DHA metabolism-related gene expression
pattern of Brine shrimp nauplii used as live
feed of marine shrimp
XINLIN ZHAO
Student number: 01801500
Promotor: Prof. dr. ir. Peter Bossier
Tutor: Tânia Margarida Lourenço
Master’s Dissertation submitted to Ghent University in partial fulfilment of the requirements for the degree of Master of Science in Aquaculture
COPYRIGHT
The author and the promoter give the permission to use this master dissertation for consultation and to copy parts of it for personal use. Every other use is subject to the copyright laws, more specifically the source must be extensively when using from this thesis. De auteur en de promotor geven de toelating deze masterproef voor consultatie beschikbaar te stellen en delen van de masterproef te kopiëren voor persoonlijk gebruik. Elk ander gebruik valt onder de beperkingen van het auteursrecht, in het bijzonder met betrekking tot de verplichting de bron uitdrukkelijk te vermelden bij het aanhalen van resultaten uit deze masterproef.
Ghent University, August 2020.
___________________________ Prof. dr. ir. Peter Bossier
Promoter
___________________________ Tânia Margarida Lourenço
Supervisor
___________________________ Xinlin Zhao
Page | II
ACKNOWLEDGEMNT
With the completion of this thesis, I am one step closer to achieving my master’s degree. I wish to express my sincerest gratitude to many individuals who have supported me.
I would like to express my gratefulness to my supervisor, Tânia Margarida Lourenço De Abreu, for her enthusiastic support, untiring guidance, valuable advices, constant encouragement and endless inspiration whenever I needed.
My deepest gratitude goes to my promoter, Prof. dr. ir. Peter Bossier, for his advice of great value, support and encouragement throughout my thesis work.
My special thanks are also expressed to all my professors and mentors, my colleagues from Master of Science in Aquaculture program 2018-2020, as well as staffs of the Laboratory of Aquaculture & Artemia reference Center.
Last but not least, I would like to express my appreciation to my families, for their dedicated assistant and support.
LIST OF FIGURES
Figure 1 – The relative contribution of aquaculture and capture fisheries to fish for human
consumption. ... 9
Figure 2 - Life cycle of Artemia franciscana. ... 11
Figure 3 – Globally distribution of Artemia species. ... 12
Figure 4 - The principles of bioencapsulation. ... 16
Figure 5 - Incorporation of DHA into the membrane. ... 17
Figure 6 - The synthetic pathways of GPLs‐containing DHA in mammalian cells. ... 18
Figure 7 - The roles of DHA‐containing GPLs in membranes. ... 19
Figure 8 – LC-PUFAs across the mitochondrial membranes. ... 22
Figure 9 - Carnitine-dependent transfer of fatty acyl groups across the inner mitochondrial membrane. ... 22
Figure 10 – Mitochondrial β-oxidation of linoleoyl-CoA ... 23
Figure 11 – HSFs up-regulate the expression of HSPs. ... 26
Figure 12 - HSP70 protects A. franciscana nauplii against V. campbelli. ... 29
Figure 13 - Expression of rainbow trout (Oncorhynchus mykiss) HSP70 mRNA in leukocytes supplemented with PUFAs. ... 47
Page | IV
LIST OF ABBREVIATIONS
ALA Alpha-linolenic acid CoA Coenzyme A
CPT Palmitoyltransferase I DHA Docosahexaenoic acid DHA- Non-enriched
DHA+ DHA enriched
EPA Eicosapentaenoic acid GPLs Glycerophospholipids
GRP78 Glucose-regulated protein 78 GSL Great Salt Lake
HSF Heat shock factors HSP70- Non-heat shocked
HSP70+ Heat shocked
HSPs Heat shock proteins
LC-PUFAs Long-chain polyunsaturated fatty acids NLHS Non-lethal heat shocked
PA Phosphatidic acid
PE Phosphatidylethanolamine PKC Protein kinase C
PLA2 Phospholipase A2
PUFAs Polyunsaturated fatty acids SFB San Francisco Bay
TABLE OF CONTENTS
COPYRIGHT ... I ACKNOWLEDGEMNT ... II LIST OF FIGURES ... III LIST OF ABBREVIATIONS ... IV TABLE OF CONTENTS ... V PREAMBULE ... 1 Abstract ... 2 1. Introduction ... 3 2. Literature review ... 8
2.1. The growing role of aquaculture in feeding the world ... 8
2.1.1. Fish consumption ... 8
2.1.2. Aquaculture production ... 8
2.1.3. Larviculture ... 9
2.2. Artemia: an instant live feed ... 10
2.2.1. Importance of Artemia in aquaculture ... 10
2.2.2. Life history of Artemia ... 10
2.2.3. Geographic distribution of Artemia ... 11
2.2.4. Global introduction of A. franciscana ... 13
2.3. The important role of LC-PUFAs in aquaculture ... 13
2.3.1. Introduction to LC-PUFAs ... 13
2.3.2. Marine fish larvae LC-PUFAs requirements ... 14
2.3.3. Supplement of LC-PUFAs ... 14
2.3.3.1. Fish oil, fish meal and microalgae ... 14
2.3.3.2. Artemia bio-encapsulation ... 15
Page | VI
2.4.2. Functions of membrane DHA ... 18
2.4.2.1. Membrane shape and fluidity ... 18
2.4.2.2. DHA altering membrane domains ... 19
2.4.2.3. Effect of DHA on cell signaling ... 20
2.5. LC-PUFAs degradation ... 20
2.5.1. LC-PUFAs across the cell membrane ... 20
2.5.2. Fatty acids oxidation in mitochondria ... 21
2.5.2.1. Mitochondria uptake of fatty acids ... 21
2.5.2.2. β-Oxidation of LC-PUFAs in mitochondria ... 22
2.5.3. LC-PUFAs oxidation in peroxisomes ... 23
2.5.4. Regulation of LC-PUFAs metabolism ... 24
2.6. Introduction to HSPs ... 25 2.6.1. General aspect of HSPs ... 25 2.6.2. Molecular chaperones ... 27 2.6.3. Moonlight proteins ... 27 2.6.4. HSP70 ... 27 2.6.4.1. HSP70 family ... 27 2.6.4.2. Membrane HSP70 ... 28 2.6.4.3. Artemia HSP70 ... 28
2.7. Membrane fatty acids composition regulation by heat stress and HSP70 ... 29
2.7.1. Membrane role in modulating cell’s response to stress ... 29
2.7.2. Phospholipid-based signaling pathways activated by heat stress ... 30
2.7.2.1. Heat stress relation to PA accumulation in plant cells ... 30
2.7.2.2. Activation of PKC ... 31
2.7.3. HSP 70 interaction with membrane lipids ... 31
2.7.3.1. Recruitment of HSP70 by membrane phospholipids ... 31
2.7.3.2. Integrity of lysosomal membrane in relation to HSP70 ... 32
2.7.4. Lipid-active enzymes regulation by HSP70 ... 32
2.7.4.1. Activation of phospholipase A2 by HSP70 ... 32
2.7.4.2. Stabilizing factors for PKC ... 33
3. Material and methods ... 34
3.2. Fatty acid composition analysis ... 35
3.3. Protein extraction and HSP70 detection ... 35
4. Discussion ... 37
4.1. DHA degradation during enrichment and starvation ... 37
4.2. Fatty acids contents of Artemia GSL and VC nauplii ... 38
4.3. Functions of heat stress and HSP70 on DHA degradation ... 39
4.3.1. Hypothesis 1: HSP70 enhance DHA degradation ... 39
4.3.1.1. HSP70 role in mitochondria ... 39
4.3.1.2. Role of PPARα in DHA oxidation ... 40
4.3.1.3. HSP70 activation of phospholipases ... 41
4.3.1.4. Regulation of fatty acid degradation by GRP70 ... 41
4.3.2. Hypothesis 2: Heat shock and HSP70 enhance DHA accumulation cells ... 42
4.3.2.1. Increase in membrane PUFAs in response to elevated temperatures ... 42
4.3.2.2. HSP70 promotes lipogenesis ... 43
4.3.2.3. Inhibition of import proteins into peroxisomes ... 43
4.3.3 Hypothesis 3: No function of HSP70 on DHA degradation ... 44
5. Conclusion ... 46
6. Further recommendations ... 47
7. Reference ... 49
Page | 1
PREAMBULE
This thesis research work was planned to investigate the effects of heat stress and heat shock protein 70 (HSP70) on DHA degradation of two geographically different strains of
Artemia –Great Salt Lake (USA) and Vinh Chau (Vietnam). During this period, I was supposed to conduct an experimental work with 4 different treatments (Non-Lethal-Heat-Shocked and Enriched; Non-Lethal-Heat-(Non-Lethal-Heat-Shocked and Non-Enriched; Non-Heat-(Non-Lethal-Heat-Shocked and Enriched; Non-Heat-Shocked and Non-Enriched), each in triplicate to verify the expression of HSP70 and consequently the associated expressed genes. The process to obtain those results was to pool hatch the Artemia nauplii of each strain, heat-shock and enrich the respective treatments and collect samples on the established sampling time points (hatching, after heat-shocked, after empty the gut, and 3, 6, 12 and 24h after empty the gut) for fatty acids composition analysis. To verify the expression of HSP70, a western blot is conducted.
However, due to the pandemic COVID-19, the Laboratory of Aquaculture and Artemia Reference Centre was closed since March and I have not been allowed to enter and conduct my practical research work. Therefore, to improve the value of my work, an extended literature review and proposed methodology were proposed by my promoter, for instance, although my research should focus mainly on the DHA degradation pathway, one chapter regarding the DHA functions in membranes and cell signaling was also incorporated in my work.
I conclude this thesis work based on the recent relevant literature following ideas and results from similar research applied to other species. Accordingly, it allowed me to propose a new methodology and different hypotheses from the initial one, which I consider important to achieve the aim of this work.
Abstract
Artemia nauplii are used all over the world’s hatcheries as live preys of marine fish and crustaceans although their poor LC-PUFAs profile. Bio-encapsulation with particulate or emulsified long-chain polyunsaturated fatty acids (LC-PUFAs) products, especially eicosapentaenoic acid (EPA; 20:5, n-3) and docosahexaenoic acid (DHA; 22:6, n-3), have been used as a solution to tailor their nutritional value and suit the marine fish and crustacean larval high LC-PUFAs requirements. Even though the bio-encapsulated methods have been applied for decades, understanding fatty acids assimilation and degradation process remains very limited. Activation of heat shock protein 70 (HSP70) is an evolved protection mechanism response to heat stress; interestingly, the moonlighting activities of this group of molecular chaperones might relate to manning membrane lipids-proteins properties and fatty acids metabolism. Therefore, a controlled experiment where two groups
Artemia nauplii were exposed to different levels of temperature (28°C or 37°C) and after subdivided and either exposed to a DHA enriched emulsion or starved was planned to investigate the effects of heat stress and HSP70 on LC-PUFAs metabolism, particularly DHA metabolism.
In this review, we discussed heat-induced HSP70 modulation of the Artemia DHA metabolism state and the involvement of HSP70 in mitochondrial β-oxidation and peroxisome profiler activated receptor α (PPARα) activity, such evens indicate an elevated DHA degradation capacity. However, heat stress also leads to changes in dynamic membrane lipids-protein properties resulting in increase in unsaturation degrees; lipogenesis can also be brought about by interaction with HSP70. Such observtions will explore different roles of heat stress and HSP70 concerning DHA metabolism, provide a new perspective for identifying the genes involved in fatty acids metabolism.
KEYWORDS:
Page | 3
1. Introduction
Seafood is widely appreciated as tasty and excellent nutrition, providing essential amino acids and high-quality proteins in an easily accessible form (Hosomi et al. 2018; FAO, 2018). Moreover, there is the additional health benefit omega-3 fatty acids, essential vitamins and minerals in many fish species (Dyerberg et al. 1978). EPA and DHA have been reported contribute to the neural fetal development, cardiovascular function, and clinical treatment for Alzheimer's disease (Swanson et al. 2012). Alpha-linolenic acid (ALA; 18:3, n-3) is a plant-derived shorter chain omega-3 fatty acid found in flaxseed, soybeans, nuts, hemp oil, and canola oils (Lee et al. 2009; Blasbalg et al. 2011; Swanson et al. 2012; Reboredo-Rodríguez et al. 2017; Calder, 2016). However, dietary plant oils rich in ALA do not provide the health benefits seen with EPA and DHA (Swanson et al. 2012). Though the elongase and desaturase in our body is able to synthesis EPA and DHA from ALA, the capacity is deficient in meeting the high LC-PUFAs requirements (Neff et al. 2011). Several studies suggested that < 10% of ALA converted to EPA, and < 1% to DHA in men (Hussein
et al. 2005; Goyens et al. 2005; Chiu et al. 2008). Therefore, it is necessary to obtain adequate amounts of DHA and EPA through fish and fish-oil products. On the supply side, aquaculture has already provided a substantial portion of fish, crustaceans and mollusks for human consumption (FAO, 2018). With the wild-catch fishery fished at or beyond capacity, the yields of stagnant ocean fishery will remain relatively stable, and aquaculture will continually bridge the gap between sustainable fisheries and global demand (FAO, 2018).
The brine shrimp Artemia (Crustacea, Anostraca) is a non-selective filter feeder inhabits hypersaline environments where predators and food competitors can hardly survive (i.e. inland salt lakes, salty coastal lagoons, and artificial solar saltworks) (Triantaphyllidis et al.
1995; Gajardo 2010; Kaiser et al. 2006; Gajardo & Beardmore, 2012). Artemia acquired different adaptive mechanisms to survive environmental stress and response to varying abiotic conditions (Persoone, 1980). For instance, the most studied different reproductive
patterns with females releasing either free-swimming nauplii in favorable conditions or dormant cysts under adverse situations (MacRae, 2005). Artemia cysts can be stored and marketed worldwide, and newly hatched nauplii from cysts can be used directly as nutritious live feed (Van Stappen, 1996). For this reason, Artemia has been extensively used in the larval culture of marine fish and crustaceans. Commercial Artemia cysts initially originated from the coastal saltworks in the San Francisco Bay (SFB), California, USA and an inland biotope, the Great Salt Lake (GSL) in Utah, USA (Lavens & Sorgeloos, 2000). New natural (i.e., China, Argentina, Australia,) and man managed (i.e., Vietnam, Thailand) Artemia
production sites were explored and established globally (Lavens et al. 2000). Moreover, the intentional introduction of the Artemia franciscana (Kellogg, 1906) from SFB and GSL worldwide has led to the establishment of permeant populations of A. franciscana along coastal regions of Asia and the Mediterranean region (Zheng & Ma, 2004; Amat et al. 2007; Van Stappen et al. 2007). Now interspecific genetic differentiationhas been reported among introduced Asian A. franciscana and American A. franciscana populations (Kappas et al.
2004; Eimanifa, 2014). Moreover, the composition and metabolization of fatty acids in
Artemia seemed to differ widely from strain to strain (Van Stappen, 1996). For instance, fatty acid compositions results suggested that VC and GSL Artemia are essentially different in fatty acids composition, especially in terms of EPA and linolenic acid contents (El-Magsodi
et al. 2014). DHA has long been thought to be absent in Artemia, though the results indicated its presence in Artemia VC and a higher content of EPA than Artemia GSL, the inherent deficiency in essential fatty acids is still a considerable limitation.
LC-PUFAs (carbon chain lengths ≥ 20, double bonds ≥ 3) are also essential fatty acids for fish and crustacean larvae. As discussed above, LC-PUFAs exert physiological functions as one of the prominent components of cellular membranes (Tocher et al. 2019); they have also been reported to improve the growth, survival, behavior, and development of marine fish larvae (Shields et al. 1999). However, the capacities of endogenous biosynthesis of LC-PUFAs by some marine fish species are insufficient to meet physiological demands, especially the high LC-PUFAs requirements of rapidly growing and developing larvae
Page | 5
(Sargent et al. 1999; Castro et al. 2016). Therefore, enrichment protocols using unicellular algae (Howell, 1979), emulsion (Leger et al. 1986; McEvoy et al. 1996), liposomes (Ozkizilick & Chu, 1994), and encapsulated diets (Southgate et al. 1995) were developed to meet the lipid requirements. Classically, Artemia nauplii are bio-encapsulated with DHA or EPA enriched particulates or emulsions to alter the nutritional quality in meeting the larval requirements (Sorgeloos et al. 1991). Further applications include the transfer of bio-encapsulated pigments (Conceição et al. 2010), probiotics (Arndt & Wagner, 2007; Seenivasan et al. 2012), medication like anti-biotics (Venkat et al. 2004; Vázquez-Silva et al. 2017), ascorbic acid (Immanuel et al. 2004; Akbary et al. 2011) and spawning hormone (Burton, 1998) to targeted animals.
Instead of being simply accumulated in the gut, enriched LC-PUFAs are digested, assimilated into Artemia nauplii. β-oxidation is the vital metabolic pathway by which fatty acid is degraded and stored energy is released; the β-oxidation rate responds to animal nutrition and hormonal status (Wakil & Abu-Elheiga, 2009). LC-PUFAs degradation is a complex balance between the degradation and biosynthesis of fatty acids; while the availability of free fatty acid substrate, activation of relevant enzymes and competition between them, uptake and transportation of fatty acids among different oxidation sites seemed to be major influences of fatty acids metabolism (Leaver et al. 2008; Schulz, 2013; lee et al. 2016; Lankinen et al. 2018). Each process independently controls the LC-PUFAs metabolism while also in conjugation with other processes. The first step of LC-PUFAs β-oxidation occurs in peroxisomes, where they might be converted to chain-shortened acyl-CoA esters in the peroxisomal matrix (Schulz, 2013). Peroxisomal β-oxidation systems contain the enzymatic activities required for the metabolism of double bonds in LC-PUFAs; in particular, peroxisomal β-oxidation is involved in remove of the Δ4 double bond in DHA, and degrade it to EPA (Calder, 2016). β-oxidation occurs in the mitochondrial matrix that fully oxidizes LC-PUFAs to carbon dioxide and water. A particular transport mechanism carnitine-dependent process is required to transport the LC-PUFAs fatty acyl-CoA across mitochondrial membranes (Schulz, 2013; Calder, 2016). Fatty acyl-CoA is reformed in
mitochondrial matrix catalyzed by carnitine palmitoyltransferase II; subsequently acetyl-CoA undergoes citric acid (Krebs) cycle and chain shortened metabolites are produced during β-oxidation, while the FADH2 and NADH pass their electrons to the mitochondrial electron transport chain (Calder, 2016).
Heat shock proteins (HSPs) are a group of highly conserved proteins in prokaryotic and eukaryotic organisms that constitutively expressed under normal physiological conditions and response to environmental and physiological stressors, such as high temperature, osmotic stress, ultraviolet and gamma radiation, inflammation, hypoxia, glucose, microbial infection and cellular toxins (Mathew & Morimoto, 1998; Arieli et al. 2003; Collins & Clegg, 2004; Todgham et al. 2005; Baruah et al. 2010). Functionally, HSPs act as molecular chaperones, performing essential biological functions under both normal and stressful conditions, assistance the folding of nascent proteins, translocation of these proteins across membranes, the assembly/disassembly of multi-subunit complexes, and refolding or degradation of proteins (Kosmaoglou et al. 2008; Baruah et al. 2010; Roberts et al. 2010; Saibil, 2013). HSPs are classified into different families based on their molecular masses, including small heat shock proteins (18–40 kDa), HSP60, HSP70, HSP90 and HSP1001 family (Whitley et al. 1999). The HSP70 family is the most extensively studied group of HSPs that involved in the “cross-protection or cross-tolerance to confer thermal resistance” (Frankenberg et al. 2000; Sejerkilde et al. 2003; Baruah et al. 2010). For instance, HSP70 has been reported protect against osmotic stress (DuBeau et al. 1998; Todgham et al.
2005), prevent oxidative toxicity and damage (Arieli et al. 2003; Todgham et al. 2005), and improve desiccation tolerance (Ma, 2005). Thus far, A. franciscana HSP70 has been identified (accession number: AF427596) (Junprung et al. 2019); evidence from previous studies suggested that Artemia HSP70 plays a vital role in host immune response to bacteria and virus infections with improved tolerance against Vibrio campbellii and Vibrio proteolyticus (Yik Sung et al. 2007, Baruah et al. 2010).
Despite the widespread use of DHA enriched products in Artemia bio-encapsulation, little is known about the enrichment process in terms of the mechanism of lipid assimilation in
Page | 7
Artemia. Rather than being simply accumulated in the gut, filtered DHA is digested and metabolized. High variability in final fatty acids composition may be affected by the differential intrinsic metabolism and a multitude of physiology. Several studies have established the diverse molecular chaperone activity of HSP70; however, knowledge of the associated effects of heat stress and induced HSP70 on Artemia LC-PUFAs metabolism remains very limited. Moreover, comparative literature information on the genetic differences between VC and GSL Artemia is scarce. Filling these information gaps will enable the development of larval culture as well as fisheries conservation programs. It will also facilitate identifying the genes involved in fatty acid degradation to extend the enrichment window. Thus, this study was conducted to determine the effects of heat stress and heat induced HSP70 on the DHA degradation of Artemia nauplii designed with 4 controlled treatments (Non-Heat Shocked and Non-Enriched treatment, Non-Heat Shocked and Enriched treatment, Non-lethal Heat Shocked and Non-Enriched treatment, Non-Lethal Heat Shocked and Enriched treatment). Expressly, this study was set up to verify the hypothesis that heat stress induced HSP70 would affect the DHA degradation in Artemia nauplii, either enhancing the oxidation of DHA or accumulation of DHA in cells. Moreover, it is hypothesized that the Artemia VC and GSL possess different fatty acids compositions, expression of HSP70 and its activities in DHA degradation.
2. Literature review
2.1.The growing role of aquaculture in feeding the world
2.1.1.Fish consumption
The increasing global population has enhanced the world's capacity to consume a variety of nutritious food (Charles et al. 2014); recent findings on consumer trends also suggest an ever-increasing focus on foods' health-related properties (Annunziata & Mariani, 2019). For those reasons, aquaculture has been rapidly developing to meet the demands of high-value protein and health benefits products. Moreover, the decline in marine reservations, and concerns about food sources, biosecurity (use of antibiotics) and sustainability, have fostered the aquaculture industry (FAO, 2018). As an affordable food component, the fish dietary contribution has been proven not only as an energy source but also crucial in high-quality digestible animal proteins, and a valuable source of micronutrients such as vitamins and minerals (Tidwell & Allan, 2001; Mohanty et al. 2014). For instance, fish contains lysine and methionine are essential amino acids for human health. Moreover, many fish are important sources of omega-3 LC-PUFAs, contributing to human visual, fetal and cognitive development (Muldoon et al. 2014). The high nutritional value and health promoting benefit of fish products reinforcing the importance of fish as an essential food group (Shao et al.
2017). In per capita terms, fish consumption increased from 9.0 kg in 1961 to 20.2 kg in 2015, an average increased rate of about 1.5% per year (FAO, 2018). The significantly increase in consumption has driven aquaculture development; it is desirable to increase fish production with high nutritional products.
2.1.2.Aquaculture production
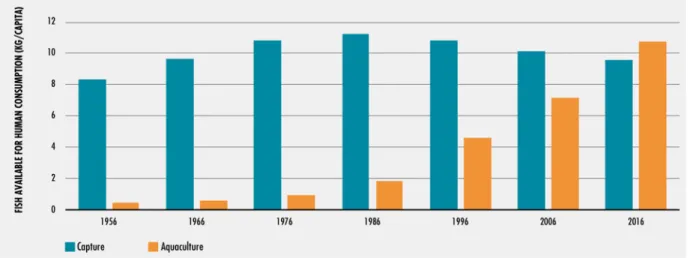
Until the 1980s, fish supply intended for human consumption was heavily dependent on wild captures, the aquaculture industry rapidly developed in the 1990s (Garcia & Grainger, 2005; FAO, 2018) (Figure 1). With the stagnation of wild captures, aquaculture production continuously increased, being one of the fastest-growing food production sectors in the main food production industries (Bondad-Reantaso et al. 2012). World fish production reached around 171 million tonnes in 2016, with aquaculture accounting for 47% of the total volume,
Page | 9
which has provided more fish for human consumption than wild captures since 2014 (FAO, 2018).
By 2030, aquaculture is expected to provide 60% of the fish for human consumption (Mimako et al. 2015). The continued growth of aquaculture will remain dependent upon the improvement of larval culture, and the development of sustainable feeds with alternative ingredients to meet the LC-PUFAs requirements (Conceição et al. 2010; Tocher et al. 2019).
2.1.3. Larviculture
Larval culture has been one of the main bottlenecks preventing the complete commercialization of many fish and shellfish farming (Dhont, 2013). Claude (2002) emphasized that survival through the larval stage is very tenuous and is generally considered the most critical period affecting fish recruitment. Given the high growth rate of marine fish larvae, the qualitative and quantitative satisfaction of larval nutritional requirements is essential for efficient production. Therefore, finding prey of suitable size and nutritional characteristics is one of the main problems in larval farming (Rao, 2003).
Figure 1 – The relative contribution of aquaculture and capture fisheries to fish for human consumption.The share of aquaculture products in total food fish consumption was 6% in 1966, 14% in 1986 and 41% in 2006, 51% in 2015, 53% in 2016 (Adapted from FAO, 2018).
2.2.Artemia: an instant live feed
2.2.1.Importance of Artemia in aquaculture
Artemia has been extensively used in marine fish and crustacean larval culture, which has become an essential breakthrough in aquaculture development (Ritar et al. 2004). This planktonic micro-crustacean constitutes a well-established live feed for the early stages of many aquaculture species, and thus it has become one of the most widely used live prey for fish and shellfish larval culture (Sorgeloos et al. 1991; Dhont, 2013). Although Artemia has been known to man for centuries, its use as live feed took place only in the 1930s when the nutritional potential for newly hatched larvae was discovered (Van Stappen, 1996). One of the features that making Artemia such a suitable live feed for marine and freshwater larvae is that it is possible to improve its natural nutritional profile throughout enrichment techniques (Akbary et al. 2011). The non-selective filter-feeding allows Artemia to transfer bio-encapsulated LC-PUFAs (Han et al. 2000) pigments (Conceição et al. 2010), probiotics (Arndt & Wagner, 2007; Seenivasan, 2012), medication like anti-biotics (Venkat et al. 2004; Vázquez-Silva et al. 2017), ascorbic acid (Immanuel et al. 2004; Akbary et al. 2011), spawning hormone (Burton, 1998) and other feed supplements like EPA and DHA to target animals.
2.2.2.Life history of Artemia
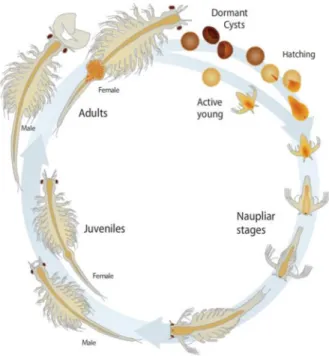
The brine shrimp, Artemia belongs to the family Artemiidae (Kaiser et al. 2006; Huys, 2009; Gajardo & Beardmore, 2012; Ogello et al. 2014). As an extreme halophile organism, they occur in hypersaline environments such as inland salt lakes and salty coastal lagoons (Gajardo 2010; Kaiser et al. 2006; Gajardo & Beardmore, 2012). According to Lavens & Sorgeloos (2000), Under optimal environmental conditions, females produce eggs to develop directly into nauplii. Contrary to favorable environmental conditions (low temperature, insufficient oxygen), females produce dormant cysts instead, which remain in a stage of arrested metabolism and can be viable for many years (Figure 2). Cysts are
Page | 11
crucial for survival; commercially dried cysts can be rehydrated and incubated in seawater whenever needed (Freeman, 2003).
Additionally, Artemia presents bisexual and parthenogenetic populations that are similar morphologically and biometrically (Triantaphyllidis et al. 2010; Agh et al. 2010; Muñoz & Pacios, 2010). Artemia populations are divided into New World populations – bisexual – (Artemia franciscana and Artemia persimilis) and Old World populations – parthenogenetic – (Artemia salina (Linnaeus, 1758), Aetemia sinica (Cai, 1989), Artemia urmiana (Günther, 1899), Artemia tibetiana (Abatzopoulos et al. 1988) and Artemia sp (Pilla & Beardmore, 1994; Triantaphyllidis, 1997; Baxevanis, 2006; Maccari, 2013).
2.2.3. Geographic distribution of Artemia
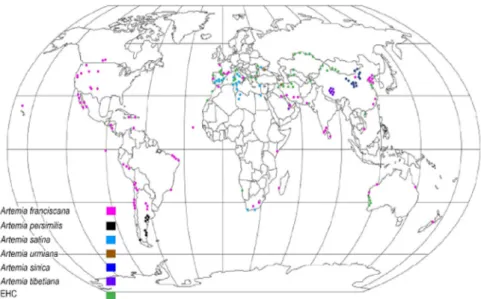
Artemia populations are globally distributed. Accordingly, over 600 locations of Artemia sites throughout the tropical, subtropical, and temperate climatic zones were identified (Kaiser et al. 2006; Naceur et al. 2009; Ogello et al. 2014) (Figure 3). Salinity is the most limiting abiotic factor of Artemia’s geographical distribution as it determines the presence or absence of
Figure 2 - Life cycle of Artemia franciscana. Two different patterns of reproduction: embryos developed in the ovisac and females release free-swimming nauplii under favorable environmental conditions (ovoviviparity); female produce dormant cysts under harsh environmental conditions (oviparity) (Sorgeloos et al. 1973; adapted from University of Utah, Genetic Science learning Centre).
Artemia. The physiological adaptation to high salinity of Artemia allows it to avoid predation and competition with other filter-feeders (Gajardo & Beardmore, 2012; Van Stappen,1996). Besides, the geographical isolation and population dynamics are also associated with environmental conditions such as temperature, predictable seasonality and ionic composition of the biotope (Vanhaecke,1984; Gajardo & Beardmore, 2012). For instance, more than 97% of the Artemia sites are located in areas where no or little water surplus can be noted at different times of the year, maintaining the salinity sufficiently high on a year-round basis.
Apart from SFB and GSL, A. franciscana is naturally distributed in North, South and Central America, the intentional introduction of this America originated species led to the establish of new Artemia production site in Asia. For instance, it was introduced in the Mekong Delta of southern Vietnam in 1982 where it has been seasonally cultivated (Quynh & Nguyen, 1987). With the capacity to tolerate and survive to a variety of environmental conditions, A. franciscana has now successfully adapted to the high temperature conditions of Vinh Chau and Bac Lieu in the Mekong Delta, reaching a production of around 50 t of raw cyst in 2001 (Van Hoa, 2014).
Parthenogenetic populations (Artemia parthenogenetica (Barigozzi, 1974) are endemic to
Page | 13
2.2.4.Global introduction of A. franciscana
Among the bisexual species of the Artemia, A. franciscana has been the most extensively studied. Compare to other strains, A. franciscana shows distinctive physiological performance and highly repetitive heterochromatin (Gajardo et al. 2001; Vikas et al. 2012). For instance, A. franciscana performed better in terms of survival and reproductive traits at a broad range of salinities (Wear & Haslett, 1986; Triantaphyllidis et al. 1995). Moreover, though tolerance to different temperatures is strain-specific, the optimal temperature for
Artemia culture and growth is around 28°C; most strains do not survive to temperatures lower than 6°C or higher than 35°C (Browne et al. 1988). A. franciscana showed a higher survival rate in both low and high temperatures (Wear & Haslett, 1986; Quynh & Lam, 1987; Gajardo & Beardmore, 1993; Kappas et al. 2004).
As mentioned earlier, natural A. franciscana from GSL and SFB have been extensively exploited as the main source of live feed for larval culture (Sorgeloos et al. 2001). Due to the intentional export of A. franciscana SFB and GSL to the world aquaculture markets since the 1950s, A. franciscana has now been broadly transplanted to different areas, leading to the rapid worldwide dispersal of those American species in non-indigenous regions (i.e. Thailand, Vietnam, Brazil, Australia) (Muñoz et al. 2013; Camara, 2020). Moreover, the introduction of A. franciscana into Portugal, Morocco, Spain, Italy, and other Western Mediterranean areas has eventually caused co-existence with autochthonous A. salina and
A. parthenogenetic (Amat et al. 2007; Naceur et al. 2010; Muñoz & Pacios, 2010; Muñoz, 2013 et al).
2.3.The important role of LC-PUFAs in aquaculture
2.3.1. Introduction to LC-PUFAs
Besides being an essential source of metabolic energy, lipids are also known as one of the main cellular membrane components. Like other fatty acids, LC-PUFAs are essential structural elements of membrane phospholipids, providing different functional properties (Alberts et al. 2002; Liu et al. 2015; Casares et al. 2019). For instance, Casares et al.(2019)
reported that changes in membrane LC-PUFAs composition affected the membranes' physicochemical properties, such as fluidity and functional proteins (i.e., receptors, transporters, and enzymes). Moreover, lipids containing high levels of EPA and DHA are preferred as energy sources in most fish species to proteins and carbohydrates (Borges, 2014).
2.3.2.Marine fish larvae LC-PUFAs requirements
Some fish naturally contain high levels of LC-PUFAs in their body tissues. However, marine fish cannot synthesize LC-PUFAs due to the lack of Δ12 (or ω6) and Δ15 (or ω3) desaturases, which convert 18:3 (n-3) to 20:5 (n-3) (Monroig et al. 2013). Fish fatty acids composition heavily depends on the interactions of diets with endogenous metabolism; therefore, they require high levels of LC-PUFAs on diets (either live or inert feed) (Tocher et al. 2019). As growth is more accentuated during the larval stage, LC-PUFAs requirements are known to be higher on diets formulates for this period than to the adult phase (Coutteau et al. 1997) . In order to store LC-PUFAs in fish, relative proportions of LC-PUFAs are particularly crucial; in general, LC-PUFAs supplementation must exceed the requirements for optimal health and growth (Coutteau et al. 1997). Additionally, the ratios between LC-PUFAs are equally relevant, especially in terms of the ratio between DHA and EPA (Tocher, 2010). DHA/EPA ≥ 2 is considered sufficient for many marine fish species (Jobling, 2011; RodrÌguez et al. 1998).
2.3.3. Supplement of LC-PUFAs
2.3.3.1.Fish oil, fish meal and microalgae
Fish oil and meal are the main sources of LC-PUFAs applied in aquaculture, which are traditionally obtained from pelagic fisheries and aquaculture by-products (Tacon et al. 2006). Together with the aquaculture industry, the fish oil and meal industry have commercially developed to overcome the demand of fish for human consumption (Martins et al. 2013). However, with the stagnation of fisheries and aquaculture over-dependency on fish oil and meal, these by-products became a limited source (Naylor et al. 2009). From a sustainable point of view, excessive use of fish oil and meal has experienced some issues and
Page | 15
difficulties; more attention has been given to other potential sources. LC-PUFAs enrichment protocols with commercial microalgae have been investigated and documented on a large volume of published literature. Ryckebosch et al. (2014) reported that microalgae could offer the ideal long term, sustainable solution to LC-PUFAs supply; different microalgae have been commonly used in hatcheries as LC-PUFAs supply, namely diatoms as EPA and flagellate as DHA supply (Budge et al. 2014).
It has been reported that organisms that over-express genes coding for enzymes involved in the biosynthesis of EPA and DHA are capable of synthesizing a sufficient quantity of LC-PUFAs from linoleic acid (Alimuddin et al. 2006). Elongase genes have been isolated from different species (Alimuddin et al. 2006). These findings allow researchers to modify the biosynthetic pathway of LC-PUFAs by overexpressing the LC-PUFAs biosynthesis-associated enzyme genes to increase the production of EPA and DHA (Ruiz-Lopez et al.
2013). Success in these protocols will offer alternatively LC-PUFAs enriched live food for marine fish larvae, thus eliminating the use of fish oil and fish meal.
2.3.3.2.Artemia bio-encapsulation
Bioencapsulation in aquaculture was initially to incorporate some essential elements into the diet, thereby improving them to guarantee adequate nourishment of aquatic animals (Fernández, 2001). Further applications include the transfer of bio-encapsulated pigments (Conceição et al. 2010), probiotics (Arndt & Wagner, 2007; Seenivasan et al. 2012), medication like anti-biotics (Venkat et al. 2004; Vázquez-Silva et al. 2017), ascorbic acid (Immanuel et al. 2004; Akbary et al. 2011), spawning hormone (Burton, 1998) to target animals depending on the desire of the aquaculturist (Figure 4).
As discussed earlier, LC-PUFAs exert not only essential physiological functions as one of the main components of biological membranes, but also affect growth, survival, behavior, and development in marine fish larvae (Shields et al. 1999; Van Stappen, 1996; Tocher et al. 2019). However, the nutritional content value of Artemia nauplii concerning LC-PUFAs is not adequate for marine fish larvae, and the ratio between DHA and EPA is largely insufficient (Bengtson et al. 1991; Shields et al. 1999). To overcome the deficiency in LC-PUFAs, a number of enrichment techniques have been developed, including the British technique with unicellular algae; the Japanese technique with ω-yeast or emulsions plus baker’s yeast; the French technique with compound diets; and the Belgian technique with microparticulate diets or self-emulsifying concentrates (Leger et al. 1986; Bengtson et al.
1991). In general, the nutritional value of Artemia is modified through incubations in seawater containing LC-PUFAs-rich particulates and emulsions, because Artemia nauplii are passive filter-feeders, enriched DHA and EPA are passively incorporate by the nauplii (Leger et al. 1986). Despite the enrichment diet used, each technique is adjusted according to the hatching conditions, the fattening time, enrichment period and temperature (Sorgeloos
et al. 1991).
Figure 4 - The principles of bioencapsulation. Bio-encapsulating specific amounts of particulate or emulsified products in Artemia nauplii to suit the larval requirements. (Adapted from Dhont & Van Stappen, 2003)
Page | 17
2.4.DHA: unique physical and functional properties
2.4.1.DHA location in the cell membrane
Cell membranes are constituted by several types of glycerophospholipids (GPLs); based on their polar head groups, GPLs are classified into phosphatidic acid (PA), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylglycerol and the phospholipids acyl inositol (Hishikawa et al. 2014). Zerouga (1996) demonstrated that DHA incorporated into membranes GPLs (Figure 5), maintaining the membrane’s physiological functions and modulating membrane protein functions. DHA is obtained from diets. Dietary supplementation can increase membrane DHA concentration if the level of DHA is below 5 mol% (percentage of total moles of DHA) of the total phospholipid acyl chains, to a maximum of 2 to 10 folds its concentration (Salem et al. 1986; Van Meter et al. 1994; Robinson et al. 1993; Stillwell & Wassall, 2003; Sugasini et al. 2017). Once saturated, no further increase of the membrane DHA is possible throughout diets; if starved, the animals maintained the membrane’s DHA concentration at the expense of other fatty acids (Salem et al. 1986; Stillwell & Wassall, 2003).
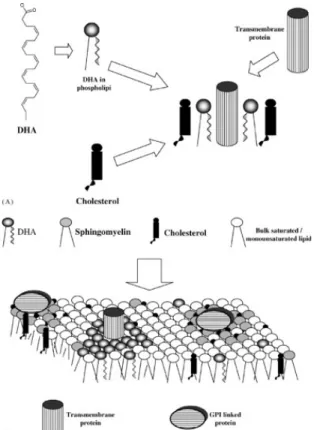
Figure 5 - Incorporation of DHA into the membrane. DHA is first incorporated with GPLs, cholesterol and GPLs-DHA are then incorporated with transmembrane proteins (adapted from Stillwell & Wassall, 2003).
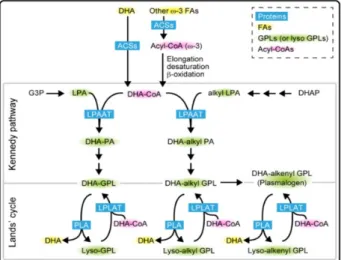
Although the mechanisms of DHA incorporation in the membranes remained obscure, Harayama (2014) reported that the action of lysophosphatidic acid acyltransferases might primarily regulate the levels of GPLs containing DHA in mammalian cells' membranes through the Kennedy pathway (Figure 6). Lysophosphatidic acid acyltransferases use lysophosphatidic acid as an acyl acceptor to regulate the production of PA; and PA is a common intermediate for GPLs and neutral lipid synthesis (Hishikawa et al. 2017). DHA is initially incorporated into PA by the action of lysophosphatidic acid acyltransferases, and subsequently, convert to other membrane GPLs.
2.4.2. Functions of membrane DHA
2.4.2.1.Membrane shape and fluidity
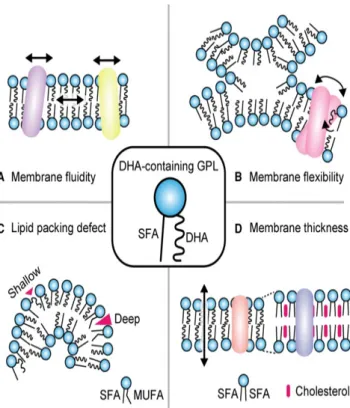
As mentioned above, LC-PUFAs is an essential component of cellular membranes, particularly GPLs-containing DHA that are predicted to have specific functions due to their structural characteristics (Figure 7). For instance, membrane thickness decreased because DHA chain occupies the area near the aqueous interface with higher volume density, while the saturated bilayer center is slightly fatter (Eldho et al. 2003). Besides, the cis double
Figure 6 - The synthetic pathways of GPLs‐containing DHA in mammalian cells. In the de novo pathway (Kennedy pathway), DHA is mainly incorporated into the PA by the action of lysophosphatidic acid acyltransferases. PA is a common intermediate for all GPLs synthesis. Alternatively, may the concerted actions of lysophosphatidic acid acyltransferases may form GPLs‐containing DHA in the Lands’ cycle (adapted from Hishikawa et al. 2017).
Page | 19
fatty acids chains decreased, and thus membrane fluidity increased (Holthuis & Menon, 2014; Ibarguren et al. 2014). Research findings from previous dietary studies on animals also reported that GPLs-containing DHA also increases membrane fluidity (Kamada et al.
1986).
2.4.2.2.DHA altering membrane domains
Lipid rafts in membranes are predicted to accumulate specific proteins, providing a platform for the functional protein activity (Edidin, 2003). For instance, the Src family of kinases accumulates in the plasma membrane's leaflet and GPI-anchored proteins are abundant in the outer leaflet (Simons & Ikonnen, 1997). Major lipid components that consist of the cellular membrane in most animals are cholesterol, which also modulates the membrane's physical properties (Finegold, 1993). Although the details have not been reported,
Figure 7 - The roles of DHA‐containing GPLs in membranes. (A) DHA incorporated with membrane GPLs may increase membrane fluidity. (B) DHA increase membrane flexibility and maintains membrane physiological properties. (C) DHA may promote formation of more shallow defects than monounsaturated fatty acids, which may affect the recruitment of several lipid packing defect‐sensing proteins. (D) Lipid bilayers composed of DHA are thinner compared to those composed of saturated phospholipids and cholesterol (adapted from Hishikawa et al. 2017).
interactions between DHA and cholesterol profoundly play an active role in forming lateral membranes domains (Mouritsen, 1998). The membrane lateral phase is separated into cholesterol-rich/PUFAs-poor and cholesterol-poor/PUFAs-rich membrane micro-domains due to the reduced affinity between cholesterol and LC-PUFAs (Brzustowicz et al. 2002; Shaikh et al. 2003)
2.4.2.3.Effect of DHA on cell signaling
Giorgione et al. (1995) reported that DHA acyl chains incorporated into membrane GPLs (phosphatidylethanolamine) increases the level of the signaling molecule Protein Kinase C (PKC) activity, and thus modulating the cellular membrane's physical properties, especially the intrinsic bilayer curvature (Slater et al. 1994, Goldberg & Zidovetzki, 1998; Stillwell & Wassall, 2003). Besides, DHA has also been reported to modulate the activities of membrane channels, including the L-type Ca2+ channels (Pepe et al. 1994; Xiao et al. 1997),
Na+/K+ - ATPase (Huang et al. 1986), Ca2+/Mg2+ - ATPase (Karmazyn et al. 1987; Stillwell
& Wassall, 2003), suggesting a vital role in cell signaling. Interestingly, many of the property changes upon the accumulation of DHA into membranes are similar to those that change in membrane properties during apoptosis (Stillwell et al. 2005). The above finding is consistent with the studies on DHA's role in enhancing apoptosis in cytosol-linked and mitochondrial-linked pathways (Siddiqui et al. 2004).
2.5. LC-PUFAs degradation
2.5.1.LC-PUFAs across the cell membrane
LC-PUFAs are transported between organs in the form of unesterified fatty acids complexed to serum albumin or triacylglycerols associated with lipoproteins (Van der Vusse et al. 1998). Many of the fatty acids across cell membranes through unassisted diffusion, LC-PUFAs have also been long thought to across cell membranes by passive diffusion as well; however, membrane-associated fatty acids-binding proteins (fatty acids transporters) were identified in facilitating and regulating the uptake of fatty acids by cells ( Schwenk et al. 2010; Zárate et al. 2017), such as CD36, plasma membrane-linked fatty acids-binding proteins,
Page | 21
and a family of fatty acid transport proteins (FATP1-6) (Glatz & Luiken, 2018). Though their specific functions in LC-PUFAs across membrane process have not been elucidated, fatty acid transporters might take function in the reversible and inducible rapid translocation of fatty acids from intracellular storage pools to cell membranes (Schwenk et al. 2010). Once across the cellular membrane, LC-PUFAs either diffuse or are transported to mitochondria, peroxisomes, and endoplasmic reticulum where the metabolism is activated (Wanders et al. 2016). Cytosolic fatty acid-binding proteins in the cytosol also facilitate the transfer of fatty acid from membranes to their activation sites (Atshaves, 2010). The activation of LC-PUFAs starts with the esterification of coenzyme A (CoA), acyl-CoA synthetases catalyze the ATP-dependent esterification of LC-PUFAs to their fatty acyl-CoA (Watkins & Ellis, 2012, Schulz, 2013).
2.5.2. Fatty acids oxidation in mitochondria
2.5.2.1.Mitochondria uptake of fatty acids
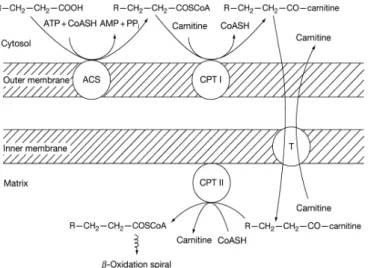
According to Schulz (2013), prior activation of LC-PUFAs in mitochondrial membranes convert them to fatty acyl-CoA; subsequently, carnitine palmitoyltransferase I (CPT I) catalyzes fatty acyl-CoA to CoA-carnitine. The fatty acylcarnitine cross the mitochondrial membrane in exchange for endo-mitochondrial carnitine (Figure 9). Once across the mitochondrial membrane, fatty acylcarnitine is converted back to fatty acyl-CoA catalyzed by carnitine palmitoyltransferase II; consequently, LC-PUFAs acyl-CoA is transferred to the mitochondrial matrix, where the β-oxidation takes place (Schulz (2013).
2.5.2.2.β-Oxidation of LC-PUFAs in mitochondria
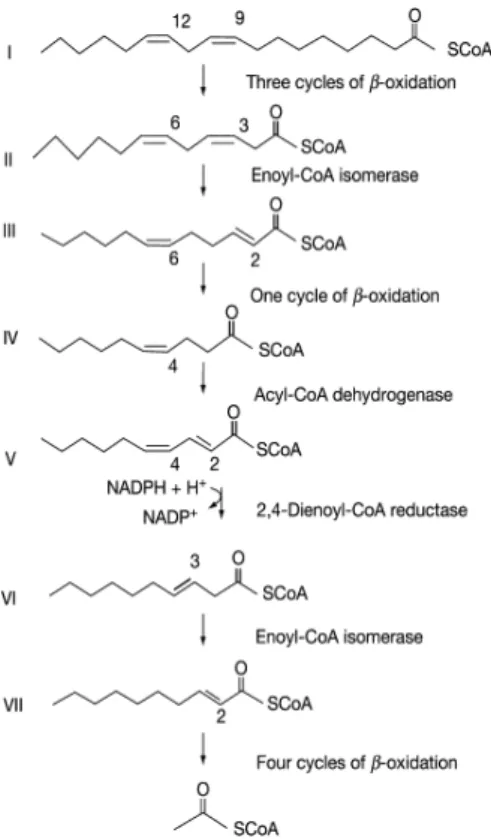
LC-PUFAs are degraded by β-oxidation, as described on Figure 10, linoleic acid contains both odd- (C-9) and even-numbered (C-12) double bonds, therefore the degradation of linoleic acid illustrates the β-oxidation of all LC-PUFAs. According to Schulz (2013), linoleoyl-CoA first undergoes three cycles of β-oxidation, yields 3-cis,6-cis-dodecadienoyl-CoA (II). Additional Δ3, Δenoyl-3-cis,6-cis-dodecadienoyl-CoA removes the Δ3 double bond and convert it to 2-trans,6-cis-dodecadienoyl-CoA (III). This metabolite completes another cycle of β-oxidation to yield decenoyl-CoA (IV) and subsequently dehydrogenated to 2-trans, 4-cis-decadienoyl-CoA (V). 2,4-dienoyl-CoA reductase active the reduction of V by NADPH and Δ3, Δ2-allyl-CoA isomerase catalyze the isomerization of Δ3 double bond to Δ2 double bond. The yield product 3-trans-decenoyl-CoA (VI) experiences another four cycles of β-oxidation to complete the degradation pathway.
Figure 8 – LC-PUFAs across the mitochondrial membranes. LC-PUFAs are convert to fattyacyl carnitine groups in the out membrane, catalyzed by acyl-CoA synthetase and CPT I. acyl carnitine are transported into mitochondrial in exchange for carnitine, and reconverted to fatty acyl-CoA. The reconversion occurs in mitochondrial matrix and active by carnitine:acylcarnitine translocase and CPT II (adapted from Schulz, 2013).
Page | 23
LC-PUFAs oxidation is a balance between enzyme specificity and the availability of fatty acid substrates. Not surprisingly, the oxidation rates of saturated/monounsaturated fatty acids are higher than LC-PUFAs oxidation rate in vitro due to double bonds in unsaturated fatty acids (Henderson, 1996). Moreover, DHA is preferentially conserved in body tissues such as liver and flesh, and membranes due to the Δ4 double bond in DHA are resistant to mitochondrial β-oxidation (Glencross, 2009). Therefore, peroxisomal oxidation is required to remove the Δ4 double bond (Madsen et al. 1999).
2.5.3. LC-PUFAs oxidation in peroxisomes
Peroxisomes contain the enzymatic activities required for the additional metabolism of double bonds in LC-PUFAs; for instance, peroxisomal β-oxidation is involved in the conversion of DHA to EPA (Calder, 2016). Unlike mitochondria, peroxisomes have different β-oxidation enzymes, and the substrates for peroxisomal β-oxidation are fatty acids-CoA derivatives and carboxylic acids such as LC-PUFAs, alkanoic acids, prostaglandins, and dicarboxylic acids, which are poorly taken up by mitochondria (Hiltunen, 1991; Monroig et
al. 2013; Schulz, 2013). The uptake and activation of fatty acids by peroxisomes remain poorly understood, while LC-PUFAs might be converted to CoA esters in the peroxisomal matrix after entering animal peroxisomes, thereby activating the peoxisomal degradation process. Palmitoyl-CoA, multifunctional enzymes MFE 2, and 3-ketoacyl-CoA thiolase are required for long-chain fatty acyl-CoA β-oxidation in peroxisomes (Schulz, 2013). The same author also emphasized that peroxisomal β-oxidation yields chain-shortened acyl-CoA, NADH, and acetyl-CoA, while little is known about transports that facilitate those products' efflux from peroxisomes. Acyl-CoA might be converted to the corresponding acylcarnitines before exiting from peroxisomes.
2.5.4.Regulation of LC-PUFAs metabolism
LC-PUFAs oxidation rate responds to animal nutrition and hormonal status (Wakil & Abu-Elheiga, 2009). According to Wakil & Abu-Elheiga (2009) and Schulz (2013), the increase in free fatty acid concentration leads to decreased cellular LC-PUFAs uptake and the oxidation rate. After feeding, carbohydrates are converted to triacylglycerol; and the activated acetyl-CoA carboxylase enhances the LC-PUFAs synthesis in cells. Acetyl-CoA carboxylase catalyzes acetyl-CoA, convert it to malonyl-CoA, the first important intermediary in fatty acids synthesis. Malonyl-CoA inhibits the uptake of fatty acids by mitochondria; therefore, limited fatty acids are oxidized by mitochondria and more fatty acids are rapidly synthesized. On the contrary, fasting results in low blood glucose levels increases in hormone glucagon concentration and decrease in insulin concentration, and thus glucagon promotes phosphorylation and inactivation of acetyl-CoA carboxylase, and consequently malonyl-CoA activity is inhibited. Therefore, fatty acids synthesis declines and oxidation increases.
On the other hand, LC-PUFAs degradation is a complex balance between the degradation and biosynthesis of fatty acids; while the availability of free fatty acid substrate, activation of enzyme and competition between them, uptake and transportation of fatty acids among different oxidation sites seem to be major influences of fatty acids metabolism (Leaver et al. 2008; Schulz, 2013; lee et al. 2016; Lankinen et al. 2018). Each process independently
Page | 25
controls the LC-PUFAs metabolism while also in conjugation with other processes (Leaver
et al. 2008). Moreover, LC-PUFAs β-oxidation rate also responds to animal nutrition and hormonal status (Wakil & Abu-Elheiga, 2009).
2.6.Introduction to HSPs
2.6.1.General aspect of HSPs
HSPs, also known as stress proteins, are a group of stress-sensitive proteins expressed response to physiological and environmental stressors (Roberts et al. 2010). Tissières et al. (1974) first discovered this protein in the salivary glands of the fruit fly (Drosophila sp.) induced by heat stress. Later researches reported that HSPs are highly preserved proteins also expressed under normal physiological conditions in many organisms, from prokaryotes to eukaryotes (Zügel & Kaufmann, 1999). Physiological stressors such as high temperature, toxins, osmotic pressure, ultraviolet and gamma rays, certain chemicals and drugs, hypoxia, glucose deprivation and microbial infections that damage the cell are able to induce the expression of HSPs (Mathew & Morimoto, 1998; Arieli et al. 2003; Collins & Clegg, 2004; Todgham et al. 2005; Baruah et al. 2010; Baruah et al. 2012 ). HSPs have been reported to assist in the folding of nascent proteins, transportation of these proteins between cellular organelles, the assembly/disassembly of multi-subunit complexes (Kosmaoglou et al. 2008; Roberts et al. 2010); also, HSPs mediated the refolding or degradation of denatured proteins produced during stress conditions (Saibil, 2013).
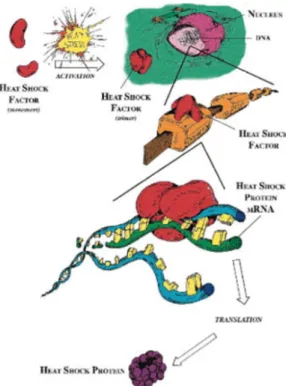
Certain stressor activates specific heat shock transcription factors; Akerfelt et al. (2010) stated that heat shock factors (HSF) interact with heat shock elements in the HSP gene promoter regions controls the transcription of HSPs (Figure 12). Monomer HSF1 can be convert a homotrimers which is capable of binding to the promoter site of the stress protein gene, and the binding initiates the transcription and translation (Whitley et al. 1999). In general, the HSF's structure and activity are different in different groups of organisms, while HSF1, HSF2, HSF4 are commonly expressed in most organisms (Roberts et al. 2010).
HSPs exert modulatory effects on host immune response to bacteria and virus infections, including apoptosis and inflammatory process (Roberts et al. 2010). For example, extracellular HSP70 has been reported to stimulate Toll-like receptors (TLR2 and TLR4) and thereby transducing inflammatory danger signals to immune cells (Asea et al. 2002; Srivastava, 2002; Yik Sung et al. 2011). Moreover, HSP70 is able to enhance chemotaxis, phagocytosis and cytolytic activity of innate immune cells and induce the expression of inflammatory cytokines. Inflammatory cytokines induced by HSP70 have been reported subsequently stimulate macrophages and neutrophils to secrete tumour necrosis factor α, interleukin-1β, and interleukin-6 (Asea et al. 2002; Campisi & Fleshner, 2003). Srivastava (2002) and Yik Sung et al. (2011) reported that HSPs might function in histocompatibility complex (MHC)-class II-peptide complex assembly in adaptive immune response, assisting
Figure 11 – HSFs up-regulate the expression of HSPs. Activation of HSF monomers (red) in response to stress combines with other monomers to form a trimer. Trimer of HSFs binds to the promotor regions on heat shock gene (DNA); the binding results in production of heat shock protein mRNA (green), and transcription of HSPs, mRNA moves to cytosol where translation occurs (adapted from Whitley et al. 1999).
Page | 27
antigen presentation and subsequently activating T cells to destroy pathogens as well as malfunctioning cells.
2.6.2. Molecular chaperones
Molecular chaperones are a diverse group of intercellular proteins that initially guide non-covalent assembly/disassembly proteins along the proper folding pathway. Molecular chaperones are not permanent components of these proteins but protect them from misfolding via shielding them from other proteins that might bind and hinder it (Hartl & Hayer-Hartle, 2002). According to the same author, molecular chaperones are also secreted and exist on the outer plasma membrane and body fluids. In bacteria and eukaryotic cells, molecular chaperones serve as receptors for a diverse set of client ligands on the outer membrane surface (Henderson et al. 2006). Besides being membrane receptors, extracellular molecular chaperones are believed to exert potential functions in signaling functions (Henderson, 2010); for instance, the same author reported that extracellular molecular chaperones control the cellular homeostasis in additional level by linking cellular stress to the physiological system.
2.6.3.Moonlight proteins
Most of the proteins with biological activities, such as enzymatic activities, have only one functional site (Alberts et al. 2002). Within moonlight proteins, a subset of multifunctional proteins, one polypeptide chain exhibits more than one physiologically relevant biochemical or biophysical functions (Jeffery, 1999). One major group of identified moonlight proteins is molecular chaperones; for instance, HSPs change their subcellular location and thereby are able to exert different functions (Hoter et al. 2018). Moreover, Pockley & Henderson (2018) reported that 'moonlighting' actives of HSP70 in membranes, which might relate to maintaining the membranes' structural and functional integrity under stress conditions.
2.6.4. HSP70
2.6.4.1.HSP70 family
HSPs are usually named according to their molecular weights; major groups of HSPs have various molecular masses, small heat shock proteins have a molecular mass of 18–40 kDa;
other groups including HSP60, HSP70, HSP90, and HSP1001 (Whitley et al. 1999; Junprung et al. 2019). HSP70 family is the largest family of stress proteins and also the one been most extensively studied; at least 121 proteins of the HSP70 family have been identified (Sanders, 1993). Although the expression of HSP70 is species-dependent, they are highly conserved as HSP70 shares 95% sequence homology (the identity of the DNA sequence) (Baruah et al. 2010), approximately 60% and 46% of amino acid sequence identity can be observed in prokaryotic and eukaryotic HSP70, respectively.
2.6.4.2.Membrane HSP70
HSPs were believed to have purely intracellular functions; because most essential groups of molecular chaperones involved in maintaining cellular homeostasis are initially intracellular. Nevertheless, further studies have found extracellular HSPs escape from cells with multiple functions (Calderwood, 2007; Zuo et al. 2016). Because in eukaryotic cells, in addition to secreting proteins through the endoplasmic reticulum-Golgi-signal peptide mechanism, there are many other mechanisms secrete proteins (Lancaster & Febbraio, 2005). The same author first described the secretion mechanism of Hsp70, released through the small membrane vesicles called exosomes. Now a large volume of literature has reported diverse extracellular HSPs. For example, nuclear-encoded proteins such as HSP10, HSP60, and HSPA9 are mitochondrial chaperones that are considered functional only in mitochondria; while they have been found on the outer surface of plasma membranes (Gupta & Knowlton, 2007). From another study using surface labeling techniques, HSP60 has also been directly observed on the outer surface of plasma membranes (Soltys & Gupta, 1997). Based on biophysical measurements of the Gram-negative inflammogen receptor lipopolysaccharides on the outer surface of cell membranes, HSP70 was also found in the outer membranes (Borges et al. 2012).
2.6.4.3.Artemia HSP70
Artemia HSP70 has been proven effective against a set of pathogens (Figure 13). As mentioned earlier, Artemia produces dormant cyst under challenging conditions; Hsp70 is still synthesized during diapause and might induce alteration of normal cellular functions
Page | 29
(Haslbeck, 2002; Iryani et al. 2020; MacRae, 2010). When Iryani et al. (2017) using RNAi to knock down Artemia HSP70, 71% of cysts containing HSP70 survived, whereas for cysts lacking HSP70, 50% survived. The author also reported that HSP70 might cooperate in proteins during diapause, preventing protein damage. HSP70 has now been employed in aquaculture operations to protect aquatic animals from infections; moreover, the identification of the HSP70 gene from A. franciscana allowed further studies that focus on the therapeutics functions of HSP70 against pathogens. Several studies have revealed that HSP70 potentially mediated the innate and adaptive immune system of A. franciscana; when knocked down HSP70, the survival of A. franciscana nauplii reduced approximately 34% in
V. campbellii challenged conditions, showing a significant difference (Srivastava, 2002). Furthermore, Yik Sung (2007) reported that NLHS induced HSP70 improved Artemia
survival and was associated with protection against virulent Vibrios.
2.7.Membrane fatty acids composition regulation by heat stress and
HSP70
2.7.1.Membrane role in modulating cell’s response to stress
Organisms have evolved a set of adaptive responses to Controlling of enzyme activities, gene transcription, translation, and modifications; reorganization of membrane lipids and
Figure 12 - HSP70 protects A. franciscana nauplii against V. campbelli. The survival rates of nauplii with HSP70 were significantly higher than those without HSP70 (P < 0.05) (Adapted from Iryani, 2017).
activation of HSPs are such evolved protection mechanisms response to heat stress (Pan, 2013; Wallis & Browse, 2002; Penfield, 2008). Hundreds of different lipids distributed in the membrane bilayer with various structural and functional properties; membrane lipid dynamics and composition are critical to restoring the optimal functions under stressful environments or altered physiological conditions (Yang et al. 2005; Van Meer et al. 2008). Specific lipids, enzymes in membranes can be targeted to alter their compositions and structures in response to physiological and pathological situations (Yang et al. 2005). Moreover, signaling proteins binds to membrane lipids to propagate the signals (messages) from to receptors to effectors; and therefore, changing in membrane lipids compositions is able to affect the functions of signaling proteins such as transcription factors of (Torres et al. 2020). Therefore, changing in membrane lipids consequently affect vital proteins' activities, interactions between lipid-protein or protein-protein in membrane micro-domains, and propagation of signals. Moreover, changes in ambient temperature cause fluctuations in membrane fluidity has also been found to modulate interactions between specific membrane domains and certain HSPs, leading to remodeling the pre-existing architecture and physical order of membranes (Los & Murata, 2004; Horváth et al. 2008).
2.7.2.Phospholipid-based signaling pathways activated by heat
stress
2.7.2.1.Heat stress relation to PA accumulation in plant cells
Synthesis of PA occurs through either the phosphorylation of diacylglycerol catalyzed by diacylglycerol kinase or transfer the phosphatidyl group of structural phospholipids by phospholipase D. Santoro (2000) demonstrated that the activity of a specific G protein coordinates the activities of phospholipase D and phosphatidylinositol (4)-phosphate kinase in plants in response to Heat stress, leading to the accumulation of phosphatidylinositol (4,5)-bisphosphate (PIP2) and PA in cells. Using radioactive label techniques, Santoro (2000) noted that incorporated PA is generated from structural phospholipids; the accumulated PA is an essential intermediate for membrane GPLs incorporated with DHA (Hishikawa et al. 2017). Further experiments using 1-butanol as an alternative substrate to
Page | 31
temperature increase (Milton & Wendlandt, 1970). Balogh et al. (2010) reported the accumulation of cholesterol and other saturated phospholipids during heat stress.
2.7.2.2.Activation of PKC
Several studies focus on cell lines illustrated that heat stress triggers an increase in the plasma membrane’s fluidity, which elicits an immediate elevation in intracellular Ca2+ (Finka
et al. 2012). Ca2+ mobilization is known to be a prerequisite for the transcription of HSP
(Price & Calderwood, 1991). Moreover, elevated intracellular Ca2+ triggers diacylglycerol
that serves as a second messenger, activating PKC. Activated PKC phosphorylates a wide variety of protein and interacts with negatively charged membranes GPLs and membrane microdomains (Newton et al. 2016; Goñi & Alonso 1999); because the C2 domain of PKC recognizes various phosphoinositides, and membranes are abundant in phosphoinositides (Escribá et al. 2015).
Conclusively, heat stress triggers the accumulation of PA generated from structural phospholipids, which might be incorporated with membrane DHA. The active PKC bind with GPLs in cell membranes might also increase the divers of GPLs containing DHA in the plasma membrane.
2.7.3.HSP 70 interaction with membrane lipids
2.7.3.1.Recruitment of HSP70 by membrane phospholipids
As mentioned above, cells reorganize the cellular membrane and remodel the membrane properties to restore optimal membrane structures and functions under stress. Rac1-mediated is activated in response to membrane physiological alteration, which is able to control the expression of HSPs, and thus active cellular defense mechanisms (Horváth et al. 1998). In turn, expressed HSPs interact with cellular membranes and stabilize the membrane structure and function (Balogi et al. 2008).
Guidon & Hightower (1986; 1989) found that HSPs bind to membrane fatty acids, suggesting the direct interaction between HSPs and membrane lipids. Moreover, they reported that HSP70 possesses a specific affinity for membrane lipids. A specific interaction between Hsp70 and membrane PS was reported in vitro studies using a liposome aggregation assay,
and results suggested that HSPs are accumulated in membrane GPLs and other regions enriched in cholesterol, GPLs, acylated proteins. (Arispe et al. 2002; Kishimoto et al. 2016; Lamprecht et al. 2018). Armijo et al. (2014) proposed that such interaction might be based mainly on the negative charge group of phospholipids. PS that constitutes the membrane GPLs is predominantly found in the plasma membrane's inner leaflet under normal conditions, whereas upon stress, PS shifts to the outer membrane leaflet (Schilling et al. 2009).
2.7.3.2.Integrity of lysosomal membrane in relation to HSP70
Maintaining the integrity of the lysosomal membranes is essential for cell homeostasis, HSP70 has been noted to present in the lysosomal membrane and in endo-lysosomal systems in a soluble form released by multivesicular bodies (Nylandsted et al. 2004; Juhász
et al. 2013). In general, membrane-bound HSP70 enters lysosome through clathrin-dependent/independent mechanisms; while cytosolic HSP70 enters lysosome through autophagic mechanism (Juhász et al. 2013; Morozova et al. 2016). Not surprisingly, lysosomal and endo-lysosomal HSP70 are resisted to lysosomal hydrolases and restore functions in such hostile environments (Nylandsted et al. 2004; Kirkegaard et al. 2010). By engineering cells to overproduce HSP70, Kirkegaard et al. (2010) noted that expressed HSP70 protects cells against stress-induced lysosomal damage. Further studies pointed out such ability is mostly attributed to HSP70’s high affinity for binding to Bis(monoacylglycerol)phosphate in lysosomes, which is an anionic phospholipid responsible for lysosomal sphingolipid catabolism (Kolter & Sandhoff, 2005; Kirkegaard et al. 2010) (Figure 15). The interactions between HSP70 and BMP enhanced the break down of sphingomyelin to ceramide and phosphocholine, suggesting HSP70 regulates lysosomal membrane integrity (Balogi et al. 2019).
2.7.4.Lipid-active enzymes regulation by HSP70
2.7.4.1.Activation of phospholipase A2 by HSP70
Phospholipase A2 (PLA2) represents a ubiquitous group of enzymes highly conserved in catalytic mechanisms and structures, with a high degree of sequence homology between
Page | 33
different species (Scott et al. 1990; Six & Dennis, 2000). PLA2 plays a role in cell signaling, antibacterial activity, and particularly phospholipid metabolism, it cleaves the sn-2 acyl chain of GPLs to produce lysophospholipids and free fatty acids (Linderoth, 2008). According to Mahalka (2011) and Sousa & Lafer (2006), HSP70 sustains the expression of PLA2, and attenuate the oligomerization of PLA2 and its transformation into amyloid fibres.
2.7.4.2.Stabilizing factors for PKC
Gao & Newton (2002) and Mahalka (2011) reported that HSP70 serves as a stabilizing factor for PKC during stress challenges, prolonging the lifetime of the active state PKC and sustaining the expression of PKC and its catalytic activity. In the Co-immunoprecipitation experiments, HSP70 bind to the unphosphorylated Thr (641) in PKC β-II, stabilizing the protein and allowing the re-phosphorylation of PKC (Gao & Newton, 2002; Kumar et al.
2016); the results also suggest that HSP70 screws binding partners for the carboxyl terminus of PKC.