Copepods as first exogenous
feed during intensive larvae
culture of pikeperch Sander
lucioperca (Linnaeus, 1758)
in RAS
Lugho D. Jonathan
Student number: 01801081
Promoter
Prof. Dr. Gilbert Van Stappen Supervisors
Prof. Dr. Wim Van Den Broeck Edson Panana Villalobos
Master’s Dissertation submitted to Ghent University in partial fulfilment of the requirements for the degree of Master of science in Aquaculture
Deze pagina is niet beschikbaar omdat ze persoonsgegevens bevat.
Universiteitsbibliotheek Gent, 2021.
This page is not available because it contains personal information.
Ghent University, Library, 2021.
ii | P a g e
Acknowledgement
I wish to extend my sincere gratitude to VLIR-UOS (Vlaamse Interuniversitaire Raad- Flemish Interuniversity council) for the financial support they offered me to undertake this masters programme in aquaculture at Ghent University. My appreciation goes to Dr. Stefan Teerlinck, the head of aquaculture research at Inagro for putting forward this topic and availing the research facility for running the experiment. I am very much indebted to the technical staff at Inagro particularly Laurens, Anne and Lukas for supporting me during and after the experiment. Special thanks goes to Dr Wim and Lobke of the laboratory of morphology at the veterinary campus for facilitating the analysis of my histology samples. I won’t forget to thank Geert of the ARC laboratory for his crucial guidance during the analysis period of especially, my HUFA samples. I also extend my appreciations to my family, friends and classmates who always wished me the best during the whole period. Teresa, Abdul and Felix, your moral support is really appreciated.
A very huge hug goes to my immediate thesis supervisor Edson Panana Villalobos of Inagro for the great energy he dedicated during the running of the experiment until the end. His dedication in making sure everything was right and giving me key insights about the whole experiment is unforgettable. Sincerely, his great sense of professionalism, enthusiasm and support made me feel comfortable despite the much tension linked to research work.
Special thanks goes to my promoter and tutor Prof Gilbert Van Stappen. Prof, your dedication and passion on mentoring me and shaping my work during the whole thesis period was something else. I personally learnt a lot from you, you are a great professor and mentor. Thank you so much for your timely, precise and scholarly guidance every time I needed it. I believe that it has shaped me for my future endeavours.
Lastly, I give God all the gratitude for making all this possible, thank you our Lord God.
iii | P a g e
List of figures
Figure 2.1 Total aquaculture production in million metric tonnes (mmt) by 2012 (World resources
institute; 2014). ... 6
Figure 2.2 Global capture fisheries and aquaculture production, 1990-2030 (Msangi et al., 2013). ... 7
Figure 2.3 Adult pike perch, Sander lucioperca (Linnaeus, 1758) fish (Zakęś, 2002) ... 8
Figure 2.4 Pikeperch production cycle (Zakęś, 2002) ... 12
Figure 2.5 Global trend of Sander lucioperca production in tonnes (T) (Zakęś, 2002). ... 14
Figure 2.6 Schematic life-cycle of a copepod (a), A.tonsa nauplii (b) (Stottrup, 2006; Dhont et al., 2013). ... 16
Figure 2.7 Schematic diagram of an automated 500-L copepod culture system. Nauplius collection and water recycling procedures are described as follows: 1 Harvest sequence initiated by pressing the ‘start’ button on the PLC device. 2 Air supply to the culture vessels turned off for 5 min to stop water movement. 3 Light turned on for 10 min to illuminate 150-µm screen submerged in culture vessel. 4 Solenoid valve opened so that water (containing nauplii) siphons through 150-µm screen into harvest vessel. 5 Float switch (FS) #1 activated which closes solenoid value, turns light off and turns pump #1 on. 6 FS #2 activated which turns pump #1 off and pump #2 on (drawing through 53-µm screen). 7 FS #3 deactivated which turns pump #2 off and air supply on. 8 Concentrated nauplii drained from harvest vessel (M. F. Payne & Rippingale, 2001)... 19
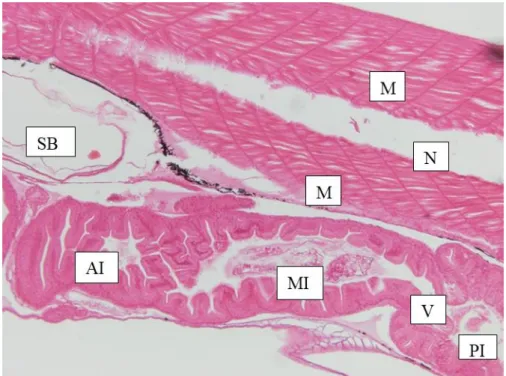
Figure 2.8 Sagittal section of (SB) swim bladder, (M) muscles, (N) notochord, (AI) anterior intestine, (MI) mid intestine, (V) valve and (PI) posterior intestine of a 15 dph pike perch larva obtained from the present study. ... 27
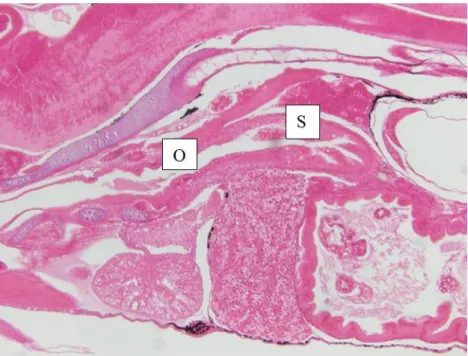
Figure 2.9 Sagittal section of the primary stomach (S), developed from oesophagus extension (O) of a 15 dph pike perch larvae obtained from the present trial. ... 28
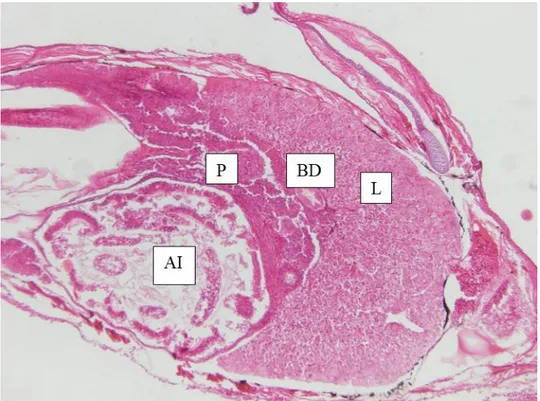
Figure 2.10 Coronal section showing the U-shaped liver (L), pancreas (P), anterior intestine (AI) and bile duct (BD) of a 15 dph pike perch larvae obtained from the present trial. ... 30
Figure 2.11 Sagittal section of a 15 DPH pike perch larva fed Artemia, displaying well developed mucosal folds (a), and weaned on day 9 characterized by a flat epithelium (b) in the anterior intestine (AI) and median intestine (MI). (Modified from Neila Hamza et al., 2015b). Scale bar 100 µm. ... 32
Figure 2.12 Sagittal section of pike perch fed formulated diet (a) with dispersed groups of necrotic hepatocytes (red arrows) in liver. Bigger hepatocytes in liver of larvae fed Artemia (b). (Modified from Neila Hamza et al., 2015b). Scale bar 10 µm (not clear from the images). ... 34
iv | P a g e
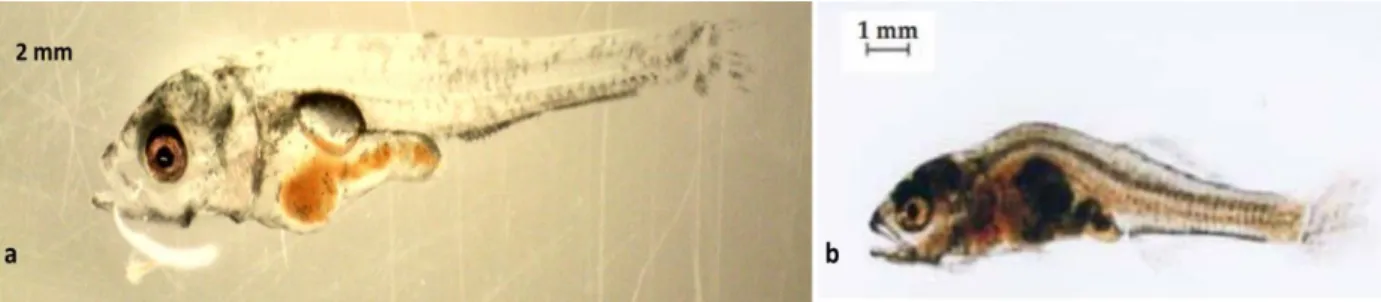
Figure 2.13 Pike perch larvae of 20 dph from the present trial with inflated swim bladder and normal morphology (scale bar 2 mm) (a), 18 dph with noninflated swim bladder and deformed spine (b) (Adapted from Zakes et al., 2003) scale bar 1 mm. ... 36 Figure 2.14 Pike perch larvae of 20 dph with lordosis (upper), with a normal body morphology (lower) (a) and 4 dph pike perch with scoliosis (b) all obtained from the present study. ... 38 Figure 3.1 Random design arrangement of the experimental tanks including three treatments and one control. Every component was connected to a joint RAS system. ... 39 Figure 3.2 Diets used to feed pike perch larvae during the 29-day experiment, indicating when each diet was used, and the transitions in between. AF- Artemia franciscana (non-enriched), GSL- Great Salt Lake A. franciscana (enriched). ... 40 Figure 3.3 Cleaned and dried tank with an outlet pipe in the middle covered with a fine plastic sieve to prevent larvae and feed from being drained out during the experiment(a) and completely set up rearing tank full of water ready for the experiment (b). ... 41 Figure 3.4 System biofilter 3/4 filled with water and black bio-plastic micro-beads as substrate for autotrophic bacteria and (b) bubbling action in the biofilter due to aeration by air stones during biofilter maturation ... 42 Figure 3.5 Drum filter of 30µm (a), buffer tank (b) and oxygen cone (c) used during the 29-day pike perch larviculture experiment. ... 44 Figure 3.6 Lansy breed essential 8 mm sinking pellets rich in DHA and EPA (a) and Skretting floating pellets (b) used for feeding the broodstock during conditioning prior to the experiment. ... 45 Figure 3.7 Image of the feed package (a) and ingredients used (b), both obtained from the supplier’s website, otohime feed inside the automatic feeder during the experiment at Inagro (c) and an image visualizing the outer texture of the feed granule (d) from the supplier’s website. ... 48 Figure 3.8 Pike perch fish larvae displaying a directional swimming pattern (arrow) during the
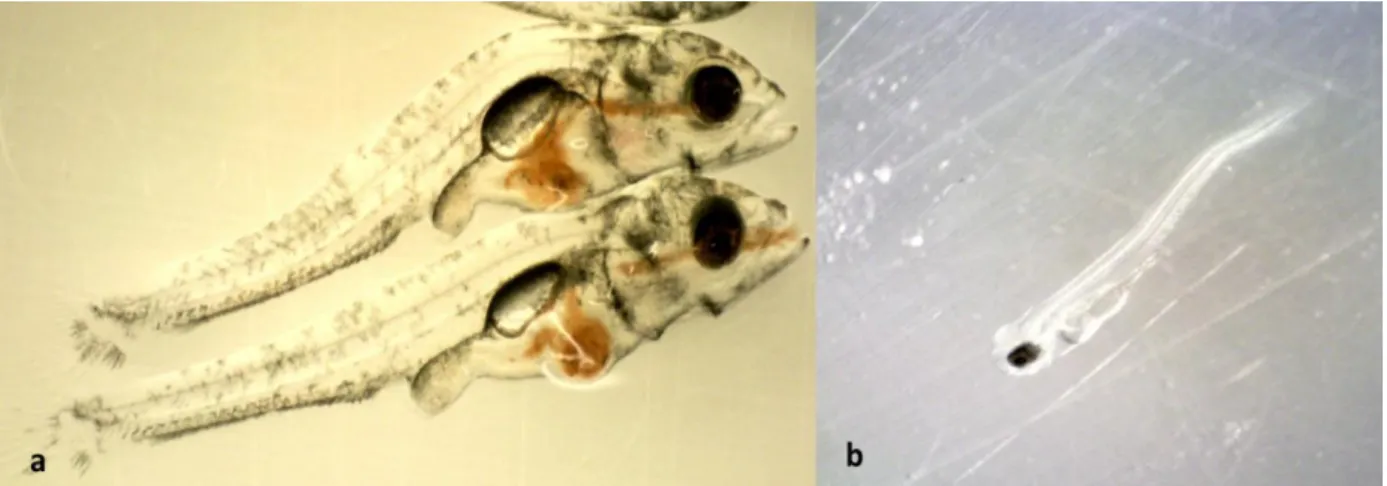
experiment (a), nylon strainer sieve (b) used for scooping out dirt collected on the water surface after automatic cleaning of tanks, also used during fish sampling. ... 51 Figure 3.9 Narrow water jets (arrow) introduced to each rearing tank so that any oil fil layer or related dirt on the water surface could be broken during the experiment. ... 51 Figure 3.10 Gadgets of the Aquaray Smart Control 8 system used to monitor temperature, pH,
dissolved oxygen and conductivity (a), equipment bearing the alarm that was triggered to go off in case the water quality parameters deviated from the desired set limits (b), JBL test kit for measuring ammonium ions and nitrite contents in the rearing water (c), and JBL kit equipment in use during the experiment (d). ... 53
v | P a g e
Figure 3.11 shows some of the equipment in use in one of the steps during the FAME analysis at the ARC laboratory. ... 54 Figure 3.12 Olympus BX61 light microscope used during histology analysis of the samples ... 58 Figure 3.13 Dinocapture microscope (arrow) connected to a laptop installed with a software Version 1.5.32.B of the digital microscope which was used for image analysis of pike perch larvae from the experiment... 59 Figure 3.14 Straightly placed, transparent 4 dph pike perch larvae on a Dinocapture- illuminated microscope slide during the preliminary sets of analysis (a), 6 dph pike perch larvae with feed visible in their gut (b) during the same procedure. ... 60 Figure 3.15 Pike perch larvae of 6 dph from the trial without a swim bladder (a) and with an inflated functional, swim bladder (arrow) below the notochord and anterior to the digestive tract. ... 61 Figure 3.16 Normal pikeperch larvae of 4 dph from the trial (a), with axial curvature of the spine in the abdomen (lordosis) (b), and axial curvature of the spine in the caudal region (kyphosis) (c). ... 62 Figure 3.17 Schematic illustration of four gut fullness intensities on the example of 15dph old pike-perch larvae. No gut content (i), little gut content (ii), average gut content (iii) and high gut content (iv). Illustrations redrawn from original photographs. Source: (Tielmann et al., 2017). ... 63 Figure 3.18 Analytical balance used for dry weight analysis (a), me, performing the dry weight analysis in the ARC laboratory. ... 64 Figure 3.19 Mettler Toledo weighing balance used during final biomass determination of the larvae at Inagro research facility. ... 65 Figure 4.1 Final survival (mean % ±SD, n=3) of 29 dph pike perch larvae fed different dietary
treatments. Treatments with same letters above error bars are not significantly different (p>0.05). .... 69 Figure 4.2 Mean total biomass (mean g ±SD, n=3) of 29 dph pike perch larvae fed different dietary treatments. Treatments with same letters above error bars are not significantly different (p>0.05). .... 69 Figure 4.3 Mean total body length (mean mm ±SD, n=30) of pike perch larvae fed different dietary treatments during the trial. ... 70 Figure 4.4 Dry weight (mean mg ±SD, n=3) of pike perch larvae fed four different dietary treatments during the trial. ... 71 Figure 4.5 Mean gut fullness index (mean ±SD, n=20) of pike perch larvae fed four different
treatment diets between 6-29 dph. Different letters above error bars on the same sampling dph
indicate significant differences (p<0.05). ... 72 Figure 4.6 Percentage (mean % ± SD, n=30) of 9-29 dph larvae with malformations. ... 73
vi | P a g e
Figure 4.7 Mean percentage of larvae with inflated swim bladder (SBI) (mean % ±SD, n=30) fed different dietary treatments on 9,12 and 15 dph. Same letters above error bars of same dph denote values which are not significantly different (p>0.05). ... 74 Figure 4.8 Sagittal section of 15 dph pike perch larvae fed Artemia nauplii, displaying thick, well developed mucosal folds (arrow) in the anterior (a) mid and posterior intestine (b), and of larvae fed a 5% replacement diet characterized by thin and poorly developed folds in the anterior (c), mid and posterior intestine (d). AI- anterior intestine, MI-mid intestine, PI- posterior intestine. ... 77 Figure 4.9 Sagittal section of 15 dph pike perch larvae fed Artemia nauplii, displaying a well
differentiated y-shaped (arrow) stomach (a), and of larvae fed a 5% replacement diet characterized by a poorly differentiated stomach without the y-shape (b). O- oesophagus, S-stomach. ... 78 Figure 4.10 Sagittal section of 15 dph pike perch larvae fed Artemia nauplii, displaying a well
differentiated y-shaped (arrow) stomach (a), and of larvae fed a 5% replacement diet characterized by a poorly differentiated stomach without the y-shape (b). O- oesophagus, S-stomach. ... 78
vii | P a g e
List of tables
Table 2.1 Taxonomic classification of Sander lucioperca fish species (Zakęś, 2002) ... 8 Table 3. 1 Feeding regime of the experiment indicating different times of the day and the type of live diet dispensed in the rearing tanks for the four treatment groups. ... 50 Table 3.2 Tissue TFA content (mg/g DW) and FA composition (% of TFA) of copepods analysed and used as live feed during the experiment and a reference AF and GSL Artemia (not analysed in the present study). ... 55 Table 3.3 A detailed summary of the sampling process during the trial ... 66 Table 4.1 Overview of water quality parameters in the recirculating system tanks over 29 days during the pike perch larvae feeding trial. Values are presented as means ±SD (n=116 for temperature, dissolved oxygen, pH and conductivity). NH4+ and NO2- were measured every 3 days (11 time points)
in a common buffer tank from 1 dph, n=11. ... 68 Table 4.2 Analysed overview of the larvae performance parameters for each feeding treatment. Mean final survival (%), final total wet weight (WW) biomass (g), initial, final body length (mm) and individual dry weight (mg), final gut fullness index, malformed and swim bladder inflated (SBI) larvae (%). Values are presented as means ±SD. Different superscripts on values of the same row denote significant differences in the parameter performance between treatments (p<0.05). Values with asterisks represent measurements taken before first feeding with treatment diets. ... 75 Table 4.3 Analysed whole body TFA content (mean mg/g DW) and selected FA composition (% of TFA) of copepods used in the present trial, and 29 dph pike perch larvae fed different diets. Values are presented as means ± SD (n=3). Different superscripts on values of the same row denote
viii | P a g e
List of abbreviations
ARC: Artemia reference center
RAS: Recirculating aquaculture system WW: Wet weight
TL: Total length
FAO: Food and agriculture organization DPH: Day post hatch
TAN: Total ammonia nitrogen FCR: Feed conversion ratio SGR: Specific growth rate UV: Ultraviolet
FAA: Free amino acids GSL: Great Salt Lake DW: Dry weight
SBI: Swim bladder inflation FAME: Fatty acid methyl esters LC: Long-chain
USA: United States of America SD: Standard deviation
ANOVA: Analysis of variance EFA: Essential fatty acid DO: Dissolved oxygen mm: millimetre
kg: kilogram g: gram
pH: Measure of the acidity or basicity of an aqueous solution mg: milligram
ix | P a g e
Table of contents
Copyright ... i
Acknowledgement ... ii
List of figures ... iii
List of tables ... vii
List of abbreviations ... viii
Abstract ... xiii
Preamble ... xiv
Chapter 1. Introduction ... 1
Chapter 2. Literature review ... 5
2.1 Global aquaculture ... 5
2.2 Pikeperch (Sander lucioperca) ... 8
2.2.1 General biology ... 8
2.2.1.1 Species description... 8
2.2.1.2 Ecology and growth ... 9
2.2.1.3 Natural life cycle ... 9
2.2.1.4 Feeding habits ... 9
2.2.2 Nutritional requirements for pike perch production ... 10
2.2.3 Pike perch production systems... 11
2.2.3.1 Pond system ... 11
2.2.3.2 RAS system ... 11
2.2.4 Major challenges and opportunities in pike perch production ... 13
2.2.5 Status and trends in worldwide production ... 14
2.3 Larval fish feeding ... 15
2.3.1 Live feeds in larviculture ... 15
x | P a g e
2.3.1.2 Artemia (general biology and use as live feed) ... 21
2.3.1.3 Rotifers (general biology and use as live feed) ... 23
2.3.2 Dry feeds application in larviculture... 24
2.4 Ontogeny and development of a functional gut and sub parts of pike perch ... 25
2.4.1 General description ... 25
2.4.1.1 Anterior, mid and posterior intestines (gut) ... 25
2.4.1.2 Stomach... 27
2.4.1.3 Liver and pancreas ... 28
2.4.2 Effect of diets on development of the gut and sub parts (criteria for larval feeding assessment) ... 30
2.4.2.1 Gut morphology ... 30
2.4.2.2 Stomach morphology ... 32
2.4.2.3 Liver and pancreas size and morphology... 33
2.5 Other criteria used to assess larval feeding ... 34
2.5.1 Body fatty acid methyl esters (FAME) ... 34
2.5.2 RNA/DNA ratios as condition factor in fish larvae ... 35
2.5.3 Initial swim bladder inflation (SBI) in fish larvae ... 36
2.5.4 Body malformations in developing fish larvae ... 37
Chapter 3. Materials and methods ... 39
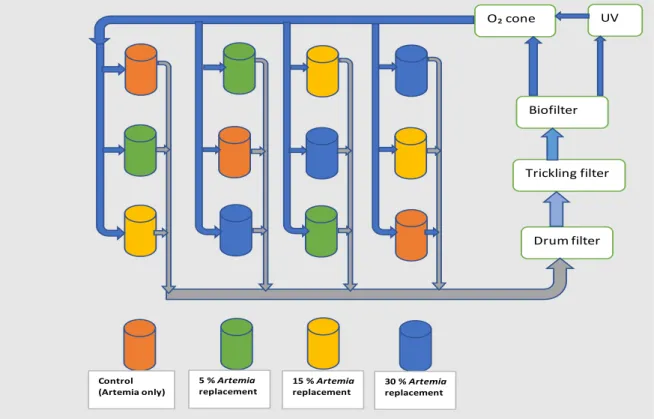
3.1. Description, design and set up of the experiment. ... 39
3.2. Initial preparations of the experiment ... 41
3.3. Description of the RAS system ... 43
3.4. Origin and stocking of the fish larvae ... 44
3.4.1. Fish larvae origin ... 44
3.4.2. Stocking ... 45
3.5. Experimental diets used ... 46
xi | P a g e
3.5.2. Artemia nauplii... 47
3.5.3. Dry feed (Otohime A2) ... 48
3.6. Fish feeding and monitoring ... 49
3.6.1. Feeding of the larvae ... 49
3.6.2. Monitoring on feeding ... 49
3.7. Water quality management ... 52
3.8.2. FAME analysis... 53
3.8.3 Determination of RNA/DNA ratios of whole fish larvae ... 55
3.8.4. Histological analysis of the gut and sub parts ... 57
3.8.4.1. Sample taking... 57
3.8.4.2. Tissue processing ... 57
3.8.4.3. Tissue sectioning and staining ... 57
3.8.4.4. Histology tissue analysis ... 58
3.8.5. Anatomical observations and growth parameters ... 59
3.8.5.1. Sedation of fish larvae ... 59
3.8.5.2. Total length (TL) determination ... 60
3.8.5.3. Swim bladder inflation analysis ... 60
3.8.5.4. Malformations ... 61
3.8.5.5. Gut fullness index ... 62
3.8.6. Individual dry weight (DW) analysis ... 63
3.8.6. Final survival ... 64
3.8.7. Final wet weight (WW) total biomass ... 65
3.9 Statistical analysis ... 67
Chapter 4. Results ... 68
4.1. Water quality ... 68
4.2. Final fish survival ... 68
xii | P a g e
4.3.3. Individual body dry weight of larvae ... 70
4.3.4. Gut fullness index in larvae ... 71
4.3.5. Malformations during larval growth ... 72
4.3.6. Swim bladder inflation in larvae ... 73
4.4. Larval FA composition ... 74
4.5. Histology ... 76
4.5.1. Gut mucosal folds ... 76
4.5.2. Subparts of the gut ... 77
4.5.2.1. Stomach... 77
4.5.2.2. Liver ... 78
Chapter 5: Discussion ... 79
5.1 Recapitulation of problem statement ... 79
5.2 Water quality parameters ... 79
5.3 Final survival of the fish ... 81
5.4 Growth performance parameters and anatomical observations ... 82
5.4.1 Gut fullness index, individual dry body weight, body length, final biomass ... 82
5.4.2 Swim bladder inflation and body malformations in fish larvae ... 85
5.5 Gut and subparts histology analysis ... 87
5.6 Condition assessment of larvae through RNA/DNA ratios ... 88
5.7 Conclusion and recommendations ... 89
xiii | P a g e
Abstract
The aim of this study was to determine the effect of feeding on growth (individual dry weight, body length, final biomass), anatomical development (swim bladder inflation, malformations), gut fullness index, condition factor (RNA/DNA ratios), nutrition status (HUFA content), final survival, and histology of the gut, stomach and liver in pike perch (Sander lucioperca) larvae. The larvae were divided into four experimental groups and fed either AF Artemia nauplii (control), 5%, 15% or 30% Artemia replacements with copepods; Acartia tonsa nauplii. After feeding the larvae with the trial diets (4-9 dph), all groups were transitioned to common diets of exclusively AF Artemia from Great Salt Lake (GSL)-enriched (13-19 dph) and a dry feed (Otohime) weaning diet (23-29 dph). At the end of the experiment, the larval pike perch in all diet groups survived similarly with the same final biomass. Swim bladder-inflated larvae were recorded in equal shares in all diet groups. Gut fullness indices of the larvae and total body length were observed to be positively influenced by the 30% replacement diet by 15 and 18 dph of the experiment respectively, but dry weight was not affected by the four diets. Body deformities, on 20 dph, and supressed levels of total fatty acid (TFA) content on 29 dph were observed in the 5% replacement diet. However, an evaluation of the larval fatty acid composition revealed that the four diets did not result to differential contents of FAs. Preliminary histological observations showed that feeding pike perch with Artemia only or a 5% inclusion of copepods did not result to differential development of the liver. However, the 5% replacement diet supressed the development of the anterior, mid and posterior mucosal folds of the intestine. RNA/DNA ratios of whole-body fish could not be determined because the corona pandemic disrupted the schedule of events and rendered the laboratories inaccessible. These results indicate that the 30% replacement diet resulted to fuller guts and better sized pike perch larvae by 15 and 18 day of rearing.
xiv | P a g e
Preamble
My thesis work involved a 29-day feeding trial in September 2019 where pike perch fish larvae received four different dietary treatments at Inagro aquaculture research facility, Belgium. The aim of the trial was to get a better overview on the application of the euryhaline copepod;
Acartia tonsa as a live feed during the early feeding stages of pike perch larvae as compared to
the widely used feed Artemia nauplii. To do this, samples were collected during and at the end of the trial and preserved for analysis of various performance parameters in the laboratories of Gent University. Most samples were collected and made available to me by Inagro company except for some digital images for swim bladder inflation, malformations and length analysis, which I took by myself. The performance parameters to be assessed included growth (dry weight, final wet weight biomass, total length), final survival, anatomical development (swim bladder inflation, malformations), nutritional status (HUFA content), gut fullness, histology and RNA/DNA ratios.
Ideally, I was supposed to perform analyses to assess all the above-mentioned parameters during the period of February to June 2020, after which I would do the write up before submitting my thesis in late August 2020. However, due to the corona situation, I did not manage to analyse and obtain all the data I needed for my thesis work. I obtained data for larval growth, final survival, anatomical development, larval HUFA composition, gut fullness and part of histology. Most samples for histological assessment of the larval gut, stomach and liver remained untouched in the laboratory of morphology, veterinary sciences campus - Gent university, Merelbeke. In fact, for histology results of this thesis, I ended up using few images that I obtained from my own practice sessions in the laboratory at the vet campus, while preparing for the actual histology work. RNA/DNA samples remain in the laboratory of aquaculture and Artemia reference center (ARC)-Gent University completely untouched. This whole scenario considerably deviated the expectations of this study because the untouched samples would have generated very crucial data for my broader discussion.
1 | P a g e
Chapter 1. Introduction
Pike perch, Sander lucioperca (Linnaeus, 1758) is one of the highly promising species which has received much attention recently. It is a fresh and brackish water fish, which inhabits the rivers, lakes, ponds, and dam reservoirs of Central, Eastern Europe and Northern Asia (Fao, 1989). It is an emerging high value freshwater fish species, which is considered relevant and interesting for up scaled production. In Europe, pike perch prices are high due to its desirable characteristics such as very tasty meat (Teresa, 2005). The zeal to culture and integrate this species into the aquaculture industry has continued to grow and this explains why this species was chosen for the present study. However, successful production of pike perch and other species in general hugely depends on the corresponding success in the hatchery operations. A hatchery is a facility where eggs of aquaculture animals are hatched under artificial conditions. They are often referred to as infrastructures for mass production of fries.
Pike perch larviculture thus is an important limiting factor of fry production and further on-growing operations of the species. Traditionally, larviculture was done in ponds, relied on natural zooplanktons as feed and was characterized by low production and less effectiveness. Later, people started embracing a more effective and productive method; recirculating aquaculture system (RAS). Yields started going up which made people perceive RAS as the only option for successful mass production of pike perch fries and subsequent development of cultures. However, RAS being a more intensive fry production method which involves more application of feed than the traditional method, the type of feed used immediately stood as a very crucial factor in pike perch laviculture success. Studies have established that provision of a correct diet to fish larvae at the start of exogenous feeding improves growthand survival and reduces deformities (Roca et al., 2018). Larval rearing diets thus have become an issue of great concern with more debate being on live feeds. Despite recommendable improvements in the development of formulated larval feeds, the worldwide use of live feed in the hatchery rearing of most fish species is essential and is expected to remain so in the future, (Dhont et al., 2013).
The standard reference live feed which has been used during the first exogenous feeding for pike perch (Perca lucioperca) larvae is nauplii of the brine shrimp Artemia (Hamza et al., 2015a) thanks to its high convenience for use. The popularity of Artemia usage as live feed and its appreciation by hatchery managers and farmers in general rose because research revealed new developments related to its use. Such developments include nutrition, microbiology and hatching related breakthroughs (Dhont et al., 2013). Despite this good news, studies have
2 | P a g e
demonstrated that use of Artemia as live feed has its drawbacks. Such drawbacks include challenges in the acquisition and production, as prices of the product vary widely from year to year, shortage of essential n-3 and n-6 polyunsaturated fatty acids (PUFAs) such as docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), arachidonic acid (ARA) and linoleic acid (LA). PUFAs have been demonstrated to be very important components of not only pike perch larvae diets but also of other species like rainbow trout (Salmo gairdneri), coho salmon (Oncorhynchus kisutch) and chum salmon (Oncorhynchus keta) (Takeuchi & Watanabe; 1982). A reduction in quantity of these PUFAs in live feed items to levels below the optimum for pike perch larvae stand as a major cause of mortality according to Lund and Steenfeldt (2011). Mortalities, deformities and nutritional deficiencies in pike perch larvae were also common.
The main objective of my thesis was therefore to provide a better overview of the potential of
A. tonsa nauplii when used as live feed for S. lucioperca larvae as compared to that of the
conventional live diet, Artemia nauplii. The hypothesis of the present study is thus, given their superior nutrition capabilities, a 30% inclusion of copepods in the diet is expected to be optimal enough to boost the health status of pike perch larvae and consequently result to a general better performance when compared to larvae fed Artemia nauplii only or suboptimal copepods inclusions. In order to test this hypothesis, a feeding trial was conducted where pike perch larvae were divided into four groups each receiving a diet of either Artemia nauplii only or combinations of Artemia and A. tonsa nauplii for 29 days. The trial was conducted in a block random design using 12 black plastic tanks of 225 L capacity connected to a joint RAS system. The effect of each diet was evaluated through assessment of the performance of larvae from each group. The parameters used in the present study to assess the larval performance include growth (individual dry weight, total length, final biomass), final survival, gut fullness, anatomical development (swim bladder inflation, malformations), histology (of the intestines, stomach and liver) and nutritional composition (larval HUFA content). It was an intention of the present study to also assess the RNA/DNA ratios of whole larval fish.
Gut fullness may be a good indicator of feed intake (appreciation) by larval fish. When rearing species like pike perch, which are known to be very sensitive in size-related selective feeding, it may be very useful to assess gut fullness. Gut fullness indices may paint a general picture on which diet was consumed more by the larvae, hence can provide useful information on species-specific feed preference. A better consumed diet is expected to result to healthy larvae with good growth. Individual growth is considered on of the most important aspects of the biology
3 | P a g e
of percids because it often influences feeding success, recruitment potential, mortality, maturation and future growth (Feiner & Höök, 2015). A combination of these factors can determine the final survival which is an important indicator of potential yields in the aquaculture sector. Because survival can easily be linked with larval growth, it seems logical to think that it is almost compulsory to monitor the parameters in order to better assess the dynamics during larval rearing. Feed intake, body weight, length, biomass and survival have been reliably used widely in assessing larval growth performance for several species and thus it is used in the present work.
Knowing the proportions of larvae with inflated swim bladder at different time points during larval rearing stands as a good water quality and husbandry indicator. Pike perch larvae may not be able to inflate their swim bladders if the rearing water surface is covered with pollutants or oil films. Non inflation of the swim bladder may affect the floating abilities of the fish hence result to severe consequences such as malformations occurrence and low growth. Thus, assessment of both swim bladder inflation as well as malformations seems important in the present study. Histological studies of the digestive system have been used as important indicators of nutrition. The morphological development of the stomach, mucosa of the intestine as well as other important organs such as the liver may reflect the effects of Artemia and copepods. The mentioned organs play the major role in feed digestion and absorption, and storage of lipids and glycogen which have enormous consequences on the health status of larval fish (Kowaslska et al., 2011). Therefore, it was one of the major intentions of this study to inspect these organs in detail. However, as earlier said, only partial work was done on the same.
Pike perch larvae are known to have high demands of PUFAs, but they possess small abilities to synthesize them using short-chain PUFAs. However, when supplemented in the diet, pike perch often utilize them for various functions such as basic metabolism, suppression of malformations and optimal growth (Schulz et al., 2005). Because Artemia and copepods are known to naturally contain varying levels of HUFAs, it seems very important to assess how these levels are reflected in reared pike perch. This would provide more information about the suitability of copepods as live diets. Biologically available phospholipids, and free amino acids are suggested to be naturally abundant in copepods. In contrary, Artemia are known to have high levels of neutral lipids, and proteins which are not biologically available to fish larvae. As such, it may be feasible to think that larvae fed copepods may have higher rates of protein synthesis which is often linked to better growth than those fed Artemia. The rate of protein synthesis may be assessed using various methods among them being RNA/DNA ratios
4 | P a g e
determination. RNA/DNA ratios determination was chosen for the present work because it known to provide information on the most recent growth unlike the other methods (Buckely et al., 1999). The exclusive set of mentioned parameters has never been used before to assess larval pike perch performance however, most of them have been used for other species. Many studies have assessed growth and/or either gut histology, swim bladder inflation, malformations or exclusively one of these parameters. The novelty of the present work is the combination of all these parameters. In fact, to the best of my knowledge, no study has ever attempted to assess the effects of live feeds on the RNA/DNA ratios of whole pike perch larvae. Nevertheless, an optimal nutritionally balanced diet, 30% copepod inclusion is expected to result to a fair trade-off of desirable performances of all the mentioned performance parameters above.
5 | P a g e
Chapter 2. Literature review
2.1 Global aquaculture
Aquaculture is the farming of aquatic organisms, including fish, molluscs, crustaceans and aquatic plants. Farming, in this sense involves some form of intervention in the rearing process to enhance production, such as regular stocking, feeding, protection from predators (Tacon, 2003). Fish aquaculture can be traced back to 2000-1000 BC when common carp (Cyprinus
carpio) culture was developed in China. Since then, the aquaculture practice has spread to
many countries especially after the second world war (Austin, 2014). Aquaculture, according to Austin (2014) was started to provide food (protein) to people, ornamental fish for the pet industry and production of specimens for biotechnology purposes of rare species. However, other studies suggest that it is the worldwide decline in fisheries stocks (Naylor et al., 2000) in conjunction with the need to feed the rapid growing population (Young, 2002) which mainly informed the onset of aquaculture. Initially, fish protein used to be obtained from the then healthy fisheries until a period when fish stocks in the wild started dwindling. The decrease in the fish stocks was gradual but steady, due to the climate anomalies which were mostly caused by negative anthropogenic factors (Pauly et al., 2002). This decline is however antagonistic to the world’s population which is at a constant rise.
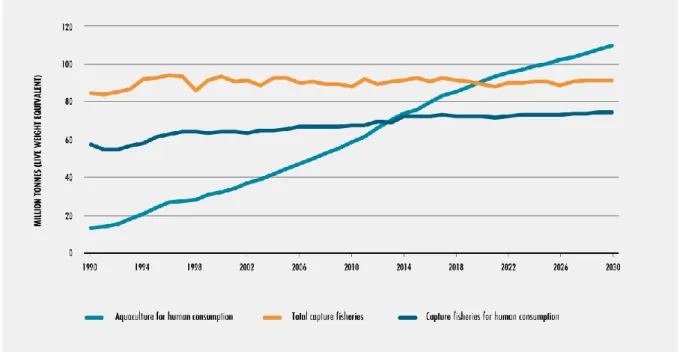
The world’s population is growing at a rate of 2.3 percent with a fish consumption rate of 3.2 percent (Juergen, 2015). This situation raises the fish world demand, and consequently, more aquaculture activities are established. Thus, a massive growth in aquaculture production has been experienced in the past few decades. The sector has so far recorded total production shifts from 45.71 million metric tonnes (mmt) in 2000 (Tacon, 2003) through 54.78 mmt in 2003 to 65 mmt in 2012 (Austin, 2014) (figure 2.1). By 2007, aquaculture was already providing 43% of the aquatic animal food for human consumption in the world (Bostock et al., 2010) with the top producing countries being China, India, Indonesia, Vietnam, Bangladesh and Norway. Generally, Juergen (2015) predicts that aquaculture will continue to grow in all areas except sub-Saharan Africa, the Arctic and Antarctic. Figure (2.2) indicates the current production since 1990 and how it is projected to evolve until 2030.
The major advantage of aquaculture in contrast to terrestrial farming is the numerous numbers of aquatic species which are viable for culture. A total of 198 aquatic species including finfish, molluscs and crustaceans were reported to be cultured by 2000 (Tacon, 2003). The large number of species cultivated reflects the wide range of candidate species available for
6 | P a g e
aquaculture. By the year 2000, the main species groups reared in freshwater were finfish (97.7%). High value crustaceans and finfish predominated in brackish water (50.5% and 42.7% of the cultured species respectively), and molluscs and aquatic plants in marine waters (46.1% and 44.0%, respectively) (Tacon, 2003). The most common cultured species in these groups include cyprinids, tilapia and catfish (freshwater), salmonids, milkfish and eels (diadromous). Brackish species include giant tiger prawn (Penaeus monodon), white leg shrimp (P.
vannamei) while marine species are cupped and flat oysters and scallops (Tacon, 2003). Studies
(e.g. Sicuro & Levine, 2011; Robles & Mylonas, 2017) have identified more viable and promising species that possess many desirable characteristics to farmers, and this gives even more greenlight on further growth of the sector.
Figure 2.1 Total aquaculture production in million metric tonnes (mmt) by 2012 (World resources institute; 2014).
7 | P a g e
8 | P a g e
2.2 Pikeperch (Sander lucioperca) 2.2.1 General biology
2.2.1.1 Species description
Sander lucioperca, commonly known as pike perch, is a spiny-rayed piscivorous fish (figure
2.3) originating from the Percidae family (Craig, 2008). The taxonomic classification of this species is summarized in table 2.1 below.
Table 2.1 Taxonomic classification of Sander lucioperca fish species (Zakęś, 2002)
9 | P a g e
2.2.1.2 Ecology and growth
Sander lucioperca is a fresh- and brackish-water species inhabiting the lakes, rivers, estuaries
and reservoirs of temperate and subarctic regions of North America and Eurasia (Nelson, 1994). Lately, the species is now acclimated to the waters of Northern Africa (Algeria, Morocco, Tunisia) (Zakęś, 2002). It is a hardy species tolerating a wide range of environmental conditions and the main limiting factor dictating its distribution is water temperature. Higher temperatures of about 31°C have effects on the metabolic processes of pikeperch while low temperatures affect the reproductive success (Craig, 2008). The thermal optimum for pike-perch growth is thus about 27-28 °C but fast growth rates are already noted at 23 °C (Zakęś, 2002). Pikeperch can attain adult lengths of 50-70 cm and a wet weight (WW) of 2-5 kg, however, larger values for these parameters were recorded. Males attain maturity earlier (2-3 years) and are smaller in size than females who attain their maturity after 3-4 years Spawning starts from spring to summer (April-June) when the water temperatures range from 8 to 15 °C (Zakęś, 2002).
2.2.1.3 Natural life cycle
Spawning activities in pikeperch are characterized by parental care and switching of roles between males and females. Females, which are nest spawners (build a nest either on sand, gravel or aquatic vegetation) deposit their eggs into these nests and males actively guard them until hatching. During this guarding period, the males display territorial behaviour in which they protect the embryo from predators, but also keep oxygen levels high by fanning water currents. Healthy and mature females can approximately produce 200 eggs/g WW (Zakęś, 2002).
2.2.1.4 Feeding habits
Pike perch fish are known to have three trophic phases; planktonic, mixed feed stage and predatory phase (Kestemont et al., 2015). They begin with the planktonic phase where larvae prefer zooplanktons as their main feed, a behaviour which goes on until they are 13-30 mm in total length (TL) (~23 days post hatch, dph). The mixed feed stage follows where juveniles feed on invertebrate fauna and fish (TL 24-70 mm) and finally the predatory phase where their feed is comprised exclusively of fish (TL 34-80 mm) (FAO, 2002). On condition of availability
10 | P a g e
of the correct diet in their life cycle, pike perch mostly end up being very successful animals within the tolerable environmental conditions (Zakes, 2002; Steenfeldt, 2015).
2.2.2 Nutritional requirements for pike perch production
Knowledge on the quantity and quality of the nutritional composition of foods is key to avoiding nutritional disorders and to set diet formulations for fish among other organisms (Ózyurt et al., 2009). However, central to this, is understanding the nutritional requirements of the animals. Unlike salmonid species which produce large eggs and consequently large fry, obtaining nutrients from the yolk during several weeks, pike perch larvae, despite them being carnivores, are rather small at hatching and possess a relatively small yolk sac (Hamza, et al, 2015b). Due to this, the onset of exogenous feeding in pikeperch comes relatively early (<5 DPH) (Ljunggren, 2002). During the embryogenic and larval stages, Hamza et al. (2015b) reported that pike perch have a higher demand of lipids and fatty acids compared to other species. Pike perch were observed to metabolize saturated and unsaturated fatty acids during this period, however, metabolism of PUFAs was relatively lower than MUFAs. Of the n-6 PUFAs utilized, LA (linoleic acid) was shown to be catabolized more in starved pike perch larvae from the first day of starving while AA (arachidonic acid) only decreased after 5 days of starvation, suggesting a preservation of AA during the early life. On the other hand, DHA, EPA and LNA (α-linoleic acid) were progressively and intensely utilized during the starvation period (Hamza et al., 2015b).
The contribution of DHA for tissue renewal and energy requirements reached up to 20.3 % of the total fatty acids catabolized by starved pike perch compared to 8.2 % in Eurasian perch larvae (Ayad et al., 2000, Ayad et al., 2004). Higher contents of PUFAs (55.9% of TFA) were found in pike perch than Eurasian perch (50.6% of TFA) and this informed the suggestion that pike perch may have a particularly high PUFA demand (Ayad et al., 2004). Thus, their supplementation in the diet seems very necessary. The major roles played by these dietary components, e.g. DHA, in pike perch larvae include reduced stressed-induced mortality (Lund et al., 2012), improved responses to mechano-sensory stimuli that assist in escape responses (Lund et al., 2014), improved growth, reduced malformations and improved digestive enzyme activity (Lund et al., 2018). Apart from lipids, larval pike perch also require at least 43 % of dietary protein for adequate growth and feed efficiency (Wamwiza et al., 2005). Vitamins and
11 | P a g e
minerals may also be important components in pike perch nutrition, however there is a general scarcity of information on this regarding pike perch (Özyurt et al., 2009).
2.2.3 Pike perch production systems 2.2.3.1 Pond system
A complete production cycle (figure 2.4) of pikeperch involves broodstock, eggs, fry, larvae, juveniles and adult and this may be done indoors through recirculation aquaculture systems (RAS) or outdoors in ponds. Pond culture started in the early 20th century where production of pikeperch was done for stocking open waters. In these systems, spawners are obtained from natural waters in fall (October-November) or spring (March-April) and stocked in earthen ponds for natural spawning (Zakęś, 2002). When the mean daily water temperatures fall to 8-9°C, spawners are removed from the ponds and transported to spawning ponds. In these ponds, subsequent controlled or uncontrolled natural reproduction is applied. For controlled natural reproduction, spawning nests (turf, sea grass, rice straw or willow roots substrates) are placed on the broodstock ponds bottom. A ratio of 1:10 female to male is used in these ponds where after successful spawning, nests with eggs are transported to other ponds for fry production. In the uncontrolled broodstock ponds, a set of spawners, usually 1:2 female to male, are released into carp ponds to spawn. After spawning, the fish were left in the pond for a maximum of 2 months until fry were obtained. Production was done using either as monoculture or polyculture with carp, depending on which type of fry are used (obtained from fall or spring spawners). The production chain then proceeds to the nursery and finally to ongrowing ponds where after 3-4 years of culture, an average fish WW of 400-1000 g, corresponding with a production of 5-50 kg/ha was obtained. The main feed for the fish used during culture in these ponds include zooplankton, zoobenthos and formulated feed (Zakęś, 2002).
2.2.3.2 RAS system
Intensive rearing of pike perch larvae has made forward strides from small research-based setups to full commercial scale operations. These operations have thus incorporated the necessary technology and capacity to support the larvae requirements in highly intensified RAS cultures for further ongrowing of the species (Steenfeldt, 2015). RAS is an aquaculture method which was initially adopted mainly due to lack of space to expand and develop new ponds,
12 | P a g e
limited freshwater availability and concerns over pollution. It has since been promoted by mainly European aquaculture producer countries as one of the possible solutions and opportunities to further develop aquaculture (Badiola et al., 2012). This culture method is applied for, among other species, pike perch production in hatcheries where fish fry are obtained through the out-of-season reproduction technique. This method of obtaining fry was deemed possible after a successful study on the reproduction cycle of pikeperch (Fontaine et al., 2008). To perform out-of-season reproduction of pike perch in hatcheries today, wild or cultured broodstock are stimulated environmentally using temperature and photoperiod which are generally considered as vital cues for gametogenesis and spawning (Miguad et al., 2002). This process lasts for around 18 weeks of cooling, chilling and warming phases.
Figure 2.4 Pikeperch production cycle (Zakęś, 2002)
After these phases, hormonal stimulation is applied and this results to production of sex products (eggs and milt) several months before the natural spawning period (Zakęś, 2002). Gametes are then transferred to incubating jars in which they hatch, and the fry is collected and
13 | P a g e
transferred to larval tanks for intermediate development. Upon attaining the juvenile stage, stocking is done in ongrowing culture tanks where fish grow until the market size is attained. All the production stages in this method take place in RAS equipped rearing tanks where maintenance of water quality parameters (temperature, TAN, nitrites, pH and dissolved oxygen) and feed are given high priority. During the culture period, efficiency and growth parameters like feed conversion ratio (FCR), specific growth rate (SGR) and survival are estimated on a regular basis. To perform these activities, the use of anaesthetics is highly recommended to reduce induced stress due to manipulation of the fish (Zakęś, 2002). After at least 7 months of ongrowing in RAS, pikeperch attain a marketable size of 500-600 g (WW) however, >1 kg can be obtained after 15 months. Current feeds used for pike perch in the respective RAS culture stages include salmon (Salmo salar), rainbow trout (Oncorhynchus
mykiss) and sea bass (Dicentrarchus labrax) feeds produced by various feed manufacturers
(Badiola et al., 2012). Both sinking and floating feeds are used, and feeding is mostly automated. Use of live feed e.g. Artemia is prominent during the earlier larval stages of pike perch i.e. at the onset of exogenous feeding.
2.2.4 Major challenges and opportunities in pike perch production
Despite the increasing trends in pike perch production, larviculture of this species still stands as a primary bottleneck for a faster breakthrough. Low survival rates (of up to 20%) in hatcheries have led to less effectiveness and consequently high production costs (Verreth et al., 1987; Zakęś, 2002). Mass mortality of larvae is mainly attributed to the critical stage in larviculture which marks the onset of exogenous feeding, a stage when larval digestive capabilities are limited and thus requires the application of live feed (Wamwiza et al., 2005). The nutritional requirements of pike perch during this stage have been sparsely examined (Lund & Steenfeldt, 2011), and as well, species-specific diets for pikeperch are currently still under development. People today are using the classical live feed, Artemia nauplii from mouth opening to weaning and later use salmon, rainbow trout and sea bass formulated diets for ongrowing (Zakęś, 2002). However, the Artemia and weaning diets hardly fulfil the pike perch nutritional requirements and thus result to poor larval performance (Dhont et al., 2013). A correct diet thus needs to be identified and developed to meet these requirements. Because challenges in determining the correct larval diets have been considerably overcome in some species such as salmon and trout (Rollin et al., 2003), the methodologies used are being
14 | P a g e
transferred to finding solutions for emerging species like pike perch (Steenfeldt, 2015). Furthermore, other potential live feed organisms are under investigation and of late, there has already been considerable application of rotifers and marine copepods with some documented success in pike perch (Støttrup, 2003; Roca et al., 2018). Full success in this may be the breakthrough in pike perch production.
2.2.5 Status and trends in worldwide production
In the beginning, the 19th century, pike perch production was done in ponds in very negligible quantities and was referred to as an additional fish to the traditional cultured carp. This practice went on until the beginning of the 21st century when production quite improved. Pike perch production increased (figure 2.5) mainly due to a shift from extensive (pond) to more intensive (RAS) production, the main producer countries being Czech Republic, Denmark, Hungary, Romania, Tunisia and Ukraine. Pike perch production rose from 50 T in 1950 through 945 T (1995) to 1358 T in 2016 (FAO, 2002). Recently, pikeperch has been a major subject of intense research in both Central (Czech Republic, Hungary, Poland) and western (Belgium, Finland, France, Germany) Europe. More focus is on how to develop methods for intense aquaculture production of this species, mainly in RAS. Researchers are refining and developing methods for artificial production, including out-of-season spawning (Miguad et al., 2002; Zakęś, 2002) and this is expected to attribute to a potential increase in pike perch production.
15 | P a g e
2.3 Larval fish feeding 2.3.1 Live feeds in larviculture
2.3.1.1 Copepods (history, taxonomy, morphology and biology)
The name copepod originates from the Greek words kope, an oar, and podos, a foot, and refers to the flat, laminar swimming legs of the animals (Dhont et al., 2013). According to Mauchline (1998), copepods are probably the most numerous multicellular organisms on earth. They are aquatic animals which are primarily marine although some species also occur in large numbers in freshwater environments. They were estimated to be around 11,500 species by the end of 1993 by Humes (1994). Copepods belong to subclass Copepoda of the Crustacea Subphylum. The most common cultured species in the laboratory include orders Calanoida, Harpacticoida and Cyclopoida. Together, they represent just a third of the known orders of copepods (Dhont et al., 2013). Of these three orders however, the Calanoida are the most abundant in the marine environment, easily identifiable (Støttrup, 2006) and thus they have received most attention with most studies done on the Acartia and Calanus spp. (Mauchline, 1998).
Copepods generally have cylindrical bodies with a narrow abdomen (planktonic forms) or broader bodies or dorsoventrally compressed (benthic and surface living forms) (Støttrup, 2003). Their trunk is composed of a chitinous exoskeleton, with a fused head and thorax. They have several appendages protruding from the ventral thorax section which are used for feeding or locomotion. Newly hatched nauplii are generally small. The width of first naupliar stages of some copepod species e.g. Acartia spp. (figure 2.6b) (Schipp et al., 1999), Parvocalanus
crassirostris (McKinnon et al., 2003) and Tisbe spp. Were found to range from ~38 to 90 µm.
Adult copepods can reach up to body length ranges of 850-1050 µm (Belmonte et al., 1994; Suchman & Sullivan, 2000). In adults, the differentiating factor among members of the three earlier mentioned orders is the size of the antennae. Harpacticoids have the shortest, cyclopoids medium and calanoids the longest antennules (as long as the body itself) (Dussart & Defaye, 2001).
Reproduction in most copepods is sexually with genital openings generally located in the first segment of the abdomen. Calanoids are open water broadcasters, they shed eggs which sink slowly through the water column while both harpacticoids and cyclopoids bear eggs in one or two egg sacs which they possess. Copepod eggs can switch to a diapause state in case of harsh environmental conditions. In that case, often the diapause eggs must be stored for some time
16 | P a g e
under species-specific conditions of temperature before they can be induced to hatch (Marcus, 1987). Nauplii develop in these egg sacs until they are directly hatched into the water. Life starts with the nauplii stage which then molts through six stages with subsequent increase in size and development of appendages. The final naupliar stage moults into a copepodite, resembling the adult copepod, but still moults through six further stages before attaining adulthood (figure 2.6 a) (Støttrup, 2006). Calanoids are primarily planktonic (pelagic), 75% are marine, 25% live in fresh water (Mauchline, 1998). They utilize phytoplankton as food while most harpacticoids are benthic grazers and are mostly found on seagrass surfaces and other substrate feeding on bacteria and bacterial films. They are primarily marine species with only 10% living in fresh water. The last order, cyclopoids, display both benthic and planktonic ecological patterns and are generally omnivorous (Dhont et al., 2013). They are divided between marine and fresh water almost equally (Mauchline, 1998). More comprehensive reviews on copepod biology and ecology exist (e.g. Dussart & Defaye, 2001; Sazhina & Banse, 2006).
Figure 2.6 Schematic life-cycle of a copepod (a), A.tonsa nauplii (b) (Stottrup, 2006; Dhont et al., 2013).
Extensive or semi-intensive culture of copepods
This is commonly done in outdoor ponds or enclosed lagoons in the rearing of for example turbot, flounder and cod, and is deemed cost-effective due to the application of low technology (Dhont et al., 2013). Scandinavian countries e.g. Denmark have so far adopted this method commercially (Stóttrup et al., 1994). Operations of these systems rely on bloom culture of
17 | P a g e
phytoplankton and zooplankton to which the fry are added. The systems operate in all seasons except winter, where there is low prevailing air and sea temperatures. To culture copepods, filtered (20-40 µm) water is filled in the lagoons or ponds, fertilization maybe done to enhance phytoplankton bloom (Drillet et al., 2011) before careful administration of nutrients whose main aim is to selectively favour growth of particular algal species e.g. diatoms (Naas et al., 1991). Later, copepods are filtered from the surrounding seas and added to the system. Collection can be done with plankton nets to selectively sieve nauplii (80-250 µm), copepodite (80-350 µm) or adult stages (250-600 µm) (Støttrup, 2006). Some systems, however, start the culture with diapause copepod eggs that hatch from the sediments of the ponds (Dhont et al., 2013). According to Svåsand et al. (1998) extensive systems usually result to a mixture of copepods of different size ranges and species representing all three orders mentioned above. Due to the low intervention and input in these systems, low fish fry densities (1/L) are usually stocked. For purposes of feeding experiments, extensively cultured copepods are harvested through a filter system, separated into different size classes and fed to intensive larval rearing systems (e.g. Busch et al., 2011).
Intensive culture- batch
In this system, start-up production is done in tanks of 100-300 L recommended size, with a low ratio of bottom area to height (Stóttrup et al., 1986) using filtered, UV-treated seawater (Marchus & Wilcox, 2007). These tanks are stocked with nauplii or copepodites, at stocking densities of 100-2000/L (Støttrup, 2003). Feed is then administered in a way and type corresponding to the natural feeding behaviour of copepods. Planktonic copepod species are supplied with suitably sized phytoplankton at densities that allow for optimal filtration and hence maximum growth rates (Støttrup & Jensen, 1990). Benthic copepod species are fed a range of formulated feeds and are not dependent on fresh phytoplankton supply. They also require a surface area to graze on and thus substrate is provided to increase the surface area per volume or per floor area. Daily cleaning of tanks is necessary for maintenance of good water quality (Støttrup, 2003).
At the end of each culture, harvesting of either eggs (mainly from broadcasting copepods e.g. calanoids) or nauplii (from egg bearing copepods e.g. harpacticoids and cyclopoids) takes place (Dhont et al., 2013). Eggs normally sediment out and are hence easily siphoned out off the bottom while nauplii are separated from adults and detritus using graded sieves in a proportion
18 | P a g e
of the culture volume. High production e.g. 3.77 nauplii/mL for the paracalanid Parvocalanus
sp. (Shields et al., 2005) and 440,000 nauplii/day for the calanoid Gladioferens imparipes
(Payne & Rippingale, 2001) have been recorded. One such comprehensive production procedure, which also includes counting of eggs and nauplii of A. tonsa is provided by Marchus and Wilcox (2007). Major challenges for batch culture include administering balanced proportions of feed sufficient enough to maintain egg production without compromising water quality (Dhont et al., 2013).
Continuous and automated culture
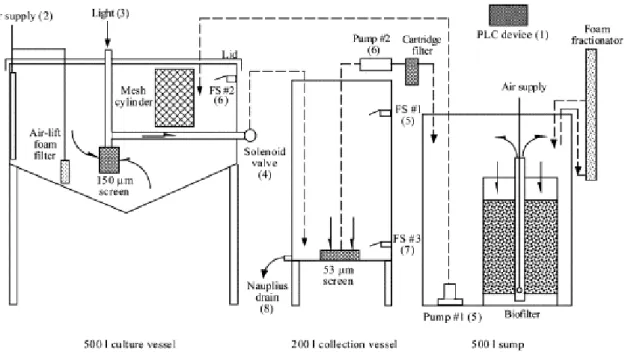
Several advances have been made towards automating copepods production and harvest systems. Such advances replaced batch culture with RAS, which involves continuous partial water exchange and automatic egg and nauplii harvesting (Støttrup & Norsker, 1997) (figure 2.7). In these systems, does not stop at harvesting then restart later like in the intensive batch culture, instead, it goes on alongside continuous harvesting. Separation of nauplii from species with egg bearing adults that was initially impossible especially in batch cultures is possible in these advanced systems. For example, nauplii are separated from debris and adult copepods using environmental cues such as light prior to the automated harvest.
In this technique, which Støttrup and Norsker (1997) developed for Tisbe holothuriae, the natural traits of nauplii and copepodites stages are utilised to do the separation. The first naupliar stages are known to swim freely in the water column but are generally weaker swimmers than adults. So, light above the tank would switch on to drive copepodites and adults (strong swimmers) away towards the tank bottom leaving nauplii behind. The flow would then be reversed so that water is drawn from the surface instead of the bottom, harvesting nauplii only, from the water column (Støttrup, 2006).
Another technique was later developed to automatically harvest eggs (Toledo et al., 2005). This allows for automatic separation of broadcasting copepods from their eggs. To do this, adults are retained in the upper water layer using a mesh filter, then eggs and algae are airlifted back into a retainer tank in which eggs are concentrated within a sieve. This allows for easy collection of clean eggs. A number of other continuous and automated systems were developed for various copepod species e.g. Amphiascoides atopus with an average production of
19 | P a g e
Figure 2.7 Schematic diagram of an automated 500-L copepod culture system. Nauplius collection and water recycling procedures are described as follows: 1 Harvest sequence initiated by pressing the ‘start’ button on the PLC device. 2 Air supply to the culture vessels turned off for 5 min to stop water movement. 3 Light turned on for 10 min to illuminate 150-µm screen submerged in culture vessel. 4 Solenoid valve opened so that water (containing nauplii) siphons through 150-µm screen into harvest vessel. 5 Float switch (FS) #1 activated which closes solenoid value, turns light off and turns pump #1 on. 6 FS #2 activated which turns pump #1 off and pump #2 on (drawing through 53-µm screen). 7 FS #3 deactivated which turns pump #2 off and air supply on. 8 Concentrated nauplii drained from harvest vessel (M. F. Payne & Rippingale, 2001).
500,000/day over five months by Sun and Fleeger (1995) and G. imparipes with a production of ~800 nauplii/L (Payne & Rippingale, 2001). Storage and preservation of harvested Storage and preservation of harvested copepod nauplii can be done in static seawater at densities of 30 to 100 ml-1 in 4-8°C refrigeration conditions for up to 1 week and can be used on days when
the production is low (Støttrup, 2003). Frozen preserved copepods were also shown to be beneficial as a supplement to Artemia nauplii as prey for the ornamental fish Amphiprion
clarkia (Olivotto et al., 2010). Lastly, despite the so far seen advancements, Dhont et al. (2013)
recommend close collaborations of experts within the industry in order to take the final step towards more effective commercial culture systems.
20 | P a g e
Nutritional value and application of copepods as live feed
Båmstedt (1986), Støttrup (2003 and Dhont et al. (2013), provide comprehensive descriptions on the nutritional profile of marine copepods, which suggest evidence that copepods possess impressive nutritional merits. This is in respect to their use in the larviculture of various fish species, especially marine. Marine copepods have lipid contents ranging from 2-73% depending on latitude, season and food availability (Bamstedt, 1986). The contents are generally high in high latitudes and low in medium and low latitudes. The detailed dynamics regarding the mentioned changes in lipids are explained by Støttrup (2003). Lipid levels are often high in newly hatched nauplii and early copepodite stages with the predominant forms being structural phospholipids (Sargent & Petersen, 1988) which are rich in n-3 polyunsaturate fatty acids. Having been suggested by McKinnon et al. (2003) that dietary phospholipids are more biologically available to developing fish larvae than neutral lipids which are in high abundance in Artemia and rotifers, their presence in copepods is no doubt a nutritional merit to larvae.
Nanton and Castell (1999), provided evidence that copepod species have the ability to synthetize PUFAs, and store most of them in their polar lipid fraction (Bell et al., 2003). This evidence coincides with the claims by Dhont et al. (2013) that DHA and EPA content are higher in both extensively and intensively cultured copepods (adults and nauplii) and proportionally higher than in rotifers or Artemia nauplii. Calanoids, especially those inhabiting high latitudes, were found to be characterized by wax esters in their copepodite IV and V stages (Sargent & Petersen, 1988). These esters are rich in various fatty alcohols, but most importantly 90% of their makeup is EPA (20:5n-3) and DHA (22:6n-3). These fatty acids have been demonstrated to be essential for reduction of growth anomalies and survival of marine larvae (Hamre et al., 2005; Busch et al., 2011).
According to Bamstedt (1986) protein contents in marine copepods range from 24 to 82% dry weight and are highest in species from medium latitudes. The author further says that the high latitude species have an increasing level of free amino acids (FAA) with increasing environmental salinity. He suggests that, the most abundant FAA in copepods include glycine, alanine, arginine, lysine, proline and taurine. Copepods themselves utilize these FAA in osmotic regulation (Støttrup, 2003) but for fish larvae, these components may be used for protein biosynthesis especially in those fish with high protein requirements. Developing fish larvae have low protein digestibility and hence rely on their high assimilation capacity for FAA.
21 | P a g e
which were reported to be as twice as abundant in copepods as in enriched Artemia nauplii (Meeren et al., 2008). Copepods also contain high levels of vitamin C, which is known to stimulate reproduction in crustaceans (Hapette & Poulet, 1990). Enzymes including proteases, amylase, esterase and phosphodiesterase were also found to be high in copepods (Eurytemora
hirundoides) (Moran et al., 1990).
The suitability of copepods as live feed has been demonstrated in several established marine species like cod (Gadus morhua) seabass (Lates calcarifer) and halibut (Hippoglossus
hippoglossus) (Shields et al., 1999; Rajkumar, 2006; Busch et al., 2011). As such, Dhont et al.
(2013) considers copepods as the most superior studied live feed in terms of nutritional composition.
2.3.1.2 Artemia (general biology and use as live feed)
Artemia is a primitive arthropod with a segmented body to which broad leaf-like appendages
called thoracopodes are attached. An adult male is ~8-10 mm and female is ~10-12 mm. Their body is divided into head, thorax and abdomen and each of these sections is segmented severally. The entire body is covered by muscles which a internally attached to a thin and flexible chitin exoskeleton (Dhont et al., 2013). There are sexually reproducing Artemia species as well as parthenogenetically reproducing populations. Endemic to Europe, Africa and Asia are the parthenogenetic species however, as well bisexual populations are found (Dhont et al., 2013). Within the Artemia genus, there is still confusion in terms of phylogenetic relationships of populations and species which requires a multi-trait approach to address (Baxevanis et al., 2006).
Commonly referred to as brine shrimp, Artemia is a zooplankton inhabiting hypersaline environments such as salt lakes, coastal salt pans and man-managed saltworks. Artemia does not exist in areas with year-round low temperatures although a lot of strains are still found in the continental areas of North and South America and Asia which are characterized by extremely cold temperatures with the condition that sufficiently high summer temperatures allow cyst hatching and subsequent colonization of the environment (Dhont et al., 2013). Brine shrimp is an extremely osmotolerant organism and thus survive in a wide range of salinities of approximately 10-340 g/L. Most abundant populations of Artemia occur within elevated salinity levels in which their predators or food competitors are eliminated. During reproduction,
22 | P a g e
Artemia are either ovoviviparous (release nauplii), especially when the environment is
favourable, or oviparous (produce cysts) under adverse ecological conditions (Criel & Macrae, 2002). Under favourable ecological conditions, hatched individuals can live for several months, developing into adult form in only eight days and reproduce up to 300 nauplii or cysts every four days. Knowledge of strain-specific genotypic and phenotypic characteristics can increase the effectiveness of the application of Artemia as live feed in aquaculture according to Dhont et al. (2013).
Today, most Artemia product reaching the world market is A. franciscana (Kellogg, 1906) from the Great Salt Lake (GSL) or parthenogenetic product from several resources in central and eastern Asia. The global demand of Artemia cysts resources is said to be widening (Shadrin & Anufriieva, 2016). Investigators adopted brine shrimp since its inception in 1930, as a convenient replacement for the natural plankton diets for larval fish, and that made the first breakthrough in the culture of commercially important fish species (Rode et al., 2011). Today, protocols for hatching, decapsulation and enrichment of Artemia have been optimized and consequently, its application in commercial hatcheries and research institutes has become a standard routine. Such protocols are comprehensively described in several studies (e.g. Vanhaecke & Sorgeloos, 1980; Dhont & Van Stappen, 2003). However, persistent efforts to get rid or replace Artemia with alternative feeds including formulated and/or other live feeds have been witnessed since its introduction.
Nutritional value of Artemia
Initially, HUFA content of Artemia was considered the most crucial factor in the larval rearing success of fish and crustaceans according to Dhont et al. (2013). That is why studies on the
Artemia HUFA profile kicked off long ago. Sorgeloos et al. (2001) provides a review of the
history of these studies on dietary HUFAs. As Artemia was becoming more popular as live feed in aquaculture, more information on the specific deficient and/or insufficient nutrients was realised through research. These deficiencies were attributed to the failure of Artemia to satisfy all requirements of predator larvae especially the marine species (Dhont et al., 2013). However, different enrichment procedures were later developed to overcome these bottlenecks.
Recent research suggests that DHA, the most important HUFA for mainly marine larvae is more important nutritionally than EPA; however, standard quality Artemia are usually higher in EPA, but lack DHA (Treece, 2000). The author reports that different strains vary in size and nutritionally, particularly in the content of EPA and DHA. Artemia nauplii from San Francisco