COMPLICATIONS IN LVAD PATIENTS
Verhaeghe Sarah
Student number: 01413604Supervisor: Prof. dr. Yves Van Belleghem
A dissertation submitted to Ghent University in partial fulfilment of the requirements for the degree of Master of Medicine in Medicine
Deze pagina is niet beschikbaar omdat ze persoonsgegevens bevat.
Universiteitsbibliotheek Gent, 2021.
This page is not available because it contains personal information.
Ghent University, Library, 2021.
Preface
The realization of this thesis was an interesting and enriching experience. Therefore, I would like to thank prof. dr. Y. Van Belleghem for his guidance and support during this process. I also wish to thank the staff of the secretariat of the Cardiac Surgery Department of Ghent University Hospital for their practical support. Lastly, I would like to thank Sophie De Decker with whom I worked on the database at Ghent University Hospital for our pleasant cooperation.
Table of contents
0. Abstract 1
0.1. English version 1
0.2. Dutch version 2
1. Introduction 3
2. Overview of left ventricular assist systems (LVADs) 5
2.1. First generation pulsatile flow LVADs 5
2.2. Second generation continuous-flow LVADs (axial-flow pumps) 5
2.2.1. HeartMate II (HMII; Abbott Laboratories, Lake Bluff, Illinois, USA) 5
2.2.2. Advantages and limitations 6
2.3. Third generation continuous-flow LVADs (centrifugal-flow pumps) 7
2.3.1. HeartMate 3 (HM3; Abbott Laboratories, Lake Bluff, Illinois, USA) 7 2.3.2. HeartWare HVAD (HVAD; Medtronic, Inc., Fridley, Minnesota, USA) 8
2.3.3. Advantages and limitations 9
2.4. Indications of CF-LVAD implantation 10
2.4.1. Bridge to transplantation (BTT) 10
2.4.2. Destination therapy (DT) 10
2.4.3. Bridge to decision 10
2.4.4. Bridge to recovery 10
3. Methodology 11
3.1. Purpose of the study 11
3.2. Literature review 11
3.3. Data analysis 11
4. Complications 11
4.1. Mortality rates 11
4.2. Thromboembolic and bleeding events 12
4.2.1. General picture of thromboembolic and bleeding events 12 4.2.2. Haemolysis and acquired von Willebrand syndrome (AvWS) 13
4.2.2.1. Haemolysis 13
4.2.2.2. Acquired von Willebrand Syndrome (AvWS) 14
4.2.3. Anticoagulation 15 4.2.4. Pump thrombosis 15 4.2.4.1. Pathophysiology 16 4.2.4.2. Epidemiology 16 4.2.4.3. Treatment 17 4.2.4.4. Risk factors 17
4.2.5. Transient ischaemic attack (TIA) or cerebrovascular accident (CVA) 18
4.2.5.1. Pathophysiology 18
4.2.5.2. Epidemiology 18
4.2.5.3. Treatment 20
4.2.5.4. Risk factors 20
4.2.6.1. Pathophysiology 21 4.2.6.2. Epidemiology 21 4.2.6.3. Treatment 22 4.2.6.4. Risk factors 23 4.3. Device dysfunction 23 4.4. Cardiac complications 23
4.4.1. Right ventricular failure (RVF) 23
4.4.1.1. Pathophysiology 24 4.4.1.2. Epidemiology 24 4.4.1.3. Treatment 25 4.4.1.4. Risk factors 25 4.4.2. Arrhythmias 25 4.4.2.1. Pathophysiology 26 4.4.2.2. Epidemiology 26 4.4.2.3. Treatment 27 4.4.2.4. Risk factors 27
4.4.3. Aortic insufficiency (AI) 27
4.4.3.1. Pathophysiology 27 4.4.3.2. Epidemiology 28 4.4.3.3. Treatment 28 4.4.3.4. Risk factors 28 4.5. Infection 29 4.5.1. Pathophysiology 29 4.5.2. Epidemiology 30 4.5.3. Treatment 31 4.5.4. Risk factors 31
4.6. Dysfunction of other organs 32
4.6.1. Renal dysfunction 32 4.6.1.1. Pathophysiology 32 4.6.1.2. Epidemiology 33 4.6.1.3. Treatment 33 4.6.1.4. Risk factors 33 4.6.2. Hepatic dysfunction 34 4.6.2.1. Pathophysiology 34 4.6.2.2. Epidemiology 34 4.6.2.3. Treatment 34 4.6.2.4. Risk factors 35 4.6.3. Respiratory dysfunction 35 4.6.3.1. Pathophysiology 35 4.6.3.2. Epidemiology 35 4.6.3.3. Treatment 36 4.6.3.4. Risk factors 36 4.7. Psychiatric morbidity 37 4.7.1. Pathophysiology 37 4.7.2. Epidemiology 37 4.7.3. Treatment 38 4.7.4. Risk factors 38
5. Discussion 41
5.1. Thromboembolic and bleeding events 41
5.2. Cardiac complications 42
5.3. Infection 43
5.4. Dysfunction of other organs 44
5.5. Psychiatric morbidity 46
5.6. Limitations and further recommendations 46
5.7. Conclusion 47
References 49
Abbreviations
AF Atrial fibrillation
AI Aortic insufficiency
AKI Acute kidney injury
AV Aortic valve
AvWS Acquired von Willebrand syndrome
BMI Body mass index
BTT Bridge to transplantation
CF-LVAD Continuous-flow left ventricular assist device
CO Cardiac output
CPB Cardiopulmonary bypass
CRP C-reactive protein
CVA Cerebrovascular accident
CVP Central venous pressure
DLCO Diffusing capacity of the lung for carbon monoxide
DT Destination therapy
EPPY Events per patient year
FEV1 Forced expiratory volume in 1 second
FVC Forced vital capacity
GIB Gastro-intestinal bleeding
HCVA Haemorrhagic cerebrovascular accident
HF Heart failure
HM3 HeartMate 3
HMII HeartMate II
HMWM High molecular weight multimer
HRAE Haemocompatibility-related adverse event
HTx Heart transplantation
HVAD HeartWare HVAD
IABP Intra-aortic balloon pump
ICD Implantable cardioverter-defibrillator
ICVA Ischaemic cerebrovascular accident
INR International normalized ratio
LDH Lactate dehydrogenase
LV Left ventricle
LVAD Left ventricular assist device
MCS Mechanical circulatory support
MPAP Mean pulmonary artery pressure
NYHA New York Heart Association
PAP Pulmonary artery pressure
PCWP Pulmonary capillary wedge pressure
PF-LVAD Pulsatile-flow left ventricular assist device
PVR Pulmonary vascular resistance
QoL Quality of life
RPM Revolutions per minute
RRT Renal replacement therapy
RV Right ventricle
RVAD Right ventricular assist device
RVF Right ventricular failure
SVA Supraventricular arrhythmia
TIA Transient ischaemic attack
TTE Transthoracic echocardiographic
VA Ventricular arrhythmia
0. Abstract
0.1. English version
Introduction:
Left ventricular assist devices (LVADs) are increasingly used as a treatment modality for end-stage heart failure (HF), refractory to medical therapy, due to the ongoing shortage of donor organs for heart transplantation (HTx). LVADs support the failing left ventricle (LV), and thereby maintain sufficient end-organ perfusion and ameliorate functional capacity. Improvements in LVAD technology have led to a shift from the pulsatile-flow LVADs (PF-LVADs) to the smaller, more durable, continuous-flow LVADs (CF-LVADs). Because the population of LVAD patients is growing and their life expectancy is increasing, a proper understanding of the principles, indications, and limitations of this treatment modality is necessary. Complications continue to constitute challenges and require insight of clinicals in their pathophysiology, epidemiology, treatment and risk factors.
Methods:
For the review of the literature on complications after CF-LVAD implantation, 85 articles from PubMed, Google Scholar and Embase were selected and reviewed. For the data analysis of Ghent University Hospital, data of 71 CF-LVAD patients, implanted as bridge to transplantation (BTT) with a HeartMate II (HMII), HeartMate 3 (HM3) or HeartWare HVAD (HVAD) between 7 December 2007 and 15 March 2019, were retrospectively studied and compared to the literature.
Results:
The literature review summarizes the current knowledge of the pathophysiology, epidemiology, treatment and risk factors of each complication occurring during LVAD support. Those complications are common and have both physiological and psychological consequences. Survival after 1 year and 2 years on LVAD support is 80% and 70%, respectively. Frequently occurring and important complications during LVAD support are thromboembolic events (pump thrombosis, transient ischaemic attack and cerebrovascular accident), bleeding events (gastro-intestinal and nasal), device dysfunction, cardiac complications (right ventricular failure, arrhythmias, and aortic insufficiency), infection (driveline infection, pocket infection and sepsis), dysfunction of other organs (renal, hepatic and respiratory dysfunction), and psychiatric morbidity. All complications found in the literature occurred in the LVAD population of Ghent University Hospital. Cardiac complications, renal dysfunction, infection, and thromboembolic and bleeding events were the most frequently occurring LVAD-related complications in this population. However, 17/71 patients did not experience any complications during LVAD support.
Conclusion:
Although technical design improvements have ameliorated the survival of LVAD patients, major adverse events are still complicating LVAD support. Meticulous management of anticoagulation and antiplatelet therapy is important in good management of LVAD patients. Identifying risk patients is needed in order to implement adequate strategies to prevent post-operative complications, especially when treatment options are limited. Further, patient education, early management and more standardized strategies are required. Lastly, more research on LVAD support and possible technical improvements is warranted to prevent these complications and to improve both physiological and psychological outcomes.
0.2. Dutch version
Introductie:
Steunharten (Left ventricular assist devices, LVADs) worden alsmaar meer gebruikt als behandeling voor eindstadium hartfalen (HF), refractair aan medische therapie, door een voortdurend tekort aan donororganen voor harttransplantatie (HTx). LVADs ondersteunen het falende linker ventrikel (LV), onderhouden daardoor voldoende eindorgaanperfusie en verbeteren de functionele capaciteit. Vooruitgang in LVAD-technologie heeft gezorgd voor een verschuiving van de pulsatiele flow LVADs (PF-LVADs) naar de kleinere, duurzamere, continue flow LVADs (CF-LVADs). Aangezien de populatie LVAD-patiënten groeit en hun levensverwachting toeneemt, is een goed begrip van de werking, indicaties en beperkingen van deze behandelingswijze noodzakelijk. Complicaties blijven een uitdaging vormen en vereisen inzicht van clinici in hun pathofysiologie, epidemiologie, behandeling en risicofactoren.
Methode:
Voor het overzicht van de literatuur over de complicaties na CF-LVAD-implantatie werden 85 artikels van PubMed, Google Scholar and Embase geselecteerd en beoordeeld. Voor de beschrijving van de data van het Universitair Ziekenhuis Gent, werden data van 71 CF-LVAD-patiënten, geïmplanteerd ter overbrugging naar een harttransplantatie (bridge to transplantation, BTT) met een HeartMate II (HMII), HeartMate 3 (HM3) of HeartWare HVAD (HVAD) tussen 7 december 2007 en 15 maart 2019, retrospectief bestudeerd en vergeleken met de literatuur.
Resultaten:
Het overzicht van de literatuur vat de huidige kennis van de pathofysiologie, epidemiologie, behandeling en risicofactoren van elke complicatie optredend tijdens LVAD-ondersteuning samen. Deze complicaties zijn vaak voorkomend en hebben zowel fysieke als psychologische gevolgen. De overleving na 1 jaar en 2 jaren LVAD-ondersteuning is respectievelijk 80% en
70%. Frequent optredende en belangrijke complicaties tijdens LVAD-ondersteuning zijn trombo-embolische complicaties (pomptrombose, transiënte ischemische aanval, cerebrovasculair accident), bloedingscomplicaties (gastro-intestinaal en nasaal), dysfunctie van het toestel, cardiale complicaties (rechter ventrikelfalen, aritmieën en aortaklepinsufficiëntie), infectie (aandrijflijninfectie, pocketinfectie en sepsis), disfunctie van andere organen (renale, hepatische en respiratoire disfunctie) en psychiatrische morbiditeit. Alle complicaties gevonden in de literatuur kwamen voor in de LVAD-populatie van het Universitair Ziekenhuis Gent. Cardiale complicaties, renale disfunctie, infectie en trombo-embolische en bloedingscomplicaties waren de meest frequent optredende LVAD-gerelateerde complicaties in deze populatie. Echter, 17/71 patiënten maakten geen enkele complicatie door gedurende de LVAD-ondersteuning.
Conclusie:
Ondanks dat de technische verbeteringen aan het ontwerp de overleving van LVAD-patiënten hebben verbeterd, wordt LVAD-ondersteuning nog steeds bemoeilijkt door ernstige complicaties. Nauwgezet management van anticoagulatie en antiplaatjes therapie is belangrijk voor goed management van de LVAD-patiënten. Het identificeren van risicopatiënten is noodzakelijk om adequate strategieën te implementeren om postoperatieve complicatie te voorkomen, vooral wanneer de behandelingsopties beperkt zijn. Verder zijn patiënteneducatie, vroeg management en meer gestandaardiseerde strategieën vereist. Tot slot, meer onderzoek naar LVAD-ondersteuning en mogelijke technische verbeteringen is nodig om deze complicaties te voorkomen en zowel de fysieke als psychologische uitkomsten te verbeteren.
1. Introduction
Heart failure (HF) is a worldwide rising problem with an important mortality, morbidity and health care cost. The number of people afflicted with HF is increasing and considering the aging population, this increase is likely to continue (1-3). Chronic HF is a complex syndrome resulting from cardiac disorders changing either the structure or the functioning of the heart. These changes lead to an impairment of the pump function of the heart, which negatively affects the physiological circulation (4).
Clinical features of HF are represented in the New York Heart Association (NYHA) classification (Table 1) of HF. The NYHA classification is used to assess the severity of HF in patients and is based on symptoms and limitation of exercise capacity. Prognosis of HF deteriorates from class I to class IV (4).
Table 1: New York Heart Association classification (NYHA) of heart failure Class Features
Class I No limitation. Normal physical activity does not cause dyspnoea, fatigue or palpitations.
Class II Mild limitation. Comfortable at rest but normal physical activity causes dyspnoea, fatigue or palpitations.
Class III Marked limitation. Comfortable at rest but less than ordinary physical activity causes marked symptoms of heart failure.
Class IV Symptoms of heart failure occur at rest and discomfort is increased by any physical activity.
According to the Rotterdam Study of S. Bleumink et al., incidence rate of HF is 14.4/1000 person-years (95% CI 13.4-15.5) and increases with age (2). Thanksto evidence-based medical therapy, the prognosis of HF has improved over the past decade, but the mortality rate still remains high, with 1-year and 5-year mortality rates of 30% and 60%, respectively (3).
Heart transplantation (HTx) is a highly effective therapy for end-stage HF, but its availability is limited due to shortage of donor organs. Therefore, left ventricular assist devices (LVADs) are becoming an increasingly important treatment modality for patients suffering end-stage HF, refractory to maximal medical therapy. (1, 5-10).
LVADs are mechanical assist devices developed to support the failing left ventricle (LV) in those patients. They are used to maintain sufficient end-organ perfusion and to ameliorate functional capacity. Because of this, the number of LVAD implantations has increased over the past decades, especially for use as bridge to transplantation (BTT) or as destination therapy (DT) (1, 7-12). However, DT is not an indication for LVAD implantation in Belgium. The different indications of LVAD implantation will be discussed later.
Despite the promising continuous-flow technology, the devices must meet safety and efficacy requirements of a large-scale primary treatment of NYHA class III or class IV HF patients (13). Although most patients with an LVAD have extended survival and ameliorated quality of life, complications do occur during LVAD support (1, 7, 9-12). Adverse events such as stroke, infection, gastro-intestinal bleeding, right heart failure and pump thrombosis are associated with higher mortality rates and significant morbidity (1, 7, 9, 14,15). Assessment of these complications forms the main part of this thesis.
First generation LVADs consisted of pulsatile-flow LVADs (PF-LVADs), but persistent complications related to the size and durability of the devices have limited the use of this design. Improvements in LVAD technology have led to the development of smaller, more durable, continuous-flow LVADs (CF-LVADs) such as HeartMate II (HMII; Abbott Laboratories,
Lake Bluff, Illinois, USA), HeartMate 3 (HM3; Abbott Laboratories, Lake Bluff, Illinois, USA) and HeartWare HVAD (HVAD; Medtronic, Inc., Fridley, Minnesota, USA) (12, 13, 15, 16).
The CF-LVADs may last >10 years in a patient. Current studies show 1- and 2-year survival after LVAD implantation of 80% and 70%, respectively (17). Given that both the number of patients implanted with LVADs and their life expectancy are increasing, a proper understanding of the principles, indications and limitations of this treatment modality is important. Adverse events, including infection, bleeding and stroke continue to constitute challenges (12, 14, 16). The evolution, principles of operations and use of different LVADs will be discussed in this section, focussing on HMII, HM3 and HVAD.
2. Overview of left ventricular assist systems (LVADs)
2.1. First generation pulsatile-flow LVADs
The first generation LVADs consisted of PF-LVADs designed to functionally resemble the native LV. As a result, the PF-LVADs could produce flow rate, stroke volume and LV dP/dt within the normal physiologic range. Survival rates and quality of life of patients suffering advanced HF enhanced after PF-LVAD implantation (13).
However, mechanical durability was limited due to the wear of moving parts in the design. Together with the large size and the association with persistent complications, this confined the application of PF-LVADs in a wide range of patients and required new designs. Continuous-flow blood pumps using axial- or centrifugal-flow have been developed for augmented durability, smaller size and reduced adverse events. Therefore, they are useful in a broader population of patients than the earlier pulsatile devices (12, 13, 15, 16).
2.2. Second generation continuous-flow LVADs (axial-flow pumps)
2.2.1. HeartMate II (HMII; Abbott Laboratories, Lake Bluff, Illinois, USA)
The HMII (Figure 1) is a CF-LVAD which provides circulatory support in patients with HF (10, 11, 14, 19). With over 22.000 patients worldwide, the HMII has been the most frequently implanted LVAD (14). Its titanium axial-flow pump (Figure 2) receives blood from the left ventricular apex through a flexible inflow cannula and delivers it through an outflow cannula to the ascending aorta (10, 11, 19). The HMII weighs 390 grams and is implanted through a median sternotomy in a small preperitoneal pocket at the level of the diaphragm (11, 19, 20).
Figure 1 (left): Main components of the HeartMate II. (Abbott Laboratories, Lake Bluff, Illinois, USA) Figure 2 (right): The internal components of the axial continuous-flow HeartMate II pump. (Abbott Laboratories, Lake Bluff, Illinois, USA)
In contrast to PF-LVADs, the HMII has only one moving part: an internal rotor that is suspended by contact bearings. The HMII can provide flow up to 10 L/min. Blood enters the pump trough the inflow cannula and is straightened by the inlet stator before entering the path of the rotor. The rotor spins the blood radially and drives it through the outlet stator into the outflow cannula. The pump runs from 8,000 to 10,000 revolutions per minute (RPM). Both the inflow and outflow cannula have sintered surfaces reducing thrombogenicity. However, anticoagulation remains necessary (19).
The external components of the HMII include a flexible percutaneous lead connecting the pump to an external system driver and power source (1, 19, 20). The external system controller operates and monitors the pump function. This is connected to power sources, including two batteries worn in holsters to enable the patient to resume normal daily activities as much as possible (19, 20).
2.2.2. Advantages and limitations
The second-generation devices offer several advantages over pulsatile-flow devices. The size is reduced, mostly due to the elimination of the reservoir needed in PF-LVADs. The smaller size reduces the risk of infections, simplifies the implantation of the LVAD and makes this technology more applicable for a wider range of patients (13, 15).
Simplicity and durability are enhanced by the reduction of moving parts and smaller blood-contacting surfaces. Absence of valves to direct blood flow and decreased energy requirements also contribute to this. Moreover, the second-generation CF-LVADs produce less device noise, improving quality of life (20).
Some important limitations of this design are thromboembolic events, infection and right ventricular failure (19). Complications occurring with the HMII will be discussed later.
2.3. Third generation continuous-flow LVADs (centrifugal-flow pumps)
2.3.1. HeartMate 3 (HM3; Abbott Laboratories, Lake Bluff, Illinois, USA)
The HM3 LVAD consists of a centrifugal-flow pump surgically positioned in the pericardial space (Figure 3). It receives blood from the LV entering the inflow cannula. Blood is pumped along a central axis and driven out at right angles through the impeller blades of a centrifugal rotating rotor. After the blood is angularly accelerated and driven around a volute, it is directed tangentially into the outflow cannula. The pressure and flow rate can be adjusted (12, 16).
Figure 3 (left): The main components of the HeartMate 3. (Abbott Laboratories, Lake Bluff, Illinois, USA) Figure 4 (right): The internal components of the centrifugal continuous-flow HeartMate 3 pump. (Abbott Laboratories, Lake Bluff, Illinois, USA)
In contrast to the HMII, the HM3 has a fully magnetically levitated rotor (Figure 4), which results in frictionless movement reducing wear of the moving parts and heat production (12). The space between the rotor and the housing is kept as large as possible, creating a wide blood flow gap (12, 15, 16). This enables blood flow with less shear stress and improves haemocompatibility, which is essential for improvement in survival and quality of life (16).
The operating speed of the HM3 ranges from 3,000 to 9,000 RPM and a maximum flow rate of 10 L/min can be generated. A principal characteristic of the HM3 is the creation of an artificial pulse: every 2 seconds, the rotor speed is decreased by 2,000 RPM for 0.15 seconds, followed by an increase of 4,000 RPM for 0.20 seconds before returning to set-speed (12, 16). The generation of a pulsatile flow is used to reduce the risk of thrombus formation (12, 15, 16).
Another way to reduce thrombogenicity are textured blood-contacting surfaces (12, 15, 16). The internal surfaces of the HM3 are covered with titanium microspheres that ensure adhesion of the patient’s own cells, creating a tissue lining similar to a blood vessel. Therefore, the internal walls are smoother and less thrombogenic (12, 16).
The power cable is externalized as a percutaneous cable and is coupled to a modular cable (1, 12, 15). The latter is connected to the external system controller and a pair of 14V lithium-ion batteries or external AC power sources. Except for the system controller, the external parts are identical to the previous generation, HMII (12, 15).
2.3.2. HeartWare HVAD (HVAD; Medtronic, Inc., Fridley, Minnesota, USA)
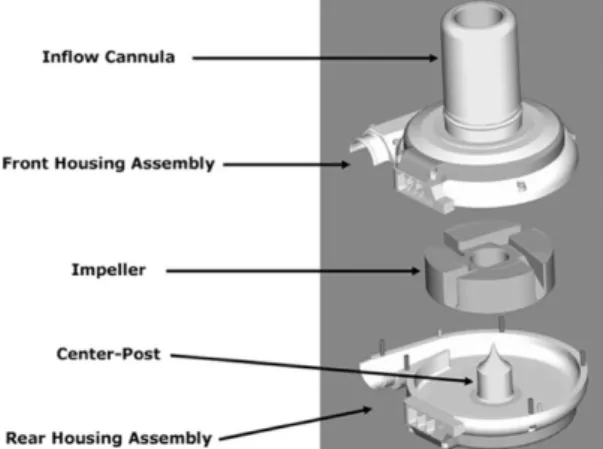
HeartWare HVAD is another CF-LVAD with a centrifugal-flow pump implanted in the pericardial space. With a 50 mL volume and a 145 g weight, it is smaller than the HMII and HM3 and suitable for patients with smaller body types (3, 13). The HVAD consists of three parts (Figure
5): 1) a front housing assembly with an inflow cannula, 2) a wide-blade rotating impeller, and
3) a rear housing assembly with a magnetic center-post (13).
Figure5: The main components of the HeartWare HVAD. (Medtronic, Inc., Fridley, Minnesota, USA)
Left ventricular blood is drawn into the pump through the inflow cannula. The rear housing is connected to an outflow cannula leading to the ascending aorta, through which blood is forced by an impeller. The wide-blade impeller is suspended using passive magnetic levitation and hydrodynamic thrust bearings. This design provides optimal blood flow paths through the system and decreases shear stress. This creates a contact-free rotation of the impeller, eliminating component wear (3, 13).
Permanent motor magnets are enclosed in the impeller and the front and rear housing both contain a motor stator. The motor stators create an electromagnetic force moving the motor magnets in the impeller around an axis of rotation. The dual stator system augments the reliability and efficiency of the system. The impeller rotational speed ranges from 1,800 to 4,000 RPM and maximum flow generated by the HVAD is 10L/min (13). Every minute, the impeller speed decreases with 200 RPM for 2 seconds and then increases with 400 RPM for 1 second before returning to set-speed. This is called the Lavare cycle, which is designed to increase both ventricular and pump washing (3, 13).
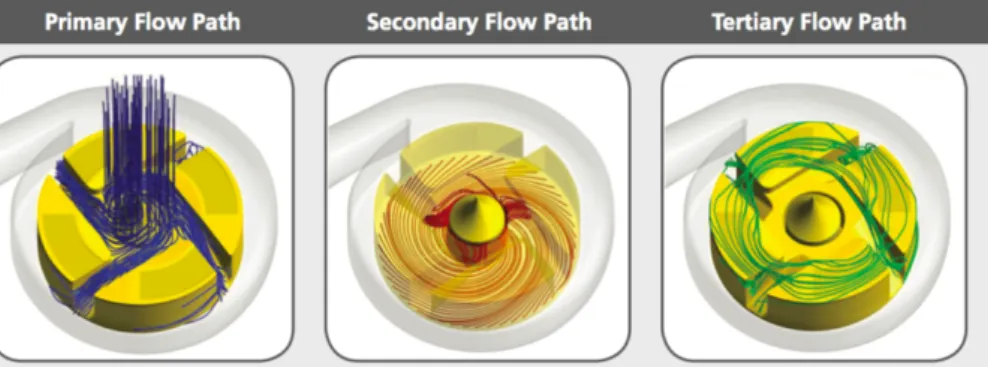
In the HVAD design, three different blood flow paths are created (Figure 6). Following the primary flow path, blood flows through the inflow cannula into four impeller flow channels
and is collected in the housing before leaving through the outflow cannula. Blood in the secondary flow path flows from beneath the impeller, upward through the space between the impeller and the center-post to merge with the primary flow path. The tertiary flow path, starting from the impeller slots and flowing along the hydrodynamic thrust bearings on top of the impeller, also merges with the primary flow path (13).
Figure 6: Different flow paths in the HeartWare HVAD. (Medtronic, Inc., Fridley, Minnesota, USA)
The connection between the pump and the external power sources and control components is formed by a flexible driveline that is externalised through the abdominal wall. The controller controls pump function and provides power regulation to the dual motors of the continuous-flow pump. The system monitor can be used to survey pump function and to adjust pump operation parameters. The pump receives power from either AC or DC sources (3, 13).
2.3.3. Advantages and limitations
The third generation relies on centrifugal continuous-flow instead of axial continuous-flow. Whereas the rotor of the HMII still needs contact bearings for its suspension, the HM3 rotor is fully magnetically levitated. This results in less component wear compared to the HMII (12, 15, 16). In the HVAD, the impeller also rotates contact-free, which enhances the device durability (3, 13). The wide blood flow gap created in the HM3 and the three different blood flow paths within the HVAD improve haemocompatibility (13, 15).
Furthermore, through washout, the generation of an artificial pulse in the HM3 contributes to the reduction of thrombus formation (12, 15, 16). In the HM3 design, this artificial pulse is also intended to reduce angiodysplasia (25). The changes in impeller speed in the HVAD are also used to decrease thrombogenicity (13).
Moreover, the HM3 and HVAD are implanted in the pericardial space, whereas the HMII is implanted in a preperitoneal pocket (11-13, 15, 16). Hence, with the HM3 and HVAD, there is no need for abdominal surgery and creation of a pump pocket (15). In addition, the HVAD is smaller than the HMII and HM3, making it more applicable for smaller patients. Its dual stators system enhances device efficiency and reliability (13).
Despite improvements in LVAD technology, the third generation also has its limitations. For example, bleedings, arrhythmias and infection can occur (12, 15). The limitations and complications of the HM3 and HVAD will be discussed in the section ‘4. Complications’.
2.4. Indications of CF-LVAD implantation
2.4.1. Bridge to transplantation (BTT)
Bridge to transplantation (BTT) is an important indication for CF-LVAD implantation. Considering the shortage of available donor organs for HTx, CF-LVADs are increasingly used as a temporary supportive therapy for patients on the HTx waiting list (5, 8, 14, 21).
According to the eighth annual report of the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS), a database including more than 20,000 patients from 185 hospitals, 26% of the implantations were BTT. About 30% of the BTT implanted patients underwent HTx within one year (17). The study of Magruder et al. showed 1-year survival exceeding 89% after orthotopic HTx after BTT with HMII or HVAD (5).
2.4.2. Destination therapy (DT)
LVADs can be used as a destination therapy (DT) in patients not eligible for HTx. The CF-LVAD is then used as an alternative for HTx (8, 9, 14, 21). The eighth INTERMACS report showed that over the past decade, DT increased as indication for LVAD implantation. By 2015, this strategy accounted for almost 50% of implantations (17).
2.4.3. Bridge to decision
Another indication for LVAD implantation is bridge to decision. Some patients who currently do not meet the criteria for a HTx, can become eligible for a HTx in the future by implanting an LVAD (8). Showed in the eighth annual INTERMACS report, bridge to decision comprised 23% of all LVAD implantations. 20% of the bridge to decision patients end up on the waiting list and receive a HTx within the first year after LVAD implantation (17).
2.4.4. Bridge to recovery
CF-LVAD implantation as bridge to recovery is indicated in patients with acute decompensated HF, typically due to a reversible cause. The implantation is used to endure the acute period of HF and to recover from it. This indication accounts only for a small proportion of the LVAD implantations (14, 21).
3. Methodology
3.1. Purpose of the study
The number of patients implanted with LVADs and their life expectancy increased over the last decades and will continue to increase. However, adverse events during LVAD support are prevalent and impact LVAD outcomes and quality of life. Therefore, a better understanding of these complications, their pathophysiology, epidemiology, risk factors and current treatments is needed and will be discussed in this study.
3.2. Literature review
For the review of the literature on complications after CF-LVAD implantation, articles were found on PubMed, Google Scholar and Embase. Search terms such as ‘LVAD’, ‘complications’, ‘adverse events’, ‘pump thrombosis’, ‘stroke’, ‘gastro-intestinal bleeding’, ‘right heart failure’, ‘arrhythmias’, ‘aortic insufficiency’, ‘infection’, ‘renal dysfunction’, ‘hepatic dysfunction’, ‘respiratory dysfunction’, ‘psychological health’ and ‘quality of life’ were used. Articles were evaluated on title, abstract and full text. The selection of 85 articles for this literature review was based on relevance, publication date, quality of the journal, study population, type of the device and implant strategy.
3.3. Data analysis
This retrospective study was conducted at Ghent University Hospital. Data of 71 CF-LVAD patients from the cardiac surgery department of Ghent University Hospital were retrospectively analysed after informed patient consent and approval of the Commission for medical ethics of Ghent University Hospital. The 71 patients were implanted as BTT with a HMII, HM3 or HVAD between 7 December 2007 and 15 March 2019. Data were collected from the electronic patient files and echocardiographic images and were compared to the literature.
4. Complications
4.1. Mortality rates
Over the last decade, survival after CF-LVAD implantation has improved significantly. According to the INTERMACS registry, collecting data on all FDA-approved LVADs implanted in the USA, overall survival after 1 year, 2 and 3 years is respectively 80%, 70% and 59%. 31% of patients implanted with an LVAD as BTT in 2013-2014 received a HTx within 1 year, and 50% by 2 years (22).
The second report of the European EUROMACS registry showed a survival rate in 2113 CF-LVAD patients, either as BTT or as DT, of 88%, 69%, 55% and 44% at respectively 30 days, 1 year, 2 years and 3 years (23).
Moreover, the study of Parameshwar et al. showed that in a population of 112 patients implanted with HMII and 230 patients implanted with HVAD, LVAD support allowed a quarter of this population to receive a HTx within 3 years. Overall survival at 3 years from LVAD implantation was 49.6%, whereas survival free of urgent listing for HTx or pump exchange was 34%. This reflects the frequency of serious complications related to LVAD support (24).
Further, Parameshwar et al. stated that mortality and complications did not differ significantly between HMII and HVAD, indicating that the problems are rather related to the nature of CF-LVAD support than to the design of the device. The nature of CF-LVAD support includes the need for anticoagulation and antiplatelet therapy, and the presence of a foreign body linked to an external driveline (24).
The risk factors for early mortality after LVAD implantation include female gender, advancing age, obesity, INTERMACS 1-2, renal dysfunction, elevated bilirubin and previous cardiac surgery or need for concomitant cardiac surgery (22).
4.2. Thromboembolic and bleeding events
Haemocompatibility represents critical interactions of foreign material with circulating blood elements which may cause adverse events. The LVAD interface disturbs the pathophysiology of activation or destruction of the blood elements. Foremost, this is manifested clinically as thromboembolic (including pump thrombosis, transient ischaemic attack, or cerebrovascular accident, and other systemic embolic complications) and nonsurgical bleeding events (mainly gastro-intestinal or nasal) (25-27).
In the section below, a general picture of thromboembolic and bleeding events will be outlined, after which anticoagulation will be discussed. Thereafter, an elaboration of some complications will be provided.
4.2.1. General picture of thromboembolic and bleeding events
Rheologic changes associated with CF-LVADs are associated with an increased risk of thromboembolic and bleeding complications (25). Among different studies of axial and centrifugal CF-LVADs, the incidence of thromboembolic events varies between 1 and 14% (28). Further, continuous blood flow may cause formation of arteriovenous malformations, especially in the gastro-intestinal tract, which may predispose to bleeding (25). Current data show bleeding events in 10% to 30% patients after LVAD implantation (17, 25).
During the early phase, surgical bleeding predominates, whereas gastro-intestinal bleeding (GIB) becomes dominant after the first 3 months (22). Stroke and pump thrombosis persist as serious complications during the first year post-implantation (22, 29). According to the EUROMACS registry, the rates of neurological and bleeding events continue to decline during the entire follow-up period after LVAD implantation (23). GIB, stroke and pump thrombosis will be discussed below.
Furthermore, Uriel et al. studied haemocompatibility-related outcomes in the MOMENTUM trial, comparing HM3 with HMII, at 6 months. Survival free of haemocompatibility-related adverse events (HRAEs; nonsurgical bleeding, thromboembolic event, pump thrombosis, or neurological event) was significantly better in HM3 than HMII patients (69±4% versus 55±4%; hazard ratio, 0.62; 95% CI, 0.42–0.91; P=0.012). Moreover, HM3 was predominantly associated with bleeding events, whereas occurrence of thromboembolic and bleeding events was equally among HMII patients (26).
In addition, independent risk factors for HRAE at 6 months after implantation are HMII LVADs, advanced age, lower international normalized ratio (INR) (<1.5) at 30 days and absence of aspirin at 30 days. Therapeutic intent (BTT or DT) and mean arterial pressure (MAP) did not appear to significantly influence the risk of HRAE at 6 months (26).
Further, Meyer et al. observed no higher rate of bleeding events among HVAD patients, despite a more intense anticoagulation regimen by adding a platelet inhibitor. Sintering the titanium inflow cannula of the HVAD to stimulate the growth of a pseudo-intima, may prevent clotting and thereby potential thromboembolic events (30).
4.2.2. Haemolysis and acquired von Willebrand syndrome (AvWS)
The cause of thromboembolic and bleeding events after LVAD implantation is multifactorial. Continuous blood flow and low pulsatility can disturb the normal circulation by increasing circulatory shear stress and thereby causing rheologic changes. Haemolysis, due to deformation of circulating red blood cells occurs (25, 31). Also, shear stress in CF-LVADs is associated with acquired von Willebrand Syndrome (AvWS) (25, 30).
4.2.2.1. Haemolysis
Haemolysis is defined by INTERMACS as a plasma free haemoglobin > 40 mg/dL associated with clinical signs of haemolysis after 72h post-implantation (29, 31, 32). Haemolysis can clinically result in haemolytic anaemia, lactate dehydrogenase (LDH) elevation and undetectable haptoglobin. Jaundice and dark urine can occur due to hyperbilirubinemia (28, 31). Thromboembolic events in HMII patients, including pump thrombosis, are associated with
more than fourfold higher LDH levels (28). Starling et al. also reported an increase in LDH preceding pump thrombosis in HMII LVADs (27).
Haemolysis shows a strong association with partial or complete pump thrombosis, elevated pump power and decreased pulsatility index, especially in context of infections. Akin et al. reported a more than threefold LDH increase associated with infection in patients experiencing a thromboembolic event or pump thrombosis (28). Also, lower INRs values were registered among patients with haemolysis. Ravichandran et al. suggested that higher INR targets in HMII patients may lower pump-associated thrombus formation (31). This will be discussed in a further section.
After the diagnosis of haemolysis, antithrombotic and/or antiplatelet administration are timely intensified to prevent thromboembolic events and pump thrombosis. Sometimes, pump exchange, urgent listing for HTx, or pump explant are used, when haemolysis is not reversible (28, 31).
4.2.2.2. Acquired von Willebrand Syndrome (AvWS)
In AvWS, loss of high molecular weight multimers (HMWMs) of the von Willebrand factor (vWF) is identified, which play a key role in primary haemostasis because of their interactions with platelets. (25, 30).
In a retrospective study of Meyer et al. of 102 LVADs, either HMII or HVAD, AvWS was present in both HMII and HVAD patients. The extent to which the HMWMs of vWF declined was similar among patients in both groups. In this study, the vWF profile also did not correlate with the incidence of thromboembolic or bleeding complications (30). In addition, in the ENDURANCE trial, comparing HVAD with HMII in DT patients, the rates of major bleeding were similar between both groups (33).
On the contrary, the HM3 has less tendency to cause vWF deficiency than the HMII (25). The HM3 is designed to enhance haemocompatibility and washout, through its fully magnetically levitated rotor, wide blood flow gaps and artificial pulse (25, 26). Considering the similar degree of residual HMWMs in HVAD patients compared with HMII patients, it is more likely that the differences observed in HMWMs in HM3 patients compared with HMII patients, are induced by the unique design characteristics of the HM3 than by the continuous-flow characteristics of the devices (25).
In addition, presence of any aortic valve (AV) opening is associated with reduced vWF degradation. It is thought that maintaining some degree of AV opening could reduce complications such as gastro-intestinal bleedings (25).
Also, it has been suggested that a shear force of 500 pN is the minimum force needed to unfold the HMWMs and reveal the cleavage sites. Consequently, a possible explanation for
average RPM speeds (25). According to Meyer et al., lowering speed in HVAD patients may have decreased the loss of HMWMs, whereas in HMII patients, speed did not appear to influence the vWF profile. This study also showed that vWF is influenced by age (p=0.011), C-reactive protein (CRP) (p<0.001), blood type (p=0.007) and number of days on the device (p=0.035) (30).
4.2.3. Anticoagulation
Anticoagulation regimens play an important role in the prevention and creation of thromboembolic and bleeding events. By advancing from the pulsatile-flow devices to the continuous-flow devices, a decrease in the frequency of thromboembolic events was observed with most devices. However, the frequency of bleeding events remained high. In order to prevent these events, anticoagulation targets were lowered (19, 20).
Slaughter et al. demonstrated in HMII patients that warfarin and aspirin therapy in the immediate post-operative period without bridging with intravenous heparin did not increase the short-term risk of thromboembolic events. On the other hand, this significantly decreased the risk of bleeding. Moreover, post-operative heparin administration, low post-operative platelet level and low baseline haematocrit value were found to be independent risk factors for bleeding between 3 and 30 days after the operation (34).
Furthermore, according to Boyle et al., a low number of thrombotic events appeared to be counterbalanced by a higher rate of haemorrhagic complications, especially in patients with higher INRs. A lower INR target of 1.5 to 2.5 in addition to aspirin therapy seems to be a good universal target to reduce the risk of both haemorrhagic and thromboembolic events (35).
However, lowering the INR target in order to lower the bleeding risk, combined with lowering speeds of CF-LVADs to allow intermittent AV opening and increased pulsatility, may result in more thrombotic events (27, 36). Also, patients who experienced a GIB had a significantly 7.4-fold increased risk of subsequent thromboembolic events. It is hypothesized that reducing anticoagulation and antiplatelet administration as a treatment for GIBs, may play a role in increasing this risk (36).
4.2.4. Pump thrombosis
Pump thrombosis is defined as the presence of otherwise unexplained HF with signs of device dysfunction and haemolysis (28). LVAD patients experiencing pump thrombosis, show increased mortality and morbidity unless pump replacement or HTx are carried out (27). Underlying mechanisms of pump thrombosis can vary from slow thrombus formation in the pump itself to fast ingestion of a pre-existing clot (38).
4.2.4.1. Pathophysiology
According to Starling et al., a possible mechanism is that of bearing-fibrin deposition: because of heat generation, deposition of fibrin and denatured protein can occur around the inflow bearing. The inflow path is narrowed, which increases shear stress on the red cells. Sometimes, the deposition is large enough to reduce the ability of the pump to unload the LV. It is theorized that in the initial stage, haemolysis is developing with beginning thrombus deposition, but no haemodynamic consequences. This may evolve to incomplete thrombosis with haemolysis and abnormal pump function. And eventually, progress to complete thrombosis and pump stoppage may occur (27).
So, any perturbation impairing flow and heat dissipation from the bearing, or inadequate anticoagulation, might contribute to the deposition. Some clinical factors important to consider in this context are: aortic insufficiency (AI), kinking of the inflow cannula, arrhythmias and hypovolemia (27).
4.2.4.2. Epidemiology
In the INTERMACS registry, pump thrombosis rate was 5%, 7% and 11% at 6, 12 and 24 months after HMII implantation, respectively. The hazard function (instantaneous rate) of pump thrombosis was at its highest at 2 months and rapidly declined to a constant hazard rate at about 9 months post-implantation. An increasing risk of pump thrombosis with the HMII was observed from 2009 through 2013. This increase has been reversed in the first half of 2014, probably because of more intense anticoagulation management, more awareness of optimal pump implantation and positioning among surgeons, and pump speed management (29).
In the ADVANCE BTT and Continued Access Protocol trial, pump thrombosis occurred in 8.1% of the patients with HVAD implant for a rate of 0.08 events per patient year (EPPY) (39). Parameshwar et al. showed pump thrombosis in 10.5% of 342 BTT patients supported with either HMII (7.1%; 0.36 per 100 patient months of support) or HVAD (12.2%; 0.58 per 100 patient months of support), with no difference in occurrence between the devices p=0.26) (24).
However, the engineering and type of LVAD may still be a contributing factor in predisposing to pump thrombosis: the incidence of pump thrombosis may be significantly reduced with HM3 support compared to HMII support. For example, Mehra et al. showed in a trial with 366 patients, 1.1% pump thrombosis in HM3 patients at 2 years after implantation, compared with 15.7% in HMII patients (hazard ratio: 0.06; 95% CI: 0.01 to 0.26; p<0.001) (38). Furthermore, comparing HM3 with HMII, less pump thrombosis requiring reoperation or medical therapy was observed at 6 months among HM3 patients (40).
These differences can be explained by changes in pump design, made to prevent thrombus formation within the device itself. The fully magnetically levitated rotor and artificial
pulse creation of the HM3 design are intended to result in less shear stress and in washout of possible thrombi (38).
However, in this HM3 trial, two cases of suspected pump thrombosis were observed. Thrombus formation outside the pump, for example in the atrial appendage or ventricular cavity, can be a possible explanation for these events when the thrombus is ingested and entrapped in the device. Stroke can occur when the thrombus passes unobstructed through the device (38).
4.2.4.3. Treatment
Medical therapy includes anticoagulation, antiplatelet, and thrombolytic therapy. Surgical therapy consists of LVAD exchange, HTx or LVAD explant. It is important to repeat that use of intensified anticoagulation and antiplatelet therapy is associated with a higher risk of bleeding (27).
Starling et al. noted a mortality rate of 50% among HMII patients treated with medical therapy after pump thrombosis. On the contrary, pump exchange or HTx resulted in mortality rates at 6 months similar to those of patients who did not experience pump thrombosis (27). Mortality rates of 50% after medical treatment were also found for HVAD patients (39).
Currently, LVAD exchange is the most applied treatment for pump thrombosis. Nevertheless, this procedure carries high risk of re-thrombosis, stroke and postoperative dialysis: 34%, 29% and 29%, respectively, according to Hanke et al. (42). Moreover, device replacement for thrombosis showed a subsequent 1-year mortality of 30% (29).
4.2.4.4. Risk factors
Younger age, higher Body Mass Index (BMI), history of non-compliance, severe right heart failure and elevated LDH during the first month after implantation were noted as risk factors for pump thrombosis (29). Identification of elevated LDH levels during the first month post-implant can be useful as clinical biomarker of haemolysis associated with pump thrombosis. This provides an opportunity for early intervention strategies (27, 29).
Moreover, the list of risk factors was expanded after analysis of the ADVANCE BTT and Continued Access Protocol trial of the HVAD with a MAP > 90 mmHg, aspirin dose < 81 mg, INR < 2 and INTERMACS profile level of ³ 3 (39). Consequently, according to the PREVENT multicentre study of HMII, in prevention of pump thrombosis are important: controlling MAP < 90 mmHg, heparin bridging after implantation, aspirin therapy (81-325 mg/day) along with warfarin (INR target of 2-2.5) and pump speed > 9000 RPM (41).
However, as stated in section 4.2.3., Slaughter et al. demonstrated in HMII patients that omitting heparin bridging in the immediate post-operative anticoagulation management did not increase the short-term risk of thromboembolic events (34).
4.2.5. Transient ischaemic attack (TIA) or cerebrovascular accident (CVA)
Neurological complications associated with LVAD implantation are transient ischaemic attacks (TIAs), and cerebrovascular accidents (CVAs). CVAs can be ischaemic (ICVA) or haemorrhagic (HCVA). Cerebrovascular complications can be seriously disabling or even fatal. They often have a dramatically negative impact on the quality of life and transplant candidacy (29, 43-45). According to Acharya et al., transplant rates are halved among patients experiencing stroke compared to patients not experiencing stroke (44).
4.2.5.1. Pathophysiology
The pathophysiology of strokes during LVAD support is not completely elucidated. Stroke risk is largely associated with the intrinsic thrombogenicity of LVADs. Shear stress, activation of the coagulation system and consequent thromboembolism are important factors, but it is difficult to detect the exact origin of the embolism in each patient. Emboli can originate from the LA, LV, LVAD, aortic root, or aortic atheromas (37, 44).
The likelihood of ischaemic stroke is increased by endothelial dysfunction, defined by high vWF and lower vWF cleaving protease ADAMTS13 (44). It is not known whether there are changes in cerebrovascular endothelial structure and function associated with continuous-flow devices that may contribute to development of haemorrhagic strokes (37, 44).
4.2.5.2. Epidemiology
INTERMACS data analysis showed that 10,57% LVAD patients had at least 1 stroke, with an incidence rate of all types of stroke of 0.123 EPPY (29, 44). Harvey et al. showed a stroke rate of 17% or 0.064 EPPY in a single-center retrospective analysis of 230 HMII patients (37). The distribution of stroke rates is almost even between ischaemic and haemorrhagic strokes (37, 44). Tahsili-Fahadan et al. described similar results, with an overall incidence of stroke of 19%. In this study, 49% of the events were ischaemic, 37% haemorrhagic and 14% combined (45).
In the study of Harvey et al., stroke-free rate after HMII implantation at 6 months, 12 months and 24 months, was 92.6%, 89.6% and 86.1%, respectively. Also, patients experiencing stroke had a mortality twice as high as stroke-free patients (37). According to the 7th Annual Report of INTERMACS, stroke dominates as the major cause of death between 6
In addition, haemorrhagic stroke results in worse survival than ischaemic stroke. Patient survival after the first ischaemic stroke compared with the first haemorrhagic stroke was 80.7% vs. 45.3% at 1 month, 65.8% vs. 34.8% at 6 months, and 52.6% vs. 30.3% at 1 year. Also, 13% of patients with a first stroke experienced a second stroke. The risk of a second stroke was higher among patients with an initial haemorrhagic stroke compared to patients with an initial ischaemic stroke (44).
Another important factor to consider is that patients with advanced HF have an intrinsic risk of thromboembolic stroke, independent of the presence of an LVAD, around 1.3% to 3.5% (35, 37).
Furthermore, it is not clear which role device design and strategy play in developing stroke. For example, the study of Parameshwar et al. showed an overall proportion of patients with a neurological complication of 30%, with no difference between the HMII and HVAD (24). Meyer et al. also found statistically similar rates of TIA as well as ischaemic CVA in HMII and HVAD patients (30).
However, in the ENDURANCE study, comparing HMII with HVAD in a DT population, stroke rates significantly differed between HMII and HVAD (12.1% vs. 29.7%, p<0.001). Yet, survival free of disabling stroke or need for device replacement at 2 years was 55% with the HVAD and 57.4% with the HMII (p=0.67) (33). In addition, the device strategy did not make any difference in terms of stroke-free survival, according to Acharya et al. (44).
The results of the HM3 trial of Mehra et al. are promising: rates of death and disabling stroke at 2 years were similar between HMII and HM3 patients, but overall rate of stroke at 2 years was lower in HM3 patients than HMII patients (10.1% vs. 19.2%; hazard ratio: 0.47; 95% CI: 0.27 to 0.84; p=0.02). No correlation with pre-existing stroke, atrial fibrillation (AF) (history at baseline or new), antiplatelet or anticoagulation regimens, or blood pressure management was found (38).
However, according to Teuteberg et al., compliant use of antiplatelet agents, adequacy of anticoagulation levels, and blood pressure management showed a correlation with lower rates of stroke with the HVAD. More specifically, prevalence of ICVA decreased from 6.3% (17 of 272) to 2.7% (3 of 110; p=0.21) due to pump design modifications and protocol changes in antiplatelet regimens. No effect was measured on the prevalence of HCVA (8.8%, [24 of 272] vs. [7 of 110]; p= 0.69) (43).
Moreover, in the initial HMII trial, a rate of ICVA of 0.13 EPPY was demonstrated. As a result of improvements in managing blood pressure control and anticoagulation strategies, this was lowered to 0.05 EPPY. On the contrary, a rate of 0.11 EPPY was shown in the initial HVAD trial due to tissue ingrowth at the outer surface of the inflow canula that could lead to embolic events. This was reduced to 0.9 EPPY by adding a sintered surface to the inflow canula and
So, controlling the risk of stroke requires chronic anticoagulation. The downside of this is the possible increase in risk of bleeding (44).
4.2.5.3. Treatment
The efficacy of treatment after LVAD-associated stroke is not well known. It is important to identify the possible events rapidly and to treat them aggressively. Ischaemic stroke in LVAD patients could be treated with mechanical intra-arterial embolectomy. According to the protocol of Willey et al., LVAD patients presenting within 8h after onset of symptoms and with no evidence of infarct on CT scan, are treated with an intra-arterial embolectomy (49).
When there is no evidence of haemorrhagic conversion, continued antiplatelet and anticoagulation therapy are used. On the contrary, anticoagulation reversal serves to treat haemorrhagic stroke. Vitamin K, prothrombin complex concentrate and fresh frozen plasma may be used (49).
4.2.5.4. Risk factors
Female sex, pre-implant systolic blood pressure, heparin-induced thrombocytopenia, intra-aortic balloon pump (IABP) and primary cardiac diagnosis (ischaemic/coronary artery disease, other, or unknown) are pre-implant predictors of stroke. For ischaemic stroke, female sex and previous cardiac operation were significant predictors, whereas for haemorrhagic stroke, female sex, heparin-induced thrombocytopenia, IABP and primary cardiac diagnosis were significant (44). In another study, diabetes mellitus was found as an independent risk factor of haemorrhagic stroke (45).
The impact of preoperative AF on stroke is not clear. Stulak et al. reported preoperative AF as an independent risk factor for thromboembolic events, including stroke. In patients with preoperative AF, freedom from thromboembolic events was 62% at 1 year and 46% at 2 years, compared to 79% and 72%, respectively, in patients without preoperative AF. They suggested a prophylactic removal of the left atrial appendage in patients with preoperative AF to reduce stroke (47). However, the INTERMACS analysis of Acharya et al. found no association between history of AF and an increased risk of stroke (44). This is consistent with results of Mehra et al., as explained above (38).
Furthermore, other post-implantation LVAD complications may alter coagulation. For example, CF-LVAD-related infections tend to increase the risk of stroke (37, 44). In patients with post-operative infections, right-sided strokes are more prevalent, probably due to anatomic alignment of the outflow canula and the arteries arising from the ascending aorta (48). GIB also significantly contributes to a higher risk of stroke due to consequent cessation
or alteration of anticoagulation therapy, whereas confirmed pump thrombosis was not found to be significantly increasing the risk (36, 44).
4.2.6. Non-surgical bleedings: gastro-intestinal bleeding (GIB) and nasal bleeding
The most prevalent surgical bleeding is the GIB. Nasal bleeding is another possible non-surgical bleeding. GIB in CF-LVAD patients is a significant event and can cause important morbidity, but is rarely life threatening (50). However, some GIBs can be small, so that sometimes, anaemia is the only clinical indication of the occult blood loss (35).4.2.6.1. Pathophysiology
The pathophysiology of GIB is multifactorial. One contributing factor to GIB among LVAD patients is the increased development of gastrointestinal angiodysplasia. Low pulsatility in CF-LVAD may lead to hypoperfusion of gastrointestinal vasculature, and consequent vasodilation, which enhances the formation of arteriovenous malformations. These arteriovenous malformations are more prone to rupture, particularly dangerous in conditions of combined antiplatelet and anticoagulation therapy (25, 50).
Another proposed cause is the development of AvWS and impaired platelet aggregation (36, 50, 51). As described in section 4.2.2.2., LVADs increase shear stress which alters the conformation of vWF and makes it more prone to cleavage by cleaving protease ADAMTS13. Consequently, a decrease in HMWM of vWF causes coagulopathy and bleeding (36).
vWF deficiency tends to occur less with HM3 than with HMII. However, Netuka et al. found no significant difference in the rate of GIB between HM3 and HMII, though the cumulative bleeding events were numerically lower in the HM3 group (25). Furthermore, in the HM3 trial of Mehra et al., no significant difference in GIB rates was observed between the HMII and HM3 group (38).
Meyer et al. also reported similar GIB and epistaxis incidences and long-term outcomes among HMII and HVAD patients (30). In addition, in the ENDURANCE trial, comparing HVAD with HMII in DT patients, the rates of major bleeding were similar between both groups (33). These findings suggest that the influence of other factors, such as anticoagulation levels, oxidative stress and microcirculatory factors, on the GIB incidence may be more important than AvWF deficiency (38).
4.2.6.2. Epidemiology
According to the INTERMACS registry, GIB is the most frequent complication during the first 3 months after LVAD implantation (17). In the EUROMACS registry, an overall rate of major
bleeding within the first 3 months after implantation of 6.45 per 100 patient-months was found (23). In the study of Boyle et al., 9.4% of the LVAD patients had a GIB (35). In some studies, a higher GIB rate, up to 30% was found (50, 52). For example, in the study of Stulak et al., GIB occurred in 30% of the LVAD patients, for an event rate of 0.45 EPPY and median to first GIB after implantation of 5 months (36). Freedom of GIB at 3 years after HMII implantation has been reported as 68% (52).
Diagnosis of GIB is mostly based on clinical characteristics, such as melena or hematochezia and a decline in haemoglobin levels with no other identifiable source. According to Holley et al. GIB occurs equally in the upper and lower gastrointestinal tract. In suspected patients, endoscopic techniques including upper endoscopy and colonoscopy, can help to diagnose GIB, but sometimes the bleeding source is not identifiable. In patients with uncomplicated and self-resolving GIB, endoscopy is seldom used. In recurrent or complicated GIB, a tagged red blood cells scan and mesenteric angiography are other possible diagnostic modalities. More recently, capsule endoscopy has been introduced as a safe and effective method to detect the bleeding source (50).
GIB is responsible for increased morbidity in LVAD patients, including higher need for blood transfusions, prolonged hospital stays, multiple readmissions, and overall mortality. On average, LVAD patients with a GIB received just more than 2 units of blood transfusions. In the first year after implantation, patients experiencing GIB had twice the number of transfusions compared with patients without GIB (50).
The more blood transfusions an LVAD patient receives, the lower chances of getting a HTx. Holley et al. showed a 27% lower HTx rate in LVAD patients with GIB than in LVAD patients without GIB. This is due to an increased risk of allosensitization, which implies more prospective cross-matches and limitation of the donor organ pool. However, nowadays, this risk is minor, because the leucocytes are removed from the blood before transfusion. On the contrary, administration of blood platelets remains potentially dangerous in the context of allosensitization. Heparinization required in the cardiac transplantation also raises concerns about possible recurrent bleedings in the perioperative period. In respect to this risk, there can also be a delay in being reactivated on the transplantation list (50).
4.2.6.3. Treatment
Management of GIB in CF-LVAD patients requires a multidisciplinary approach, with collaboration of cardiologists, cardiothoracic surgeons, and gastroenterologists. Primary, anticoagulation and antiplatelet therapy are reduced or mostly ceased for a while (36, 47, 50). Medical treatment and endoscopic treatment of the source of bleeding are often enough, but sometimes long-term cessation of anticoagulation or surgery are needed. For recurrent GIBs,
As described previously, presence of GIB is associated with a subsequent increased rate of thromboembolic events. A 7.4-fold increase is determined by Stulak et al.. Age also plays a role: in patients older than 70 years, an almost 15-fold increased rate should be considered. Reductions in antiplatelet and anticoagulation managements are thought to contribute to this increase. Because, in order to treat GIB, INR targets are lowered, and antiplatelet and anticoagulation therapy are withdrawn, placing the patients at risk of thromboembolic events (36).
Medical management of GIB determines the consequent risk of thromboembolic events. This risk depends on the approach of cessation of anticoagulation: active reversal of INR versus simple cessation, complete versus incomplete discontinuation of antiplatelet therapy, and timing of resumption of anticoagulation after treating the GIB (36, 47).
4.2.6.4. Risk factors
Stulak et al. stated that older patients show a higher tendency towards GIB. Therefore, they treated them less aggressively with anticoagulation therapy. On the other hand, they practiced more aggressive anticoagulation in younger LVAD patients, because their tendency to have a GIB is lower (36).
In addition to older age, lower preoperative albumin level and lower preoperative BMI are other risk factors of the development of GIB. Female gender was also found to be an independent risk factor (50, 53).
4.3. Device dysfunction
Pump stoppage, suction events, percutaneous lead site and insulation tearing all contribute to LVAD dysfunction and may require removal of the LVAD. Pump thrombosis is an important cause of pump stoppage. For a discussion of pump thrombosis, see 4.2.4..
4.4. Cardiac complications
Cardiac complications often occur after LVAD implantation. Right ventricular failure (RVF), arrhythmias and aortic insufficiency are the three most important cardiac complications and will be discussed in the section below. Other cardiac complications that can occur after LVAD implantation include volume overload, dehydration, tamponade, hypotension or hypertension, and recurrence of HF symptoms.
4.4.1. Right ventricular failure (RVF)
RVF is a major cause of morbidity and mortality after LVAD implantation (54). INTERMACS defined acute RVF as documented elevation of central venous pressure (CVP) and clinical
signs, such as ascites, oedema, weight gain, jugular venous distention, renal or hepatic dysfunction. Severe RVF is defined as the need for extended inotropes after LVAD-implantation, inhaled nitric oxide or intravenous vasodilators, or the need for a right ventricular assist device (RVAD) (17, 54-56, 58).
In addition, RVF has a severe prognostic impact, because RVF results in worse overall survival and a higher mortality in the perioperative period. Due to RVF, congestion in the patient’s kidneys, liver and gastro-intestinal tract may develop, which may worsen the function of these organs (17, 55, 57). Also, according to Kormos et al., RVF is associated with a longer hospital stay (55).
Chronic or late RVF is less well defined than acute RVF. According to Kurihara et al., it is defined as RVF occurring after 30 days post-LVAD implantation (54). Strengthening the diuretic therapy, inotropes or even RVAD implantation may be necessary. Patients with chronic or late RVF are frequently hospitalized and their general condition is often not good (54, 58).
4.4.1.1. Pathophysiology
By unloading the LV, the LVAD decreases the LV end-diastolic pressure, and optimally, reduces the pulmonary artery pressure (PAP). This reduces the right ventricle (RV) afterload and ameliorates the RV function. However, RVF may occur after LVAD implantation (55). There is a variety of potential underlying mechanisms.
Sometimes, pre-existing RVF can be unmasked by the increased venous return to the RV provided by higher cardiac output (CO) after LVAD implantation. Too much LV decompression can also contribute to RVF: the intraventricular septum excessively shifts leftward, which reduces RV contraction, resulting in RVF (55).
Apart from changes in the shape of the RV related to septal shifting, perioperative acute kidney injury (AKI), bleeding and infection can contribute to RVF in the early phase after LVAD implantation. On the other hand, ventricular arrhythmias (VAs), worsening of tricuspid regurgitation and pulmonary hypertension are some factors associated with the development of late RVF (58).
4.4.1.2. Epidemiology
LVAD implantation is complicated in 10-40% with RVF (33, 38). Despite lower rates of RVF and overall better outcomes with CF-LVADs over PF-LVADs, RVF still remains a major complication. Its occurrence is associated with higher mortality rates than seen in LVAD patients without RVF: there is less flow to the LVAD, resulting in reduced pump output, increased venous pressure, and, ultimately, impaired perfusion to vital organs (55, 57). In
addition, Kurihara et al. did not found significant differences in the incidence of early and late RVF between HMII and HVAD patients (54).
4.4.1.3. Treatment
RVF requires chronic inotropic drugs or RVAD support (54, 55, 59). Preoperative identification of high-risk patients is required, because they might benefit from preoperative optimization of the RV function or planned biventricular support (55). RVAD implantation at the same time of the LVAD implantation leads to a better survival than delayed RVAD implantation (55, 59). Moreover, Kurihara et al. showed that RVF did not significantly impact the success of bridging to HTx, unless the failing RV needed RVAD support (54).
Treatment of chronic RVF is focussed on underlying causes and symptom control. Meticulous regulation of pump speed, fluid balance, inotropes and pulmonary vasodilators may be useful. Concomitant tricuspid annuloplasty (TAP) with LVAD implantation may be necessary to avoid postoperative increases in RV preload because of progressing tricuspid regurgitation (60, 61).
4.4.1.4. Risk factors
In the context of prevention of post-operative RVF, multiple risk scores were developed to detect high risk patients. These may guide the decision whether a patient only needs LVAD support or needs biventricular support (54, 57).
Possible risk factors for RVF are: pre-existing RV dysfunction, prior cardiac surgery, renal failure, need for preoperative IABP, inotrope dependency and need for mechanical ventilation. Also, biochemical parameters, such as elevated bilirubin, aspartate aminotransferase, serum creatinine and blood urea nitrogen can predict a higher risk for RVF (54, 57, 61, 62).
Moreover, imaging with echocardiography may help predicting RVF. Some haemodynamic parameters are associated with RVF, e.g. elevated CVP, CVP/pulmonary capillary wedge pressure (PCWP) or lower PAP (54, 61).
In addition, risk factors for chronic RVF are not well described, because chronic RVF is not well defined. Diabetes mellitus, high BMI and high blood urea nitrogen level are potentially predictive (58).
4.4.2. Arrhythmias
Arrhythmias are common after LVAD implantation and are associated with worsened prognosis (10). Arrhythmias can be ventricular or supraventricular, e.g. AF.