initial cell
CEID cortex/endodermal initial daughter cell
cGMP cyclic GMP
CIPK CBL-interacting protein kinase
CLSM confocal laser scanning microscope
CNGC14 CYCLIC NUCLEOTIDE GATED CHANNEL 14
COP1 CONSTITUTIVE PHOTOMORPHOGENIC
C(D)PK calcium-dependent protein kinase
CRK CDPK-related kinase
EMS ethyl methanesulfonate
ER endoplasmic reticulum
ET ethylene
ETI effector-triggered immunity
GFP green fluorescent protein
GRAS GIBBERELLIN INSENSITIVE REPRESSOR OF GA1-3 SCR
List of abbreviations
IP3 inositol triphosphate
JA jasmonate
K+ potassium
LAX AUX1-LIKE
LR lateral root
LRC lateral root cap
LRP lateral root primordium
LUC luciferase
N nitrogen
NAA naphthalene-1-acetic acid
NB-LRR nucleotide-binding leucine-rich repeat
NH4+ ammonium
NLP NIN-like protein
NPA N-1-naphtylphtalamic acid
NRE nitrate responsive element
NRT1.1 NITRATE TRANSPORTER 1.1
PAMP pathogen-associated molecular pattern
PAT polar auxin transport
PBS phosphate buffered saline (pH 7.4)
PCD programmed cell death
PHR1 PHOSPHATE STARVATION RESPONSE1
PIN PIN-FORMED protein
PLT PLETHORA
PM plasmamembrane
PRR pattern-recognition receptor
PT pollen tube
PTI PAMP-triggered immunity
QC quiescent center
RAM root apical meristem
RBOHB respiratory burst oxidase homolog B
SA salicylic acid
SAM shoot apical meristem
List of abbreviations
SHR SHORT ROOT
TF transcription factor
TIR1/AFB TRANSPORT INHIBITOR RESPONSE1/AUXIN SIGNALLING F-BOX
TMK transmembrane kinase
WGS Whole Genome Sequencing
WT wild type (Col-0)
WUS Wuschel
Preamble
Preamble
In this research project we wished to explore the role of group III calcium-dependent protein kinases (C(D)PKs) in root growth and the potential link with auxin transport and nitrate signalling. Therefore, we tried to identify novel players in this pathway via a suppressor screen on an inducible CPK30-overexpression line, screening for restoration of normal root growth in this line. Detailed phenotypic characterization on diverse growth media would allow measuring the auxin response, ethylene response and nitrate response of the different EMS candidates. Immunolocalization showed that PIN internalization is strongly impaired upon induction of CA-CPK30 (constitutive active CPK30) expression. Therefore, we assessed whether the localization and BFA sensitive trafficking of PIN1 (PIN-FORMED 1) has been restored in the suppressor candidates, allowing us to determine the extent to which the phenotypic rescue is coupled to the effects on PIN trafficking. A backcross was made to
Rx>>CPK30#21 (RPS5A::XVE>>CPK30#21) to determine if the mutation is recessive or
dominant, and provide the starting materials for mapping via whole-genome sequencing of DNA from bulked back-crossed F2 segregants. Due to the measurements taken with regard to the corona crisis, we did not manage to map the causal mutations of the most interesting candidates. BC2 seeds could only be preliminarily analyzed. However, most phenotypic characterization and immunoscreening of the candidates was performed, allowing us to analyze these results. Moreover, we could also not perform the planned nitrate sensitivity assay of the suppressor mutants.
To identify molecular targets of CPK30, we planned to perform a phosphoproteomics analysis of the phosphorylation changes associated with induction of CA-CPK30. Seeds were bulked, and ready to perform the experiments that would allow outlining the optimal timing of induction, but due to time restrictions this was not possible.
The second part of the research project was focused on the characterization of the subclade III CPKs, allowing for a better understanding of the role of this subgroup in root growth and the potential link with auxin transport and nitrate signalling. Promoter reporters
(proCPK::NLS-GFP-GUS) and translational fusions (proCPK::CPKCDS-YFP) for the different target CPK genes
(CPK7, 8, 10, 13, 30 and 32) were generated and analyzed to study the expression domain and the subcellular localization of the respective CPKs. However, the time restrictions due to the corona crisis limited the screening of the promoter reporter and translational fusion lines to a first, simple assessment of expression domains and subcellular localization under control conditions. We still aimed to explore the response to altered nitrate availability, and possible other stimuli, as inferred from public expression databases. Using these data, we would have revisited the available mutants for specific phenotypes.
We were still in the process of cloning the CPK7 and CPK30 translational fusions with YFP using Gibson cloning, which could also not be finalized.
The material that was available from work by the student in the first semester and part of the second semester, was sufficient to draft the master dissertation, and did not need reorientation. Due to the corona crisis, certain follow-up experiments or repetitions could not be performed and therefore, an in-depth study of the results was not always possible.
This preamble was drawn up after consultation between the student and the supervisor and is approved by both.
English summary
English summary
Calcium (Ca2+) is a second messenger that mediates plant responses to a variety of developmental and environmental stimuli through variation of its intracellular concentration (so-called Ca2+ signatures). Upon recognition and decoding by specific Ca2+ sensors, such Ca2+ signatures result in the activation of physiological and biochemical responses. In a recent phenotypical screen in our lab, it was found that overexpression of constitutive active versions of a subfamily of calcium-dependent protein kinases (C(D)PKs) (CPK10-13-32) interferes with root growth and gravitropism in Arabidopsis. Via immunolocalization, it was found that the ectopic activity of these CPKs inhibits internalization of auxin-transporting PIN (PIN-FORMED) proteins, which could largely explain the observed root phenotypes. Interestingly, this subfamily of CPKs was recently implicated in a nitrate signalling cascade that controls root architecture. This raises the possibility that these CPKs represent a mechanism by which nitrate regulates auxin transport to steer root development for a more efficient mining of the soil. In this project, we will focus on dissecting the role of these CPKs in root growth regulation via forward and reverse genetics. On the one hand, we screened and characterized suppressors of the CPK overexpression phenotype. On the other hand, we generated and used new tools for functional characterization of this CPK subfamily, such as promoter reporter lines and fluorescent translational fusions. Together, this work will provide the basis for a better understanding of how CPKs could regulate root growth.
Introduction
Part 1: Introduction
1.1 Root development
Roots are plant organs that are essential for sensing and the uptake of water and nutrients. The root system typically lies below the surface of the soil, where it plays a role in plant-environment interactions. Various external stimuli, such as gravity, touch, biotic- and abiotic conditions can influence plant root architecture and morphology (Okada & Shimura, 1990; Malamy, 2005). The subterranean location of the root system, however, makes the study of its development challenging and extraction of roots from soil causes a loss of information. Furthermore, many crop root systems are highly complex and large in size, complicating characterization of basic developmental mechanisms (Petricka et al, 2012; Eshel & Beeckman, 2013).
The study of root development took major leaps through the use of the model organism
Arabidopsis thaliana. Roots of Arabidopsis are relatively thin (100 – 150 μm) and nearly
translucent, facilitating microscopic observation. Additionally, Arabidopsis roots can be easily grown in non-soil media, along the surface of agar plates, for detailed characterization of their growth and development. One of the fundamental problems faced by developmental biologists in multicellular organisms is that organ development occurs in four dimensions, being three spatial dimensions and time. Furthermore, the study of organogenesis can be complicated by the location in which it occurs. Plant organs, however, develop largely post-embryonically and continuously through populations of stem cells (meristems) of which two are formed during embryogenesis and will give rise to the organs of the shoot and root systems (Jürgens, 2001). The root apical meristem (RAM) contains a set of initial (stem) cells that form a single layer around the quiescent center (QC), a group of less mitotically active cells. Collectively these are called the stem cell niche (Spradling et al, 2001; Scheres, 2007). Through asymmetric cell division of the initial cells at the root tip, the stem cell population is maintained while daughter cells are produced, that will extend along the root’s longitudinal axis and form distinct tissue layers. Consequently, the further they are from the root tip, the more mature they become, making it possible to subdivide the longitudinal root axis in three developmental zones (meristematic, elongation and differentiation). Since a mature tissue type is restricted to a specific cellular lineage and cell file, each cell can be traced back to a single initial cell. However, it is the cell’s position rather than its lineage that determines cell identity (Kidner et al, 2000; van den Berg et al, 1995). Stem cells on the shootward and lateral sides of the QC produce epidermal, cortical and endodermal tissues forming concentric layers around a central stele (Scheres et al, 1994; Dolan et al, 1993). Whereas the root’s outer tissues exhibit radial symmetry, the cell types within the stele show a bilateral symmetric organization (Parizot et al, 2008). Together, this allows for the conceptual reduction of root development from four dimensions to two, with different cell types in the radial axis, and developmental time along the longitudinal axis (Petricka et al, 2012; Eshel & Beeckman, 2013).
1.1.1 The root apical meristem regulates root growth
In most plants, the first structure to emerge from the germinating seed is the radicle (primary root). Once this is formed, root growth relies on divisions in the root apical meristem (RAM). Unlike the shoot apical meristem (SAM), the RAM is a subapical structure that is covered at its apex by protective layers of cells, known as the root cap. When cells in the RAM divide, their daughter cells are displaced either apically (rootward) to contribute to the root cap or basally
Introduction
(shootward) to contribute to the body of the root (Ball & Clowes, 1962; Eshel & Beeckman, 2013).
The RAM is established during embryogenesis, providing new cells for the growing root. It contains a set of multipotent stem cells, known as initials or stem cells, surrounding centrally located organizing cells (QC). The QC functions both as a reservoir for cell replacement, for example when initial cells are lost due to wounding or aging, and maintains the stem cell initials in an undifferentiated state. The hypothesis is that the QC produces a short-range signal that affects cell fate and competence in a concentration-dependent manner. In this way, cells that are in close proximity to the QC receive the highest concentration of this signal causing them to be specified as initials (Scheres, 2007; Eshel & Beeckman, 2013; Hoermayer & Friml, 2019). This hypothesis was tested via ablation experiments in Arabidopsis roots. When all QC cells were ablated, the QC was rapidly restored by cells in the stele, in a region that correlated with the new auxin maximum (van den Berg et al, 1997; Xu et al, 2006). However, when only one of the QC cells was ablated, the restoration process was delayed. Columella initials that were in contact with the ablated QC differentiated as columella instead of dividing, whereas initial cells contacting the intact QC cells behaved normally (van den Berg
et al, 1997; Hoermayer & Friml, 2019). One of the candidates that might be the short-range
signal is WOX5, a member of the Wuschel (WUS) family of homeodomain transcription factors that is expressed in the QC and acts downstream of SHORTROOT and SCARECROW (see further). Loss of WOX5 function results in differentiation of the columella initial cells and an abnormal QC, suggesting that WOX5 works in a non-cell autonomous manner (Sarkar et al, 2007; Petricka et al, 2012). These data support the hypothesis of the QC as “organizer”, meaning that initial cells achieve their progenitor cell status in the RAM based upon their position relative to the QC and not based upon some inherent quality (Eshel & Beeckman, 2013).
Downstream of auxin in the specification of the QC are two parallel pathways: the PLETHORA (PLT) pathway and the SHORT ROOT (SHR)/SCARECROW (SCR) pathway. Loss-of-function alleles of these genes result in loss of QC identity and premature termination of root growth. The PLT genes (PLT1-4) encode AP2 class transcription factors with partially overlapping expression domains. Loss-of-function alleles of both PLT1 and PLT2 result in the loss of the QC, causing differentiation of the initial cells (Aida et al, 2004). Analysis of PLT1 and PLT2 expression in the presence of exogenously applied auxin showed that both genes act downstream of auxin, in turn a feedback mechanism is present in which PLTs upregulate PIN proteins affecting auxin activity. Remarkably, there is a significant delay in PLT expression after auxin application indicating that neither PLT1 nor PLT2 work directly downstream of auxin (Galinha et al, 2007; Eshel & Beeckman, 2013; Mähönen et al, 2014). SHR and SCR encode members of the GRAS (GIBBERELLIN INSENSITIVE, REPRESSOR OF GA1-3, SCR) family of transcription factors. During embryogenesis SCR is expressed in QC precursor cells, after which it extends to the initial cells for the ground tissue (cortex and endodermis) and the endodermis itself (Wysocka-Diller et al, 2000). SHR is expressed in the vascular cells and may move as a protein to the surrounding cell layer, including the QC, where it activates SCR transcription. In the QC SCR expression is required to maintain QC and stem cell identity in a cell-autonomous manner. However, the role of SHR in QC specification and stem cell maintenance is not confined to its function in SCR transcription in the QC region, but both SHR and SCR are required for QC identity (Sabatini et al, 2003). In conclusion, it is the overlap between the
Introduction
highest expression level of PLT1 and PLT2 and the SHR and SCR protein expression domain that defines the stem-cell niche at a cellular level (Scheres, 2007; Petricka et al, 2012).
1.1.2 Developmental zones of the root
As mentioned above, young growing Arabidopsis roots have a relatively simple radial organization of the epidermis and the ground tissue layers (endodermis and cortex), surrounding the bilateral symmetric vascular cylinder (Parizot et al, 2008; Motte et al, 2019). The root tip is surrounded by the root cap, a protective tissue, composed of both the columella and the lateral root cap (LRC), which makes up the exterior surface of the root together with the epidermis (Dolan et al, 1993). Aside from its radial structure, the root can also be subdivided in three longitudinal, developmental zones: the meristematic, elongation and differentiation zones (Motte et al, 2019).
When the stem cell initials in the RAM divide, their daughter cells go through three distinct developmental phases on their way to maturity, with the exception of root cap cells. After a few rounds of division, the cells exit the root meristem and enter the elongation zone, where cell expansion takes place. Finally, cells reach the differentiation zone where they adopt their respective developmental fates (Petricka et al, 2012; Eshel & Beeckman, 2013). These boundaries, however, do not really exist, as each cell file or tissue layer tends to act independently. Therefore, the transition of the meristem to the elongation zone is not the same in cells of the epidermis, for example, compared with cells of the cortex or the vasculature (Rost, 2011). A better way to define these boundaries is as transition points, which was originally described by Ivanov (1973). This concept suggests that within each cell file there are developmental switches, for example the point where cell division is turned off can determine the meristem/elongation transition point. Other such points would exist at the elongation/maturation transition and at the end of maturation. Consequently, each cell file or group of files may act in an independent way. Since the position of transition points is not fixed, the root may adapt its growth rate, specified by cell division and elongation, spatially to its cell differentiation. For example, protoxylem maturation occurs farther from the root tip in fast growing roots compared to slow growing roots (Reinhardt & Rost, 1995).
After the primary root has been established and the individual cell types have begun to differentiate, the root starts to form a branched system of lateral roots (LR) which occurs continuously during the life of a plant. LRs form in the differentiation zone where they are derived from the pericycle layer deep within the primary root tissues. Once stimulated, the pericycle cells positioned at the xylem poles (founder cells) dedifferentiate and proliferate to form a lateral root primordium (LRP). Thereafter, ordered periclinal and anticlinal cell divisions and developmental transitions of the founder cells result in a highly organized LRP. A total of seven developmental stages in LRPs can be distinguished to produce all of the cell layers present in a mature root. Following stage VII, the LRP increases in length primarily by cell expansion leading to the emergence of the LRP through the parent root epidermis. Similarly to embryonic root development, the LRP includes a group of cells that function as an apical meristem allowing continued growth of the organized lateral root (Malamy & Benfey, 1997; Petricka et al, 2012). Further establishment of the root system occurs through strictly acropetal LR initiation, with no de novo initiation events between already developed LRs or LRPs. However, once LR primordia are initiated in Arabidopsis, their pace of development can be variable even in two successively initiated primordia (Dubrovsky et al, 2006).
Introduction
1.2 Auxin is a regulator of root development
1.2.1 Auxin signalling and transport
The plant hormone auxin is one of the most important endogenous regulators of plant development, such as lateral root initiation and morphogenesis, inhibition of root elongation and regulation of gravitropic bending (Vanneste & Friml, 2009). In each of these examples, local accumulation is generally recognized as an important trigger of the event, involving a surprisingly short signalling pathway.
The best characterized auxin perception mechanism is defined by the F-box family of TRANSPORT INHIBITOR RESPONSE1/AUXIN SIGNALLING F-BOX (TIR1/AFB) proteins. High auxin levels stabilize its interaction with Auxin/INDOLE-3-ACETIC ACID (Aux/IAA), resulting in de-repression of AUXIN RESPONSE FACTORs (ARFs), that effect transcriptional responses, and ultimately developmental reprogramming (Weijers & Wagner, 2016; Zhou & Luo, 2018). Recently, TIR1/AFB auxin perception was also implicated in auxin-induced Ca2+ signalling via the Ca2+ permeable cation channel CYCLIC NUCLEOTIDE GATED CHANNEL14 (CNGC14) (Dindas
et al, 2018). Additional auxin perception mechanisms are also emerging, such as ARF3/ETTIN
(ETT), that directly binds auxin (Simonini et al, 2017), or TMKs (transmembrane kinases) that control Aux/IAA stability through auxin-dependent phosphorylation (Cao et al, 2019). Jointly, they make up a complex auxin perception network that is responsible for a highly specific, finely tuned cellular auxin response.
Given the pleiotropic effects of auxin, it is of fundamental importance that local auxin homeostasis is tightly controlled at the level of auxin transport, biosynthesis, metabolism, and signalling (Robert et al, 2010; Zažímalová et al, 2007). Generally, two distinct auxin transport pathways are distinguished: first, a fast, long distance, non-polar stream in the phloem from the main auxin source (shoot), and second, slow and directional cell-to-cell polar auxin transport (PAT) (Adamowski & Friml, 2015; Himschoot, 2018). PAT distributes auxin in a precise manner that is important for the local accumulation of auxin, mainly in developing tissues (Adamowski & Friml, 2015). To facilitate PAT, several auxin transporters were identified, such as the auxin influx proteins AUXIN RESISTANT1 (AUX1) and AUX1-LIKE (LAX), and the auxin efflux proteins PIN-FORMED (PIN) (Taylor-Teeples et al, 2016). PIN proteins are particularly important in auxin-regulated development as their polar localization instructs directional, intercellular auxin transport (Robert et al, 2010; Zažímalová et al, 2007). These polarly localized auxin transporters undergo dynamic subcellular trafficking, allowing to adjust cellular auxin direction according to endogenous signals, such as auxin (Geldner et al, 2003; Paciorek et al, 2005), and environmental cues, such as gravistimulation (Kleine-Vehn et al, 2010) and light perception (Ding et al, 2011). Together with local auxin biosynthesis, conjugation and degradation, auxin minima or maxima are created that are translated into appropriate developmental responses.
1.2.2 Auxin-regulated root growth
Root development is established by the joined action of multiple plant hormones. To do so, several phytohormones converge at the level of auxins, modulating its distribution, levels or signalling (Chaiwanon et al, 2016).
Already during embryogenesis, auxin is crucial for establishing the apical-basal axis determining the position around which the embryonic root will develop. The first asymmetric
Introduction
division of the zygote produces a smaller apical cell that will give rise to all cells of the proembryo, and a larger basal cell that will form the extra-embryonic suspensor. The apical cell of the suspensor is specified to become the hypophysis, which is correlated with an auxin maximum in the basal region of the embryo leading to a model in which auxin accumulation and transport establishes not only the hypophysis, but specifies the position of the future root in the developing embryo (Friml et al, 2002). Later in development, auxin mediates the organization of the root apical meristem, regeneration after damage, tropic root growth, lateral root development and root hair growth (Motte et al, 2019).
Auxin plays a key role in the zonation of the root meristem, exerting fast and slow effects on root meristem activity. On the one hand, the auxin distribution in the root meristem has a profound impact on the position of the PLT expression domain, and thus the PLT gradients that instruct root meristem zonation. On the other hand, auxin has fast effects on cell differentiation and elongation (Mähönen et al, 2014). These fast effects on elongation are particularly important for the root growth behavior in the soil. The root will follow the orientation of the gravity vector (gravitropism), whilst finding its way through pores in the soil (Friml et al, 2002; Rakusová et al, 2011). Additionally, the root will try to avoid adverse environments, such as salt (halotropism) and pathogens (Galvan-Ampudia et al, 2013; Kunkel & Harper, 2018). Each of these responses can be explained by the induction of differential elongation at two sides of the root, where high auxin levels inhibit elongation, and low auxin levels stimulate elongation (Went, 1974). Such asymmetric auxin accumulation patterns during tropistic root growth depend largely on the shootward auxin transport, which is established by the AUX1 auxin importer and the PIN2 auxin efflux carrier (Müller et al, 1998). In the case of gravitropic root growth, asymmetric auxin flux towards the elongation zone depends on the relocalization of PIN3 in the columella, and the coordinated stabilization of PIN2 on the lower root side, and degradation of PIN2 on the upper root side (Friml et al, 2002; Kleine-Vehn et al, 2010). In the case of halotropism, PIN2 degradation is triggered on the salt-exposed side, also resulting in asymmetric auxin transport activity, and thus differential auxin distribution for avoiding growth in the high salt environment (McLoughlin et al, 2013). Most examples of root growth regulation can be reduced to similar mechanisms. However, the underlying regulatory signals are not always well understood. An important effector of stress conditions is the plant hormone ethylene. Ethylene perception also leads to inhibition of root growth, which can be explained by excessive auxin accumulation inhibiting elongation in the epidermis (Růzicka et al, 2007). This inhibitory effect could be partially reversed in pin2 and
aux1 mutants, implying an important link between ethylene and auxin transport regulation
during root growth regulation (Van de Poel et al, 2015).
Outside the root meristem, auxin plays a key role in regulating root branching, by induction of lateral roots, which have their own meristem, and their own capacity to branch (Casimiro et
al, 2003; Dharmasiri et al, 2005). This allows for iterative expansion of the root system in the
soil. In Arabidopsis, auxin and its transport play a central role at all stages of lateral root development. Pulses of auxin in the pericycle, generated by programmed cell death in the lateral root cap, instruct the positioning of zones with high capacity to form a lateral root, so-called pre-branch sites (Moreno-Risueno et al, 2010; Xuan et al, 2016). Sustained auxin signalling converts a pre-branch site into a lateral root initiation site (Dubrovsky et al, 2008). Subsequent progression through the lateral root developmental program involves auxin transport during the organization of the incipient meristem, as well as communication with
Introduction
overlaying layers to accommodate its passage through the root tissues (Stoeckle et al, 2018). Modulation of auxin transport thus allows the integration of environmental stimuli in the root system development.
1.2.3 Environmental cues alter root growth
As plants are sessile organisms, they have acquired developmental mechanisms to adapt to environmental challenges, such as the search for sunlight and avoidance of shade, as well as adjustment to water and nutrient availability. In order to cope with these abiotic stresses and optimize the uptake of nutrients, the plant adjusts the spatial arrangement of its root system, also known as root system architecture (RSA) (Koevoets et al, 2016; Mroue et al, 2017). Light exposure of shoots and leaves has major effects on root growth. First of all, since plants are photoautotrophic organisms, they must achieve a balance between the photosynthetic carbon assimilation, carbon storage, and use of carbon energy for growth and development. So, when plants are grown in the dark or under shade, carbon resources are limited, inhibiting root growth in order to improve light exposure by promoting growth of stems and petioles. To regulate root growth, plants have sophisticated mechanisms in place that rely on the direct effect of carbohydrate availability to the root meristem, and the role of carbohydrates as signalling molecules triggering auxin signalling (Chaiwanon et al, 2016; Mroue et al, 2017). Additionally, light signalling also directly alters root growth, not indirectly through sugars. This process occurs through the master photomorphogenesis repressor COP1 (CONSTITUTIVE PHOTOMORPHOGENIC), whose high activity in darkness tunes shoot-to-root auxin transport and reduces auxin levels in the root, permitting rapid and precise adaptation of root growth to the light environment (Sassi et al, 2012; Chaiwanon et al, 2016).
Plasticity of the root system is also important to acquire nutrients in a generally heterogeneous soil. Certain nutrients, particularly mobile nutrients such as nitrate, are more abundant deeper in the soil, while immobile nutrients, for instance phosphate, are more found in the upper layers. It is known that deficiency of different nutrients induces changes in root architecture and morphology to efficiently mine the soil. These changes are, to some extent, nutrient specific (Giehl & von Wirén, 2014; Motte et al, 2019). In Arabidopsis, phosphate deficiency, for instance, inhibits primary root growth and promotes the growth of lateral roots and root hairs in order to increase topsoil exploration (Zhang et al, 2014). A strategy to increase phosphorus (P) acquisition is the secretion of organic acids that help release the anionic phosphate from bound cations (Balzergue et al, 2017). However, these acids also release iron that, when taken up in the roots, causes callose deposition at the plasmodesmata, which results in root growth inhibition (Müller et al, 2015). Furthermore, increased iron levels also result in ROS signalling in the elongation zone, inhibiting primary root growth (Balzergue et al, 2017). A central role in phosphate starvation signalling is reserved for the transcription factor PHOSPHATE STARVATION RESPONSE1 (PHR1) targeting a broad range of phosphate starvation-responsive genes, including auxin-signalling components (Bustos et al, 2010; Castrillo et al, 2017; Motte et al, 2019).
Similar to the effect of phosphate deficiency, lateral root initiation and elongation are stimulated in response to nitrogen supply, whereas primary root growth is inhibited. In the absence of nitrogen, root growth is probably suppressed through the activation of NITRATE TRANSPORTER 1.1 (NRT1.1), preventing auxin transport in lateral root tips and thus their
Introduction
further development (Krouk et al, 2010; Bouguyon et al, 2016; Chaiwanon et al, 2016). This will be discussed in more detail in section 1.3.
1.3 Nitrate-regulated root growth
Light, CO2, water and mineral elements are the basic requirements for plant growth and biomass production. Nitrogen (N) is essential as it is a constituent of amino acids, which are the building blocks of proteins, as well as chlorophyll, nucleotides and other metabolites. In plants, nitrate and ammonium are taken up by the roots and assimilated in the roots or the shoots. Assimilation of inorganic nitrogen is energetically costly, especially when N is present in the highly oxidized form of nitrate. When N is assimilated in roots, energy and carbon skeletons are provided by sucrose respiration, which must be imported from source leaves. Alternatively, nitrate and ammonium can be transported via the xylem to the shoot, where it is assimilated. Nitrate stimulates a regulatory machinery that coordinates N assimilation and transport with carbon metabolism, allowing the plant to respond to nutrient availability and environmental changes by controlling gene expression and enzyme activity (Nunes-Nesi et al, 2010).
1.3.1 Nitrate signalling
Nutrients such as nitrate also have an important role in LR development, since LRs preferentially proliferate in nutrient-rich patches of soil or media. During LR development in
Arabidopsis, different nitrogen-related regulatory mechanisms are apparent, which might be
explained by different nitrogen-monitoring mechanisms (Casimiro et al, 2003). When plants are grown on high-nitrate medium, LR inhibition occurs. Seedlings, however, have similar numbers of LR primordia to those on low-nitrate medium, yet they fail to elongate (Zhang et
al, 1999). A high sucrose-nitrogen (C:N) ratio also causes repression of LR development, but
this occurs at the level of initiation because of an obstruction in auxin movement from shoot to root (Malamy & Ryan, 2001). Lastly, when plants are grown on low nitrate, localized nitrate application stimulates LR development (Zhang & Forde, 1998). In this case, nitrate itself is the stimulus for LR formation, since the effect is independent of nitrate metabolism and only occurs in LRs that are in direct contact with nitrate (Zhang et al, 1999).
The inhibitory effect of high nitrate is mostly mediated by the plant hormone abscisic acid (ABA) (Signora et al, 2001). Both mutants in ABA biosynthesis and ABA-insensitive mutants,
abi4 and abi5, show much less LR growth inhibition in response to high nitrate. Furthermore,
exogenous ABA mimics the effect of high nitrate. The levels at which ABA acts to inhibit LR growth in high nitrate are at least ten times lower than what is observed in the inhibition of seedling germination. Moreover, mutants known to be insensitive regarding seed germination are ABA sensitive in respect of high-nitrate LR-growth inhibition, indicating a different mechanism by which ABA acts. Morphological analysis shows that ABA-inhibited LRs have the same cellular pattern as a pre-activated LR primordium, indicating that ABA inhibition specifically occurs before activation of the meristem (De Smet et al, 2003; Casimiro et al, 2003). Research of Celenza et al (1995) also suggested that ABA-induced LR inhibition might be mediated in an auxin-independent way, since phenotypic rescue could not occur by exogenous auxin application or elevation auxin synthesis.
As mentioned above, localized nitrate application stimulates LR development. A MADS-box transcription factor, ARABIDOPSIS NITRATE REGULATED 1 (ANR1), was shown to be upregulated by a localized N supply and is involved in LR development in response to local N
Introduction
availability (Zhang & Forde, 1998). Another MADS-box transcription factor, AGAMOUS-like 21 (AGL21), also plays an important role in regulating LR development in response to various signals. AGL21 overexpression plants produced more and longer lateral roots, while agl21 mutants developed fewer and shorter lateral roots, especially under nitrogen-deficient conditions. The latter phenotype could be rescued by exogenous auxin application. Furthermore, AGL21 positively regulates auxin accumulation and cell division activities in LRPs and LRs, indicating AGL21 may regulate LR formation and growth in response to N-changes through auxin signalling (Yu et al, 2014). It has also been noted that low N leads to enhanced shoot-to-root auxin transport and increased local auxin biosynthesis via transcriptional repression of TAA1 and TAR1 in shoots, and upregulation of TAA1 and TAR2 in roots, respectively (Ma et al, 2014).
Interestingly, other studies indicated that high nitrate stimulates LR growth via the NRT1.1-dependent signalling pathway (Krouk et al, 2010). The plasma membrane nitrate transporter NRT1.1 (CHL1) was initially characterized as an influx carrier in the roots mediating the uptake of nitrate from the soil (Tsay et al, 1993), however it can also function as a nitrate sensor (Wang et al, 2009; Krouk et al, 2010). NRT1.1 was found to regulate root branching because it facilitates shootward auxin transport in the LR tips in response to low N, thereby preventing auxin accumulation and thus lateral root growth (Krouk et al, 2010; Mounier et al, 2014; Mroue et al, 2017). Downstream factors in nitrate signalling have been identified by studying the binding of transcription factors to nitrate responsive elements (NREs) in the promotor sequences of primary NO3- response genes. Arabidopsis NLP6 and NLP7 were suggested as important transcription factors (Castaings et al, 2009; Konishi & Yanagisawa, 2013; Marchive
et al, 2013; Konishi & Yanagisawa, 2014). However, many questions remain unanswered about
primary nitrate signalling.
1.4 Ca
2+signalling in plant development
Plants are sessile organisms that evolved different mechanisms to adapt to environmental fluctuations and developmental cues. Signalling pathways regulating this are composed of protein elements, including receptors, enzymes and transcription factors, and non-protein elements, referring to second messengers. A second messenger that is known to be involved in signal transduction networks mediating plant development and responses to (a)biotic stress is calcium (Ca2+) (Shi et al, 2018). In plants, the free Ca2+ concentration is maintained at nanomolar levels in the cytosol in order to avoid toxicity, whereas the organelles and the cell wall can accumulate concentrations up to several millimolar (Harper et al, 1994). Apart from its function as a second messenger, calcium is an essential macronutrient element in plants having structural roles in the cell wall and membranes, helping in the formation of microtubules and functioning as a counter-cation in the vacuole (Burstrom, 1968; Tuteja & Mahajan, 2007). Ca2+ is taken up from the soil via the roots and transported to the shoot through the xylem. Since the concentration of free Ca2+ is low in the cytosol, because of its high affinity for Ca2+ binding proteins, Ca2+ moves predominantly apoplastically through the root rather than symplastically (Bush, 1995). This active transport from the cytosol to the apoplast, against the electrochemical gradient, requires transport proteins. The key proteins catalyzing this movement are Ca2+-ATPases and H+/Ca2+ antiporters (Hirschi, 2001).
The Ca2+ ion is an important signalling molecule that contributes to many different signalling pathways occurring both at the single-cell level and the multicellular level. The former including stomatal closure and opening (Ng et al, 2001) and pollen tube and root hair growth
Introduction
(Hepler et al, 2001). The latter compromising abiotic stress responses (McAinsh & Pittman, 2009) and response to mechanical stimuli (Braam & Braam, 2004). To cope with the environmental stress, plant cells reprogram their cells by triggering a network of signalling events that start with the perception of the stress signal by membrane receptors. This leads to the generation of second messenger signalling, causing a cascade reaction of phosphorylation/dephosphorylation events that may target transcription factors controlling multiple stress responsive genes. The product of these reactions ultimately leads to plant adaptation and stress tolerance both directly and indirectly (Mahajan & Tuteja, 2005).
Overall, the stress response requires a coordinated action of many genes and signalling molecules. In plants, many secondary messengers have been reported to work in signalling pathways, including Ca2+, IP3 (inositol triphosphate) and cyclic GMP (cGMP). However, no second messenger is as widespread as cytosolic Ca2+ (Mahajan & Tuteja, 2005; Mahajan et al, 2006; Tuteja & Mahajan, 2007). An additional level of regulation and specificity in the stimulus response is achieved by Ca2+ binding proteins that act as Ca2+ signal sensors. These Ca2+ binding proteins decode and relay the information encoded by Ca2+ signals by targeting diverse proteins, including cellular transporters, enzymatic and signalling proteins and transcription factors (Dodd et al, 2010; Kudla et al, 2010).
1.4.1 Ca2+ signatures
The cytosolic Ca2+ in plant cells increases in response to various environmental challenges like abiotic and biotic stresses and developmental cues (Tuteja & Mahajan, 2007). These stimulus-induced changes in cytosolic free Ca2+ ([Ca2+]cyt), also known as Ca2+ signatures, encode the specific signal information and in this way define the nature and magnitude of the response. These specific Ca2+ signatures are defined by precise control of spatiotemporal and concentration parameters of [Ca2+]cyt, including amplitude, frequency, oscillation duration and subcellular distribution (Dodd et al, 2010; Kudla et al, 2010).
An increase in [Ca2+]cyt is established by Ca2+ influx to the cytosol either from the apoplast, across the PM or from the intracellular organelles such as vacuoles, endoplasmic reticulum (ER) and mitochondria. The Ca2+ influx is mediated by ion channels whose subcellular localization, type and number is critical for the targeting of different cellular processes. Since movement of Ca2+ within the cytoplasm is low, the opening of Ca2+ channels produces a local elevation of [Ca2+]cyt that is restored to basal levels within minutes. Moreover, a coordinated cellular responses is effected by successive recruitment of certain Ca2+ channels, producing so-called calcium “waves” in the cytoplasm (Trewavas, 1999; Tuteja & Mahajan, 2007).
1.4.2 CPKs as calcium sensors
In plants multiple classes of Ca2+ sensors can be found that provide specificity in the signalling pathway: the eukaryotic, highly conserved calmodulins (CaM), the calcineurin B-like (CBL) proteins that mostly regulate the class of CBL-interacting protein kinases (CIPKs) and the calcium-dependent protein kinases (C(D)PKs) and their relatives CDPK-related kinases (CRKs) (Yip Delormel & Boudsocq, 2019). CaM is highly conserved in all eukaryotes, whereas CBLs and CPKs are only identified in plants and some protists (Shi et al, 2018; Reddy & Reddy, 2004; Day
et al, 2002). Among these families, CPKs are the best characterized, having both
serine/threonine protein kinase, auto-inhibitory junction and calmodulin-like domains in a single polypeptide. As a result, CPKs directly bind calcium, leading to kinase activity that is independent of calmodulins (Cheng et al, 2002; Roberts & Harmon, 1992). The
calmodulin-Introduction
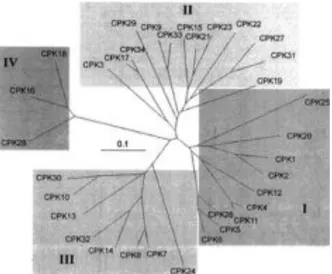
like domain consists of two lobes, displaying low and high calcium affinity respectively, that each compromise two EF-hand motifs. A pseudo-substrate mechanism keeps the kinase in an inactive state through an intramolecular interaction between the junction region and the catalytic center. The binding of Ca2+ to the low-affinity-N-terminal lobe of the calmodulin-like domain induces a conformational change that releases the auto-inhibition (Boudsocq & Sheen, 2013; Harper et al, 2004; Harper & Harmon, 2005). The Arabidopsis genome encodes for 34 CPKs divided into four subfamilies (Figure 1) (Yip Delormel & Boudsocq, 2019; Hrabak
et al, 2003). Within each subgroup the kinases show similar Ca2+ sensitivities. Between groups,
however, strong differences in Ca2+ sensitivity can be observed. For example, subgroup III CPKs seem not to be activated by Ca2+ binding alone, which could be explained by an altered sequence in the first EF-hand of the C-terminal CaM-like regulatory domain (CaMLD). These variable calcium sensitivities were measured in vitro, however the in vivo regulation by calcium has barely been investigated. It was suggested that there might be additional regulatory processes that activate CPKs or that Ca2+ binding regulates other aspects besides activity, such as protein-protein interactions. Furthermore, Ca2+ sensitivity can be influenced by the substrate, which may be related to the variable N-terminal domain of CPKs (Yip Delormel & Boudsocq, 2019; Boudsocq et al, 2012).
CPKs have a widespread distribution in plants and while some have a ubiquitous expression in most tissues, others are expressed in specific tissues (Monaghan
et al, 2014; Hong et al, 1996; Ye et al,
2009). Their subcellular localization is highly variable, including the cytosol, nucleus, plasma membrane (PM), ER, tonoplast, mitochondria, chloroplasts, oil bodies and peroxisomes. Widespread distribution of CPKs in plants supports the hypothesis that they might play significant roles in numerous signal transduction pathways (Simeunovic et al, 2016; Boudsocq & Sheen, 2013; Rudd &
Franklin-tong, 2001). Membrane
targeting of 28 CPKs is established in vitro by myristoylation, a co-translational modification, on the N-terminal peptide (Saito et al, 2018) and confirmed in vivo by MS analysis for 13 CPKs (Majeran et al, 2018). Reversible palmitoylation, counteracted by phosphorylation, controls membrane association and allows in this way the shuttling of proteins between membranes and the cytoplasm (Chehab et al, 2004). This has been observed for AtCPK10/30/32 as it translocates to the nucleus in response to nitrate (Liu et al, 2017). Mutations inhibiting myristoylation or palmitoylation were reported to alter membrane localization and suppress CPK functions (Stael et al, 2011; Monaghan et al, 2014; Chen et al, 2018). For example, AtCPK6 is known to mediate stomatal closure induced by for instance ABA, via activation of SLAC1. Non-myristoylatable (G2A) CPK6 was not able to complement the cpk3 cpk6 double mutant phenotype, which displayed impaired ABA-induced stomatal
Figure 1: Relatedness of Arabidopsis CPKs. The Arabidopsis genome encodes 34 CPKS divided into four
subfamilies (I-IV). The branch lengths are proportional to divergence, with the scale of “0.1” representing 10% change (Cheng et al, 2002).
Introduction
closure resulting from impaired SLAC1 activation at the PM (Saito et al, 2018; Yip Delormel & Boudsocq, 2019).
After stimulus-specific Ca2+ signatures are sensed by CPKs, the signals are decoded and induce various responses in plants via different targets and pathways. Despite the progress that has been made in this field, the molecular, cellular and genetic links between Ca2+ signatures and the downstream events are largely unknown. Major research has been done to uncover the role of CPKs in triggering downstream responses in plant immunity and stress signalling (Boudsocq et al, 2012).
1.4.3 Biological functions of CPKs in plant immunity
Plants sense potential pathogens through the recognition of pathogen-associated molecular patterns (PAMPs) such as the bacterial peptides of flagellin, flg22, and elongation factor elf18 or fungal chitin. Recognition can occur both on the cell surface by pattern-recognition receptors (PRRs), known as PAMP-triggered immunity (PTI), or intracellularly via effector proteins that are bound by nucleotide-binding leucine-rich repeat (NB-LRR) immune sensors, known as effector-triggered immunity (ETI). Both pathways cause the activation of overlapping and distinct signalling cascades that involve CPKs, including protein kinase activation, Ca2+ influx, hormone biosynthesis, oxidative burst, transcriptional reprogramming and programmed cell death (PCD) (Boudsocq & Sheen, 2013; Yip Delormel & Boudsocq, 2019). AtCPK4/5/6/11 from subgroup I were identified as early transcriptional regulators in PAMP signalling using a flg22-responsive reporter NHL10-LUC (NDR1/HIN1-like10-luciferase) in a cell-based functional genomic screen with 25 constitutively active AtCPKs (Boudsocq et al, 2010). Other studies confirmed their function as positive regulators of defense responses downstream of different PAMPs, including elf18, PEP3 and oligogalacturonides. Furthermore,
cpk5,6 double mutant and cpk5,6,11 triple mutant showed hypersensitivity to Pseudomonas syringae DC3000 and Botrytis cynerea (Boudsocq et al, 2010; Dubiella et al, 2013; Ma et al,
2013; Gravino et al, 2015). A common way in which PTI is established by CPKs from subgroup I is through phosphorylation of the NADPH oxidase RBOHB (respiratory burst oxidase homolog B) causing an oxidative burst. Transcription factors regulated by those CPKs in PTI remain largely unknown (Dubiella et al, 2013; Gao & He, 2013; Kadota et al, 2014). CPKs from subgroup I also mediate the plant innate immune response by transcriptional reprogramming, both transient and sustained, and regulating salicylic acid (SA), ethylene (ET) and jasmonate (JA) signalling. Overexpression of AtCPK1 or AtCPK5 triggers SA accumulation by activating SA induction-deficient2/isochorismate synthase1 (SID2/ICS1), an SA-biosynthesis gene, leading to broad-spectrum pathogen resistance (Coca & San Segundo, 2010; Boudsocq & Sheen, 2013; Dubiella et al, 2013; Yip Delormel & Boudsocq, 2019).
1.4.4 Biological functions of CPKs in stress signalling
As sessile organisms, plants undergo continuous exposure to biotic and abiotic environmental stresses. As a coping mechanism plants have evolved phytohormones such as salicylic acid (SA), jasmonic acid (JA), ethylene (ET) and abscisic acid (ABA), to protect the plant against both biotic and abiotic stresses via a so-called signalling crosstalk. The first three hormones, SA, JA and ET, are mainly involved in biotic stress signalling upon pathogen infection. ABA, however, is involved in responses to abiotic stresses such as drought, low temperature and osmotic stress. It also plays a role in developmental processes, including seed development, dormancy, germination and stomatal movement (Walley et al, 2007; Fujita et al, 2006). Due to climate
Introduction
change these stresses will increase in the near future. Therefore, understanding abiotic stress responses has been and still is one of the most important topics in plant science. Many abiotic stress-inducible genes have been isolated so far and their functions characterized (Hirayama & Shinozaki, 2010).
In the last couple of years research identified several common transcriptional responses to stress (Fujita et al, 2006; Walley et al, 2007; Hirayama & Shinozaki, 2010) which has led to the idea that plants have a universal stress response (Walley & Dehesh, 2010; Ma & Bohnert, 2007). However, the transcriptional responses of whole organs consisting of multiple cell types often obscure the more complex and subtle changes that occur at cell type-level (Birnbaum et al, 2003; Brady et al, 2007; Gifford et al, 2008; Dinneny et al, 2008). Each cell type in the root has its own transcriptional profile (Brady et al, 2007) and Dinneny et al (2008) showed that a cell’s identity plays an important role in the response to environmental stress. This study, however, did not allow a full analysis of the root’s response to stress, since it only examined two stimuli (salt stress and iron deprivation) limiting the ability to identify gene expression patterns within cell types across many stresses. By combining different datasets of transcriptional stress profiles (Kilian et al, 2007; Dinneny et al, 2008) Iyer-Pascuzzi et al (2011) identified common stress responses in the whole root. Evidence for a universal stress response at either whole root or cell-type resolution could not be found. They showed that although different stresses influence root spatiotemporal transcriptional programs in a unique way, in specific cell types there is a set of genes enriched regardless of environmental stress. This suggests that cell-cell communication plays a major role in the root’s response to stress (Iyer-Pascuzzi et al, 2011).
Arabidopsis CPKs have been reported to function mainly in drought and salt stress signalling.
Plants respond primarily to abiotic stress by closing their stomata, mediated by the drought-inducible phytohormone ABA to limit water loss. Stomatal closure results from activation of anion and potassium channels which allows ion efflux and cell shrinkage. Twenty CPKs have been detected in guard cells with quite variable levels regulating S-type and K+ channels, but also mediating a large transcriptional reprogramming. CPKs may also function in stress responses by regulating ABA signalling via modulation of factors upstream of ABA-responsive element (ABRE)-binding factors. Compared to drought and salinity, much less is known about the involvement of CPKs in temperature stress signalling (Yip Delormel & Boudsocq, 2019). Despite the increasing knowledge on the functions of CPKs in stress signalling and plant immune responses, not much is known about their role in plant development.
1.4.5 Biological functions of CPKs in development
Several CPKs are known to be involved in different aspects of plant development such as shoot and root development, pollen tube and root hair growth and metabolism.
A pollen tube (PT) is a specialized plant cell that grows exclusively at the tip, which involves ion (H+, K+, Cl-) and water fluxes regulated by a calcium gradient. These dynamic ion fluxes and gradients are molecularly controlled by eight out of 13 CPKs that are expressed in pollen. The double mutant cpk17cpk34 displayed a normal morphology, however pollen tube growth and pollen transmission efficiency were reduced compared to wild-type plants. The downstream targets need to be further identified (Myers et al, 2009). Similar results were found for the
cpk2cpk6cpk20 triple mutant, exhibiting impaired slow-type and rapid-type anionic currents.
Introduction
while the cytosolic Ca2+ homeostasis is correlated with the anion concentration gradient at the tip. CPK2/6/17/20/34 were shown to localize to the PM of the PT tip, however only CPK2/6/20 interact specifically with SLAH3, a pollen tube anion channel. CPK2/6 also stimulate the R-type anionic channel ALMT12, thus promoting pollen tube growth by activating NO3 -and malate efflux through SLAH3, ALMT12 -and potentially related channels ALMT13/14 (Geiger et al, 2011; Gutermuth et al, 2013, 2018). Another pathway in PT growth that was identified, is the CPK11-CPK24-SPIK pathway. Path-clamp analysis showed that elevated cytoplasmic Ca2+ levels inhibit the K+ influx of PT protoplasts. CPK11 is able to bind and phosphorylate CPK24 in vivo and together inhibit inward K+ channel SPIK/AKT6, thereby inhibiting pollen tube growth (Zhao et al, 2013; Shi et al, 2018; Yip Delormel & Boudsocq, 2019).
1.4.6 Biological functions of CPKs in nutrient sensing
Mineral nutrients are necessary for plants but sufficient uptake of these elements from the soil is often challenging. AtCPKs, however, are not often reported to have a correlation with nutrient uptake (Chiou et al, 2017). A recently discovered pathway in nitrogen sensing is the nitrate-CPK-NLP (NIN-like protein) signalling pathway, identified by Liu et al (2017) in
Arabidopsis. They employed a single-cell system of mesophyll protoplasts to investigate live
Ca2+ signalling stimulated by nitrate. Using an ultrasensitive Ca2+ biosensor GCaMP6 a specific and dynamic Ca2+ signature was observed in the nucleus and cytosol. To search for candidates of intracellular Ca2+ sensors, constitutively active AtCPKs (CPKac) were co-expressed with a luciferase (LUC) reporter gene NIR-LUC that exhibits a physiological nitrate response in transgenic Arabidopsis plants. When 0.5 mM KNO3 was added to the incubation medium, subgroup III constitutively active CPKs (CPK7ac, CPK8ac, CPK10ac, CPK13ac, CPK30ac and CPK32ac) were detected with strong LUC activation, suggesting that nitrate triggers unique Ca2+-CPK signalling. However, single mutants of these CPKs lacked overt growth phenotypes and affected nitrate-responsive gene expression. To overcome embryo lethality of the
cpk10cpk30 double mutant, the authors used a chemical-sensitive variant of AtCPK10 to be
reversibly inhibited by 3MBiP (1-isopropyl-3-(3-methylbenzyl)-1H-pyrazolo[3,4-d]pyrimidine). This allowed them to analyze the cpk10cpk30cpk32 triple mutant which showed reduced expression of nitrate marker genes and retarded plant growth. Interestingly, the Root Development group of professor Beeckman (Ghent University, VIB Center for Plant Systems Biology, Belgium) managed to generate a viable cpk10cpk30 double mutant which does not show embryo lethality (Ren Wang, Steffen Vanneste, Tom Beeckman, unpublished results). The nitrate-CPK-NLP regulatory network was discovered using an in vivo assay that showed nitrate-triggered NLP7 phosphorylation causing transcriptional reprogramming in the nucleus. These results were confirmed using mass spectrometry, bimolecular fluorescence complementation (BiFC) assay, etc. (Liu et al, 2017; Shi et al, 2018; Yip Delormel & Boudsocq, 2019).
Aim of Research Project
Part 2: Aim of Research Project
Roots show a tremendous developmental plasticity, allowing to make optimal use of available water and nutrients and stay clear of adverse environments, such as biotic and abiotic stress. Nitrate is the primary nitrogen source for most plants and the limiting factor for growth in aerobic soil (Nunes-Nesi et al, 2010; Konishi & Yanagisawa, 2014; Kiba & Krapp, 2016; Liu et
al, 2017). In order to respond and adapt to fluctuating environments, more specifically in
terms of nitrogen availability, it is important to proliferate the root system locally, close to a patch of high nitrate soil, and thus economically mine the soil (Yamazaki et al, 2019). Currently, not much is known of how this physiological response is regulated. Riveras et al (2015) reported that nitrate induces a rapid increase in cytoplasmic Ca2+ (calcium) levels in roots that required NRT1.1 (NITRATE TRANSPORTER 1.1). This indicates a role for Ca2+ as a second messenger downstream of nitrate perception (Armijo & Gutiérrez, 2017). Furthermore, Liu et
al (2017) found that Ca2+ sensing CPKs (calcium-dependent protein kinases) function as master
regulators that orchestrate nitrate-activated signalling. Nitrate-coupled CPK signalling phosphorylates transcription factors that coordinate the primary nitrate response. One of these nitrate-responsive transcription factors, NIN-LIKE PROTEIN7 (NLP7), was shown to be phosphorylated by CPK10, indicating a link between Ca2+, CPKs and NLPs. This signalling cascade was essential for lateral root primordium initiation and emergence, as well as lateral root elongation. Nearly all aspects of lateral root development are regulated by auxin. Therefore, one can expect that this nitrate signalling cascade converges on auxin homeostasis and/or signalling. Indeed, the nitrate transceptor NRT1.1 has been suggested to transport auxin (Krouk et al, 2010).
The second messenger Ca2+ is required for normal transport of the plant hormone auxin (dela Fuente & Leopold, 1973). However, not much is known about the role of Ca2+ in regulating root development. Asymmetric distribution of auxin, which is important for root development and gravitropism, is caused by local auxin biosynthesis and directional intercellular auxin transport facilitated by PIN auxin efflux carriers (Zažímalová et al, 2007; Robert et al, 2010). The Root Development group of professor Beeckman (Ghent University, VIB Center for Plant Systems Biology, Belgium) found that the auxin-induced Ca2+ signature is required for inhibition of PIN endocytosis by auxin. The link between auxin-induced Ca2+ and auxin-inhibited PIN endocytosis has not yet been resolved. The group, however, identified a role for subclade III CPKs in root growth and gravitropism, via a gain-of-function approach. Using immunolocalization, subclade III CPKs were found to be able to suppress PIN internalization, as overexpression of constitutively active Ca2+ signalling by CPK13 or CPK30 was sufficient to prevent PIN endocytosis. Therefore, it was postulated that the defects in nitrate-regulated lateral root growth and root architecture of the cpk10 cpk30 cpk32 triple mutant (Liu et al, 2017) could be explained by defective endocytosis of PINs causing an associated impaired auxin distribution.
This project aims at understanding how subclade III CPK activity regulates auxin-regulated root growth. We will try to unravel the downstream molecular targets via a forward genetics screen for suppressors of the short, agravitropic root phenotype of lines overexpressing constitutive active CPK30, as well as systematically characterizing the expression domain and subcellular localization of the CPKs of this subgroup. Given that this subclade of CPKs was recently shown to be involved in nitrate sensing, we will benchmark our findings in the context of nitrate signalling and control of root architecture (Liu et al, 2017).
Results
Part 3: Results
A reverse genetics screen in the Root Development group of professor Beeckman (Ghent University) identified a role for subclade III calcium-dependent protein kinases (CPKs) in root growth and gravitropism. Here, we set out to better characterize the mechanism by which these CPKs have an impact on auxin-regulated root growth. Moreover, given the previously proposed involvement of subclade III CPKs in nitrate sensing we will explore if and how this effect on auxin transport reflects a thus far unknown aspect of nitrate signalling (Liu et al, 2017). As a tool we used an overexpression line of a constitutive active (CA) version of CPK30 that is estradiol-inducible (RPS5A::XVE>>CPK30; Rx>>CPK30) and causes a very strong inhibition of root elongation and gravitropism.
3.1 Characterization of CPK30 (master 1 project)
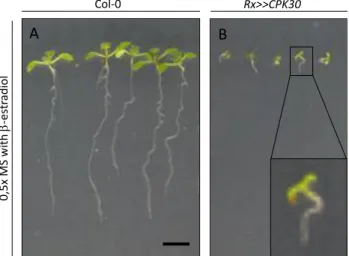
The effect of constitutive active Ca2+ signalling by CPK30 on the macroscopic phenotype, being root growth, was studied using wild type Arabidopsis plants (WT; Col-0) and an estradiol-inducible overexpression line of a constitutive active variant of CPK30 (RPS5A::XVE>>CPK30; Rx>>CPK30).
Seeds were grown for 10 days on 0.5x MS medium with β-estradiol (5 µM). Wild type seedlings had long, gravitropic roots that formed lateral roots. In contrast, Rx>>CPK30 seedlings had very short, agravitropic roots that did not have emerged lateral roots (Figure 2), reminiscent of mutants defective in auxin signalling and/or transport.
To test this hypothesis, we analyzed the dynamics of PIN (PIN-FORMED) trafficking in induced Rx>>CPK30 lines. Seedlings were germinated on control medium and transferred to medium supplemented with 5 µM β-estradiol for 2 days. To visualize PIN internalization rates, we applied a short-term treatment with the fungal toxin Brefeldin A (BFA, 25 µM) that blocks recycling and causes aggregation of the corresponding endosomes in so-called BFA bodies (Geldner et al, 2001). As a control for the internalization inhibitor treatment we used the synthetic auxin NAA (naphthalene-1-acetic acid; 10 µM) (Paciorek et
al, 2005). Two-day estradiol treatment gave robust inhibition of PIN internalization in the Rx>>CPK30 samples as indicated by the absence of BFA bodies in comparison to WT (Figure 3
A, B). A similar inhibitory effect on PIN internalization was observed in samples that were co-treated with BFA and NAA (Figure 3 C).
Previously, it was found in the lab that extracellular Ca2+ availability was necessary for the inhibitory effect of NAA on PIN internalization (Himschoot E., Wang R., Beeckman T. and Vanneste S., unpublished results). Therefore, we evaluated if the Rx>>CPK30 effect on PIN
Figure 2: Qualitative assessment of the phenotype caused by overexpression of constitutive active CPK30. 10-day-old
seedlings germinated on 0.5x MS medium containing β-estradiol (5 µM). (A) Wild type seedlings have a long gravitropic root. (B) The Rx>>CPK30 line has a short, agravitropic root. Scalebar = 5 mm.
A Col-0 B 0,5 x M S w ith b -e st rad io l Rx>>CPK30 A B