Effect of Pain Neuroscience Education
Combined With Cognition-Targeted Exercise
Therapy in Chronic Whiplash Associated
Disorders:
A Randomized Controlled Trial
De Backer Ann-Sophie
Student number: 01508953Deknudt Gaëlle
Student number: 01506524Paelinck Eva
Student number: 01406180Promotor:
Prof. Dr. Mira Meeus
Copromotor: MSc Dorine Lenoir
A dissertation submitted to Ghent University in partial fulfilment of the requirements for the degree of Master of Rehabilitation Sciences and Physiotherapy.
Effect of Pain Neuroscience Education
Combined With Cognition-Targeted Exercise
Therapy in Chronic Whiplash Associated
Disorders:
A Randomized Controlled Trial
De Backer Ann-Sophie
Student number: 01508953Deknudt Gaëlle
Student number: 01506524Paelinck Eva
Student number: 01406180
Promotor:
Prof. Dr. Mira Meeus
Copromotor: MSc Dorine Lenoir
A dissertation submitted to Ghent University in partial fulfilment of the requirements for the degree of Master of Rehabilitation Sciences and Physiotherapy.
AWKNOWLEDGEMENTS
This study was realized with the kind support, help and encouragement of many individuals. We would like to extend our sincere thanks to all of them.
First of all, we would like to extend our gratitude to our promoter Prof. Dr. Mira Meeus and our copromotor MSc Dorine Lenoir who helped a lot during this period, providing us with support, clear guidance, advice, valuable comments, suggestions and provisions. Especially the quick and clarifying communication during the COVID-19 crisis, when physical consultation was impossible, was very much appreciated.
Furthermore, we also thank our parents and friends for reassuring and supporting us and for always believing in us. Lastly, we are grateful for each other and for the pleasant and fruitful collaboration over the past two years.
TABLE OF CONTENT
LIST OF TABLES AND FIGURES ………. 6
LIST OF ABBREVIATIONS ………... 7 RESEARCH Abstract (English) ……….. 8 Abstract (Nederlands) ………... 9 Introduction ……….. 11 Method Design ………... 13 Participants ………... 13 Research procedure ……….. 14 Intervention ………... 16 Statistical analysis ………... 17 Results Baseline characteristics ……….. 18
Within group differences ……… 20
Between group differences ……….. 21
Post hoc power analysis ………. 22
Discussion Outcome compared to existing literature ………. 23
Practical Implications and Recommendations for Research ………. 25
Limitations and strengths ……….. 26
Conclusions ………. 27
References ……….. 28
ABSTRACT IN LEKENTAAL ……… 33
VERIFICATION OF SUBMISSION WITH THE ETHICAL COMMITTEE ………... 34
ATTACHMENTS Appendix 1: Figures of therapy effect ………. 41
LIST OF TABLES AND FIGURES
Table 1 In- and exclusion criteria
Figure 1 Flowchart showing the recruitment Table 2 Baseline characteristics
Table 3 Paired Samples t-Test Table 4 Repeated Measures ANOVA Table 5 Post hoc power analysis Appendix 1: Figures of therapy effect
LIST OF ABBREVIATIONS
Prof. Professor
Dr. Doctor
MSc Master of Science
cWAD Chronic Whiplash Associated Disorders PNE Pain neuroscience education
CS Central sensitization
QST Quantitative Sensory Testing NDI Neck Disability Index
NRS Numeric Rating Scale
CSI Central Sensitization Inventory PCS Pain Catastrophizing Scale EDT Electrical detection threshold EPT Electrical pain threshold
TS Temporal summation/temporele summatie CPM Conditioned pain modulation
t Value used in T-tests
p Probability value
EDD Elektrische detectiedrempel EPD Elektrische pijndrempel MRI Magnetic resonance imaging
WAD ( ׀׀/ ׀׀׀) Whiplash Associated Disorders (of the second or third degree) ICC Intraclass Correlation Coefficient
CSS Central sensitivity syndrome
Hz Hertz
ms Millisecond
mA Milliampere
°C Degrees Celcius
BMI Body mass index
Std. Standard Sig. Significant y Years cm Centimetre kg Kilogram df Degrees of freedom Noncent. Noncentrality e.g. Exempli gratia
8
RESEARCH
Abstract (English)
Background: Effective treatments for patients with chronic whiplash associated disorders (cWAD) are essential in reducing socioeconomic costs and for improving patients’ functionality.
Objectives: The aim of this study was to investigate if pain neuroscience education (PNE) combined with cognition-targeted exercise therapy is superior to current best-evidence physiotherapy for reducing symptoms of central sensitization (CS), frequency of pain and improving functionality in patients with cWAD. Furthermore, it was investigated whether the electrical thresholds, measured with Quantitative Sensory Testing (QST), had increased after the interventions were applied.
Study Design: This study is a multicenter double-blind randomized controlled trial conducted from September 30, 2019, to May 16, 2020 on 53 patients with cWAD.
Methods: Patients were randomly allocated in two groups. One group received an experimental pain neuroscience treatment, while the control group received best-evidence physiotherapy. The primary outcome measure in this study was the Neck Disability Index (NDI). The Numeric Rating Scale (NRS), Central Sensitization Inventory (CSI), Pain Catastrophizing Scale (PCS) and QST, consisting of the electrical detection threshold (EDT), electrical pain threshold (EPT), temporal summation (TS) and conditioned pain modulation (CPM) were considered as secondary outcome measures. The efficacy of both therapies was determined by comparing baseline assessment results with post-therapy assessment results using Repeated Measures AVONA. A p-value lower than 0.01 was considered significant.
Results: Fifty-three patients participated in the current study: 25 in the control group and 28 in the experimental group. There was one significant difference uncovered in the baseline data between the two intervention groups, which was the CSI [t(51)= -3.095; p=0.003]. From the within group analysis in the control group, only the CSI [t(21)=3.583; p=0.002] and the EDT of the right arm [t(21)= -3.477; p=0.002] showed significantly better results. The group with the experimental intervention clearly showed some differences. The NDI [t(20)=3.205; p=0.004], the CSI [t(20)=3.831; p=0.001], the PCS [t(20)=4.282; p<0.001], average pain scored with the NRS [t(20)=5.363; p<0.001] and heaviest pain scored with the NRS [t(20)=3.337; p=0.003] had all significantly improved. There was no significant result found searching for between group differences.
Conclusion: Best-evidence physiotherapy and the experimental modern neuroscience approach have similar short-term effects. However, significant within group differences were found, as patients who received the experimental treatment showed improvements on functionality in daily life, somatic and
9
emotional symptoms linked to CS, pain intensity, handling pain and improvement of the negative assumptions towards harmful stimuli. In comparison, patients who received the control intervention only showed significant progression for pain sensitivity of the right arm and somatic and emotional symptoms linked to CS.
Keywords: whiplash associated disorders, central sensitization, treatment, exercise therapy, neuroscience education
Abstract (Nederlands)
Achtergrond: Effectieve behandelmethoden voor chronische whiplashpatiënten zijn essentieel in het verminderen van socio-economische kosten en om de functionaliteit van patiënten te verbeteren. Doelstellingen: Het doel van deze studie was het onderzoeken of pijn neurofysiologische educatie gecombineerd met cognitief georiënteerde oefentherapie superieur is ten opzichte van hedendaagse kinesitherapie in het verminderen van centrale sensitisatie (CS), pijnfrequentie en het verbeteren van de functionaliteit bij chronische whiplashpatiënten. Er werd ook nagegaan of er hogere drempels met Quantitative Sensory Testing (QST) na de interventie plaatsvonden.
Onderzoeksdesign: Deze studie is een multicenter dubbelblinde gerandomiseerde gecontroleerde trial uitgevoerd tussen 30 september 2019 en 16 mei 2020.
Methode: Patiënten werden willekeurig verdeeld in twee groepen. De ene groep kreeg de experimentele pijnbehandeling met neurowetenschappelijke aanpak, terwijl de controle groep de huidige kinesitherapeutische behandeling met de meeste evidentie kreeg. De primaire uitkomstmaat was de Neck Disability Index (NDI). De Numeric Rating Scale (NRS), Central Sensitization Inventory (CSI), Pain Catastrophizing Scale (PCS) en QST, bestaande uit de elektrische detectiedrempel (EDD), elektrische pijndrempel (EPD), temporele summatie (TS) en geconditioneerde pijn modulatie (CPM) waren de secundaire uitkomstmaten. Het effect van beide behandelingen werd bepaald door de baseline resultaten te vergelijken met de resultaten die verkregen waren na het beëindigen van de behandeling door middel van een ANOVA met herhaalde metingen. Een p-waarde kleiner dan 0.01 werd vooropgesteld als significant.
Resultaten: Drieënvijftig patiënten namen deel aan deze studie: 25 in de controle en 28 in de experimentele groep. Er werd één significant verschil tussen de twee interventiegroepen ontdekt in de baseline data, namelijk bij de CSI [t(51)= -3.095; p=0.003]. Uit de analyse binnen elke groep werden in de controle groep significante resultaten bij de CSI [t(21)=3.583; p=0.002] en EDD van de rechterarm
[t(21)= -3.477; p=0.002] gevonden. De groep die de experimentele interventie kreeg, toonde duidelijke verschillen. De NDI [t(20)=3.205; p=0.004], de CSI [t(20)=3.831; p=0.001], de PCS [t(20)=4.282;
10
p<0.001], gemiddelde pijn gescoord met de NRS [t(20)=5.363; p<0.001] en hevigste pijn gescoord met de NRS [t(20)=3.337; p=0.003] waren significant beter. Er werd geen significant resultaat gevonden wanneer getest werd op verschillen tussen de twee groepen.
Conclusie: Op korte termijn is er geen verschil waargenomen tussen de experimentele en controle behandeling. Desondanks werden er significante verschillen binnen elke groep gevonden. Patiënten uit de experimentele groep toonden verbeteringen wat betreft functionaliteit in het dagelijks leven, somatische en emotionele symptomen gelinkt aan CS, pijnintensiteit, omgaan met pijn en negatieve opvattingen tegenover schadelijke stimuli. Dit in tegenstelling tot de patiënten die de controle behandeling kregen. Zij toonden enkel significante verbeteringen wat betreft pijn sensitiviteit van de rechterarm en somatische en emotionele symptomen die gelinkt worden met CS.
Keywords: chronische whiplashpatiënten, centrale sensitisatie, behandeling, oefentherapie, neurowetenschappelijke educatie
11
Introduction
Chronic pain is defined as pain persisting longer than normal healing time and is characterised by the lack of immediate warning function of physiological nociception [1]. Pain is usually recognized as chronic when it endures or recurs for more than three to six months. In today’s society, chronic pain is a frequent condition with a prevalence up to 23% in Belgian adults [2]. It is estimated that 50% of patients with an acute whiplash injury develop ongoing pain and long-term disability, also defined as chronic whiplash associated disorders (cWAD) [3,4]. Whiplash injury is a collection of acute neck-related symptoms and is often caused by a motor vehicle accident which can result in injuries to bones or soft tissues [5]. When several symptoms including chronic neck pain, fatigue, headaches and dizziness persist for at least six months, the diagnosis of cWAD can be made [6]. However, the majority of these patients present no physical signs even when advanced imaging techniques are used [5]. Factors such as abnormalities in post-injury magnetic resonance imaging (MRI), radiological findings, motor dysfunctions and collision factors were identified as not being associated with the prognosis of whiplash [7]. Hence, a patient suffering from chronic pain and disability after whiplash injury can possibly be involved in a minor car accident with no motor dysfunction or tissue damage. Increased responsiveness of the central nervous system and central pain processing are likely to play a key role in the transition from acute whiplash towards cWAD [8]. Moreover, research has already implied an important contribution of several predictors such as post-injury pain and disability, post-injury anxiety, catastrophizing and cold hyperalgesia [7].
Central sensitization (CS) is indeed seen as one of the possible mechanisms underlying chronic pain disorders including cWAD [3,5,9]. Clifford Woolf stated in 1983 that chronic pain arises from both peripheral sensitization as from changes in the activity of the spinal cord [10]. Peripheral sensitization is defined as the reduction in threshold and amplification in the responsiveness of nociceptors. This occurs when the peripheral ends of these neurons are exposed to noxious stimuli, inflammatory mediators and damaged tissue [11]. In contrast, CS is operationally defined as “an amplification of neural signalling within the central nervous system that elicits pain hypersensitivity” [12]. Various mechanisms contribute to CS, such as impaired descending pain inhibitory pathways, increased activity of pain faciliatory pathways and wind-up [9]. Clinically, CS is characterized with generally decreased pain thresholds, as well as with an increased sensitivity for non-mechanical stimuli [13]. Given the rising prevalence of CS [2], there is a definite need for proper screening tools. Therefore, a systematic review summarizing the available methods for detecting CS in various chronic pain patients was conducted between October 2018 and May 2019. Another aim of this review was to investigate if
12
conclusions could be made concerning the psychometric properties of these tests. Quantitative Sensory Testing (QST) was found to be an experimental assessment tool investigating somatosensory perception in a non-invasive way, using mechanical, thermal and electrical stimuli at controlled intensities [14]. QST measures the function of thin, non-myelinated C fibers and myelinated Aδ fiberfs and is able to evaluate the excitability of different pain mechanisms [15,16]. The Central Sensitization Inventory (CSI) was used as a clinical method to assess CS. This self-reported method has strong psychometric properties and may be a clinically useful outcome measure. It is important to highlight that the CSI’s main goal is to quantify symptom severity and not to diagnose patients with particular conditions [17,18]. As identified above, measuring CS remains a complex challenge due to the different contributing mechanisms. Hence, this may explain why, at present, there is still no golden standard for measuring CS in humans [12].
Given the rising evidence supporting the clinical significance of CS in cWAD, the awareness is growing that CS should be a treatment target in these patients [5,7]. Current best-evidence physiotherapy focusses on input (treatment of peripheral structures) and output (motor control training) mechanisms, yet pays minor attention to the central or processing component [13]. Pain neuroscience education (PNE) combined with a cognition-targeted exercise program has been introduced as a potential and alternative approach in chronic spinal pain and in patients with persisting complaints after whiplash injury because it tackles the presence of both peripheral dysfunctions, as well as alterations in brain structure and function [13].
This modern neuroscience approach has previously been investigated and compared with current best-evidence physiotherapy in a randomized clinical trial in patients with chronic nonspecific spinal pain [19]. It appears to be more effective than current best-evidence physiotherapy for improving pressure pain sensitivity, symptoms of CS, disability, mental and physical functioning, and pain cognitions. According to the researchers’ knowledge, no study has made a comparison between these two therapies in cWAD. Therefore, the aim of this multicenter randomized controlled trial was to investigate whether PNE combined with a cognition-targeted exercise program is different when compared to current best-evidence physiotherapy in reducing frequency of pain, improving functionality, decreasing symptoms of CS and whether higher thresholds with QST occurred in individuals with cWAD.
13
Method
Design
This study was a multicenter double-blind randomized controlled trial.
Participants
1. In- and exclusion criteria
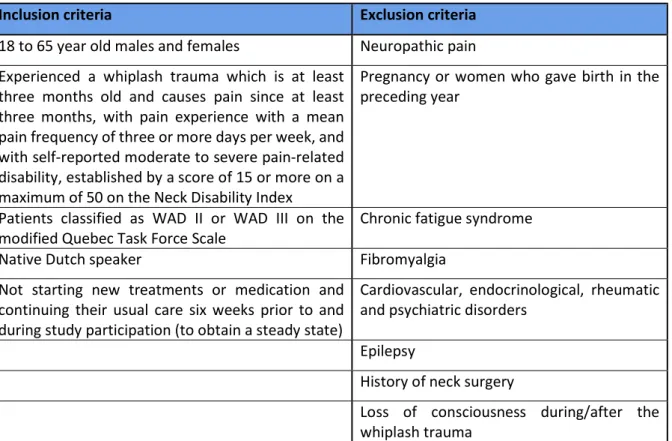
The in- and exclusion criteria can be found in table 1.
Patients were asked to refrain from non-opioid analgesics in the previous 48 hours of the assessments as well as caffeine, alcohol and nicotine in the 24 hours prior to the assessments.
Table 1 In- and exclusion criteria
Inclusion criteria Exclusion criteria
18 to 65 year old males and females Neuropathic pain Experienced a whiplash trauma which is at least
three months old and causes pain since at least three months, with pain experience with a mean pain frequency of three or more days per week, and with self-reported moderate to severe pain-related disability, established by a score of 15 or more on a maximum of 50 on the Neck Disability Index
Pregnancy or women who gave birth in the preceding year
Patients classified as WAD II or WAD III on the
modified Quebec Task Force Scale Chronic fatigue syndrome
Native Dutch speaker Fibromyalgia
Not starting new treatments or medication and continuing their usual care six weeks prior to and during study participation (to obtain a steady state)
Cardiovascular, endocrinological, rheumatic and psychiatric disorders
Epilepsy
History of neck surgery
Loss of consciousness during/after the whiplash trauma
2. Recruitment
Recruitment started at the end of May 2019. Letters with information, along with flyers and posters regarding the study were sent to general physicians, hospitals, pharmacists and physical therapists throughout Flanders. Flyers and posters were also spread throughout these regions and were distributed to public places such as sport club cafeterias, cafes, restaurants etc. After contacting the researchers through mail, possible participants received an informed consent. After approval of the consent, they were asked to fill in the study participation questionnaire. Depending on these answers, they were considered eligible or not.
14
Research procedure
Pre- and post-therapy differences were evaluated through questionnaires and QST. The questionnaires were filled in within two weeks of pre- and post-therapy assessment, while QST was administered during therapy assessment.
1. Primary outcome measure
As a primary outcome measure, the self-reported functional status or disability was measured with The Dutch version of the Neck Disability Index (NDI). The NDI is a short and easy to use questionnaire, which contains ten items [20]. Its purpose is to assess self-rated disability in patients with neck pain [21]. The NDI was translated in Dutch and has acceptable reliability (ICC = 0.84) [22], good internal consistency (Cronbach’s α = 0.83) and good overall validity in chronic neck pain patients [20].
2. Secondary outcome measures
2.1 Questionnaires
Self-reported pain assessment was measured by a 0-10 Numeric Rating Scale (NRS). This questionnaire assesses the average and heaviest intensity of pain. Ferraz et al. (1990) [23] demonstrated that the NRS has excellent reliability in both literate and illiterate patient groups (Pearson’s correlation = 0.963 and 0.947). Salaffi et al. (2004) [24] examined the minimal clinically important difference in pain intensity on the NRS that is most associated with improvement of the patient’s global impression of change. They observed a consistent relationship between NRS changes and the patient’s global impression of change. Results showed that a reduction of one point (or 15%) in the NRS equalled a minimal clinically important difference in pain, while reduction of two points or 33% equalled with much better improvement. These study results show that a one-point reduction on the NRS corresponds to significantly less pain in the patient.
Self-reported symptoms of CS were measured with the Dutch version of the CSI. The CSI consists of two parts [17]. Part A contains 25 statements which are related to current health symptoms. Part B questions previously diagnosed central sensitization syndromes (CSS) and related conditions. The CSI has excellent test-retest reliability (ICC = 0.88), excellent internal consistency of all 25 statements (Cronbach’s α = 0.91) and good discriminative power (p <0.001) in chronic musculoskeletal pain patients [25].
Pain catastrophizing was measured with the Dutch version of the Pain Catastrophizing Scale (PCS). The PCS is a self-report questionnaire which measures pain catastrophizing by looking at three different
15
aspects of pain: rumination, magnification and helplessness [26]. A meta-analysis by Wheeler et al. (2019) [27], which included both healthy individuals as well as individuals who suffered from any health condition, indicates good internal reliability (α = 0.92) and test-retest reliability (Spearman’s coefficient = 0.88), as well as excellent internal validity scores (coefficient α’s = 0.87-0.93) for total PCS scores.
2.2 Quantitative sensory testing
QST comprises multiple psychophysiological tests that measure the function of the somatosensory nervous system [28]. In this study, three different tests were applied: measurement of the electrical detection and pain threshold (EDT and EPT), temporal summation (TS) and conditioned pain modulation (CPM). A DS7A Digimeter, which is an electrical stimulator, was used to deliver the electric stimuli.
Electrical detection and pain threshold
To determine the electrical detection and pain threshold, consecutive electrical stimuli were administered on three different locations: at both median nerves (at the height of the wrist) and at the sural nerve of the dominant leg (at the height of the ankle). The dominant leg was considered to be the same side of the worst neck complaints. In case of bilateral pain, the dominant leg was the same as the dominant hand. The testing locations were randomized a priori and this sequence was maintained for the different QST-sub tests. A train of five stimuli at 250 Hz was administered. Each stimulus lasted 1 ms. The intensity of the stimuli gradually increased with 0.05 mA until patients could feel something (EDT) and until it became an unpleasant, but not yet painful sensation (EPT). Three measurements were taken for each threshold, with the final value being the average of those three measures. There was a 30 seconds rest period in between two measurements.
Temporal summation
TS of electrical pain measures endogenous pain facilitation and was assessed by delivering 20 electrical stimuli at the intensity of the electrical pain threshold. The NRS was measured on the 1st, 10th and 20th
stimulus. To detect the often increasing pain sensation, the final TS score was determined as followed: [NRS(10th) – NRS(1st)]+[NRS(20th) – NRS(10th)].
Conditioned pain modulation
Endogenous pain inhibition was measured by a CPM paradigm. An electrical stimulation was used as the test stimulus while the cold pressor test (immersing hand into 12°C water) was used as a condition stimulus. The intensity of the test stimulus was 1.4 times the average of the pain threshold from the
16
above-mentioned test location. Patients were automatically stimulated for three minutes through a randomized and arbitrary interval from eight to twelve seconds and received 20 stimuli in total. The CPM protocol lasted three minutes and finished automatically. The pain elicited by the electrical stimuli was questioned with an NRS score during and after immersion in the cold water. To determine the CPM effect, the pre-score was deducted from the post-score. A higher value represented an effective CPM effect.
Intervention
Participants of the study were randomized in two groups. The first group received an experimental treatment with a modern pain neuroscience approach. This treatment included three sessions of therapeutic PNE, 15 sessions of dynamic and functional cognition-targeted exercise therapy and stress management techniques. PNE educates patients about the neurophysiology of pain and aspires to reconceptualize pain as an interpretation of input signals by brain and nervous system rather than being an indicator of damage [29]. Dynamic and functional cognition-targeted exercise therapy followed the next principles: exercises were performed in a time contingent way, meaning that the emphasis lied on the duration of exercise, rather than the pain [13]. Treatment goals were made together with the patient and were based on functionality [30]. The physical therapist constantly assessed and challenged the patients’ pain cognitions and perceptions as well as the anticipated outcome of each exercise in order to alter maladaptive cognitions and perceptions into good ones. Stress management consisted of four phases [7]. In the first phase, patients were educated about stress and what it does to the body. Next, patients were introduced to different kinds of relaxation techniques in the initiation phase. One of these techniques was chosen and practised in the skills training phase. Lastly, patients were gradually exposed to their stressors in the confrontation phase, where they had to use the techniques they had learned to handle stressful situations.
The second group received current best-evidence physical therapy, consisting of 18 sessions. Three of those were educational sessions, where the concept of pain was approached in a (bio)mechanical way and patients received a basic explanation about the human anatomy. The remaining 15 sessions consisted of graded and active exercise therapy which focused on strength, flexibility, endurance and ergonomic principles. In this group, exercise therapy was handled in a symptom-contingent way, meaning that exercises were guided by the amount of pain rather than time [30].
Both therapies were distributed over the course of 16 weeks. The efficacy of both therapies was determined by comparing baseline assessment results of the NDI, NRS, CSI and QST with the follow-up assessment results.
17
Statistical analysis
All data were analysed using SPSS Statistics 26. Descriptive analyses of gender, age, height, weight, Body Mass Index (BMI), time since whiplash, primary (NDI) and secondary outcome measures (CSI, NRS, QST and PCS) were presented. These outcome measures were compared between pre- and post-therapy within each group and between experimental and control treatment differences. In preparation of the statistical analysis, the data was tested for normality and parametrics to select the correct consecutive tests. The pre- to post-therapy evolution was examined using the Paired-Samples t-Test. To evaluate the between group interaction, the groups were analyzed through Repeated Measures ANOVA. An a priori sample size calculation was not conducted, however the post hoc power was nevertheless measured to calculate the effectiveness of the study. A p-value lower than 0.01 (2-sided) was considered to be significant.
18
Results
Baseline characteristics
In this study 53 patients with cWAD were included and were allocated to either the experimental intervention (n=28) or the control intervention (n=25). The inclusion and randomization process is illustrated in a flowchart together with the loss of follow-up (Figure 1). The mean age and its standard deviation was 40.80 years ± 12.86 in the control group and 37.25 years ± 11.09 in the experimental group. Women were largely represented in this study, as 80% of the control population and 71.4% of the experimental group were female. Both of these components were not significantly different when comparing both groups. There was one significant difference uncovered in the baseline data between the two intervention groups, which was the CSI [t(51)= -3.095; p=0.003] (see Table 2).
19
Table 2. Baseline characteristics
1. Abbreviations: std., standard; sig., significance; y, years; cm, centimeter; kg, kilogram; NDI, Neck Disability Index; CSI, Central Sensitization Index; PCS, Pain Catastrophizing Scale; NRS, Numeral Rating Scale; mA, milliampere; EDT, electrical detection threshold; EPT, electrical pain threshold; TS, temporal summation; CPM, conditioned pain modulation.
2. Significant result in bold
Control intervention Experimental intervention Independent samples test Mean Range Std.
Deviation Mean Range
Std. Deviation t Sig. (2-tailed) Age (y) 40.80 18 - 62 12.86 37.25 24 - 62 11.09 -1.079 0.286 Length (cm) 171.08 162 - 195 7.95 172.75 158 - 200 8.98 0.713 0.479 Weigth (kg) 72.56 55 - 122 15.29 72.93 47 - 118 17.43 0.081 0.935 BMI (kg/m²) 24.64 19.96-32.08 3.70 24.29 16.65 - 36.81 4.75 -0.296 0.769
Time since whiplash (days) 3136 295 - 16331 3875.33 1393.93 165 - 5582 1316.02 -2.241 0.029 NDI pre 18 10 - 32 6 16 7 - 32 6 -1.287 0.204 CSI pre 52 32 - 83 13 41 21 - 62 12 -3.095 0.003 PCS pre 25 4 - 47 11 20 1 - 36 11 -1.438 0.157
Average pain pre (NRS) 5 3 - 9 2 5 1 - 9 2 -0.030 0.976
Heaviest pain pre (NRS) 7 4 - 10 2 7 1 - 10 2 -0.123 0.903
EDT left arm pre (mA) 1.17 0.50 - 2.17 0.43 1.10 0.5 - 2.17 0.36 -0.598 0.552
EDT right arm pre (mA) 1.13 0.50 - 2 0.41 1.05 0.5 - 3 0.49 -0.636 0.527
EDT ankle pre (mA) 1.63 1 - 3.33 0.56 1.53 0.67 – 3.17 0.53 -0.645 0.522
EPT left arm pre (mA) 5.42 1.33 -
22.67
4.82 4.65 1.50 - 13 3.12 -0.699 0.488
EPT right arm pre (mA) 5.77 1 - 19.33 4.57 4.94 1 – 13.67 3.40 -0.752 0.456
EPT ankle pre (mA) 8.85 1.83 -
21.17
5.55 7.27 2.67 -
20.83
4.56 -1.141 0.259
TS left arm pre (NRS) 1 -3 - 6 2 0 -3 - 4 2 -0.832 0.409
TS right arm pre (NRS) 1 -2 - 6 2 0 -3 - 3 1 -1.338 0.187
TS ankle pre (NRS) 2 -2 - 8 3 1 -2 - 4 1 -1.493 0.142
CPM left arm pre (NRS) 4,5 0 - 9.5 3 3.2 0 - 8 2.5 -1.302 0.200
CPM right arm pre (NRS) 4.9 2 - 8 2.0 3.7 0 - 9 2.5 -1.504 0.140
CPM ankle pre (NRS) 4.1 0 - 9 2.2 3.8 1 - 8 2 -0.382 0.704
Frequenties
Sex Women 20/25 (80%) 20/28 (71.4%) 0.714 0.479
20
Within group differences
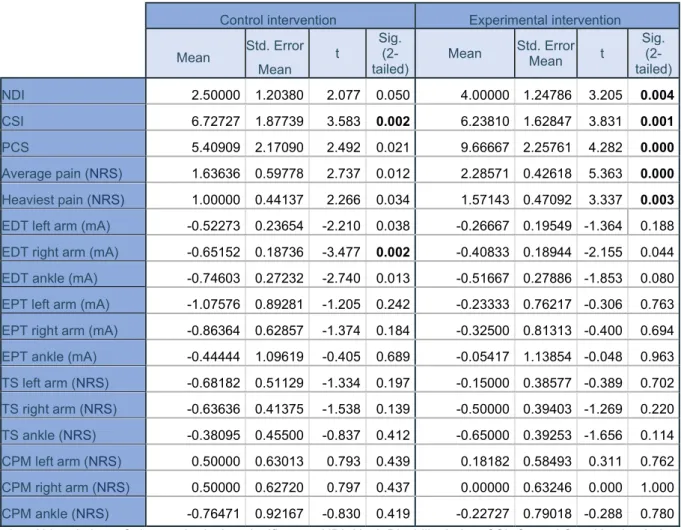
Within group differences after the therapy period, also known as time effect, were evaluated using
Paired Samples t-Test, as a normal distribution and parametric data were found. The patients who had received the control intervention only showed significantly better results for the CSI [t(21)=3.583; p=0.002] and the EDT on the right arm [t(21)= -3.477; p=0.002].
The group with the experimental intervention clearly showed some differences. The NDI [t(20)=3.205; p=0.004], the CSI [t(20)=3.831; p=0.001], the PCS [t(20)=4.282; p<0.001], average pain scored with the NRS [t(20)=5.363; p<0.001] and heaviest pain scored with the NRS [t(20)=3.337; p=0.003] were all significantly better after the intervention was applied.
Both of these conclusions can be extracted from table 3 with the significant results in bold. Convenient graphics of these improvements can be found in appendix 1.
Table 3. Paired Samples t-Test
Control intervention Experimental intervention Mean Std. Error
Mean
t Sig. (2-tailed)
Mean Std. Error Mean t Sig. (2-tailed) NDI 2.50000 1.20380 2.077 0.050 4.00000 1.24786 3.205 0.004 CSI 6.72727 1.87739 3.583 0.002 6.23810 1.62847 3.831 0.001 PCS 5.40909 2.17090 2.492 0.021 9.66667 2.25761 4.282 0.000 Average pain (NRS) 1.63636 0.59778 2.737 0.012 2.28571 0.42618 5.363 0.000 Heaviest pain (NRS) 1.00000 0.44137 2.266 0.034 1.57143 0.47092 3.337 0.003 EDT left arm (mA) -0.52273 0.23654 -2.210 0.038 -0.26667 0.19549 -1.364 0.188 EDT right arm (mA) -0.65152 0.18736 -3.477 0.002 -0.40833 0.18944 -2.155 0.044 EDT ankle (mA) -0.74603 0.27232 -2.740 0.013 -0.51667 0.27886 -1.853 0.080 EPT left arm (mA) -1.07576 0.89281 -1.205 0.242 -0.23333 0.76217 -0.306 0.763 EPT right arm (mA) -0.86364 0.62857 -1.374 0.184 -0.32500 0.81313 -0.400 0.694 EPT ankle (mA) -0.44444 1.09619 -0.405 0.689 -0.05417 1.13854 -0.048 0.963 TS left arm (NRS) -0.68182 0.51129 -1.334 0.197 -0.15000 0.38577 -0.389 0.702 TS right arm (NRS) -0.63636 0.41375 -1.538 0.139 -0.50000 0.39403 -1.269 0.220 TS ankle (NRS) -0.38095 0.45500 -0.837 0.412 -0.65000 0.39253 -1.656 0.114 CPM left arm (NRS) 0.50000 0.63013 0.793 0.439 0.18182 0.58493 0.311 0.762 CPM right arm (NRS) 0.50000 0.62720 0.797 0.437 0.00000 0.63246 0.000 1.000 CPM ankle (NRS) -0.76471 0.92167 -0.830 0.419 -0.22727 0.79018 -0.288 0.780
1. Abbreviations: Std., standard; sig., significance; NDI, Neck Disability Index; CSI, Central Sensitization Index; PCS, Pain Catastrophizing Scale; NRS, Numeral Rating Scale; mA, milliampere; EDT, electrical pain detection threshold; EPT, electrical pain threshold; TS, temporal summation; CPM, conditioned pain modulation. 2. Significant result in bold
21
Between group differences
These characteristics were evaluated using a Repeated Measures ANOVA, since the data was found to have a normal distribution and was parametric (Table 4). There was no significant result found searching for between group differences when comparing the control intervention to the experimental intervention.
Table 4. Repeated Measures ANOVA
*Measurements using the Greenhouse-Geisser correction
Abbreviations: df, degrees of freedom; sig., significance; NDI, Neck Disability Index; CSI, Central Sensitization Index; PCS, Pain Catastrophizing Scale; NRS, Numeral Rating Scale; mA, milliampere; EDT, electrical pain detection threshold; EPT, electrical pain threshold; TS, temporal summation; CPM, conditioned pain modulation.
Tests of Between-Subjects Effects: Time * Group
Type III Sum of
Squares df Mean Square F Sig.
Partial Eta Squared NDI 12.087 1 12.087 0.749 ,392 ,018 CSI 1.286 1 1.286 0.038 ,846 ,001 PCS 97.380 1 97.380 1.849 ,181 ,043 Average pain (NRS) 2.265 1 2.265 0.770 ,385 ,018 Heaviest pain (NRS) 1.754 1 1.754 0.785 ,381 ,019
EDT left arm (mA) 0.451 1 0.451 0.889 ,352 ,022
EDT right arm (mA) 0.455 1 0.455 1.246 ,271 ,031
EDT ankle (mA) 0.269 1 0.269 0.346 ,560 ,009
EPT left arm (mA) 3.200 1 3.200 0.425 ,518 ,011
EPT right arm (mA) 1.324 1 1.324 0.238 ,628 ,006
EPT ankle (mA) 0.780 1 0.780 0.061 ,806 ,002
TS left arm (NRS) 0.545 1 0.545 0.269 ,607 ,007 TS right arm (NRS) 0.026 1 0.026 0.015 ,903 ,000 TS ankle (NRS) 0.371 1 0.371 0.199 ,658 ,005 CPM left arm (NRS) 0.338 1 0.338 0.121 ,731 ,005 CPM right arm (NRS) 0.835 1 0.835 0.287 ,596 ,011 CPM ankle (NRS) 0.965 1 0.965 0.167 ,686 ,006
22
Post hoc power analysis
The post hoc calculated power (1-β), using α=0.01, is 0.66 (Table 5). This implies that there is a 66% chance that the null hypothesis will be rejected when it is truly false. It also means that β, or the chance of a type 2-error, is 0.34, meaning that there is a 34% chance that the null hypothesis is false but would not be rejected.
Table 5. Post hoc power analysis
Multivariate Tests Value F Hypothesis df Error df Sig. Partial Eta Squared Noncent. Parameter Observed Powerb Effect Pillai's Trace 0.793 3.162a 17 14 0.017 0.793 53.761 0.660
Wilks' Lambda 0.207 3.162a 17 14 0.017 0.793 53.761 0.660 Hotelling's Trace 3.840 3.162a 17 14 0.017 0.793 53.761 0.660 Roy's Largest Root 3.840 3.162a 17 14 0.017 0.793 53.761 0.660 a. Exact statistic
b. Computed using alpha = 0.01
23
Discussion
Outcome compared to existing literature
This study was developed to investigate whether two types of interventions have a similar effect in reducing pain frequency, symptoms of CS and improving functionality and pain thresholds measured with QST. No significant results were revealed that could reject this null hypothesis, as no significant between group differences were obtained in patients with cWAD. However, significant within group effects were found in the experimental intervention group. There were clear improvements on functionality in daily life, handling pain and on somatic and emotional symptoms linked to CS. Furthermore, pain intensity decreased and an improvement of the negative assumptions towards harmful stimuli was noted. This is a disparity compared to the patients who received the control intervention. In this latter group, only significant improvements were found for the somatic and emotional symptoms linked to CS and for pain sensitivity of the right arm.
The modern neuroscience approach which was implemented in this study consisted of PNE and cognition targeted-exercise therapy. It has been proven that PNE, which aims to reconceptualize pain as a product of the brain rather than the result of damage in the body, may reduce pain and catastrophizing and increase function and movement in patients with chronic musculoskeletal pain [31]. However, a study by Nijs et al. (2011) proved that, when used as a sole treatment, PNE only shows small effect sizes [32]. In order to obtain larger responses, it is crucial that PNE is combined with cognitive-targeted exercise therapy, where the new pain beliefs and cognitions that were learned in the educational sessions, can be employed [30]. For this reason, both therapy forms were combined in the experimental intervention. A recent study by Malfliet et al. (2018) [19] compared current best-evidence physiotherapy including biomedical pain education with a modern neuroscience approach in chronic spinal pain patients. They found that, although both groups showed improvements in pain and function, only the group with the modern neuroscience approach showed significant changes. Similar effects were perceived during this study, as the NDI, the CSI, the PCS and NRS provided significantly better results after having received the experimental intervention. However, the current study did find some significant improvements in the control group, namely for the EDT of the right arm and the CSI. An important discrepancy with the work of Malfliet et al. (2018) [19], is the use of pressure thresholds in QST compared to the electrical thresholds which were used in this study. Aasvang et al. (2014) [33] concluded an equal or superior reliability for electrical stimulation in comparison to pressure algometry. This suggests that there would be little consequences when a different testing technique was selected. When specifying a pain sensation, Beissner et al. (2010) [34] found that the descriptors
24
“dull” and “pressing” were related to pain caused by C fibers and “pricking” was associated with pain induced by Aβ fibers. Hereby, the derivation can be made that pressing pain (C fibers) is stimulated by measuring pressure thresholds and pricking pain (Aβ fibers) is provoked by electrical thresholds. With these conclusions in mind, it can be mentioned that there is a likelihood that both types of QST measure pain conducted by different fibers, which may explain the small differences of outcome between the present study and the study by Malfliet et al. (2018) [19]. As far as current best-evidence physical therapy concerns, several studies have proven either minimal or even no effect on pain, functionality and psychological distress in chronic pain patients [35, 36, 37], which is in line with the minimal significant improvements found in this study.
In a population of chronic spinal pain patients, a superiority of a modern neuroscience approach over current best-evidence physiotherapy was previously established [19]. A similar conclusion cannot be made in this study, as none of the between group differences were significant. However, when a comparison is made between the number of significant results in the control group and the number of significant results in the experimental group, a cautious assumption can be made that there are more significant effects in the experimental group, although these between group differences are not significant by itself. An explanation for this finding may be found in the statistical analysis. The p-values of the within-group analysis are fairly similar but, in the experimental group, just low enough to be considered as significant and, in the control group, just not low enough to be significant. This may explain why in the end, no between-group differences were found. Also, just because statistical within-group significances have been found, does not mean that they are clinically relevant to the patient [38]. The fact that statistically significant differences were found within-groups, but not in between-groups analysis, does not particularly mean that the perceived changes were not clinically relevant after all, as statistical significance and clinical relevance cannot be seen as an equal concept. Another important thing to keep in mind is that the CSI already showed significant differences between the two intervention groups after baseline analysis, as the mean of the experimental group was 11 points lower than the control group. While merely coincidental, it may have contributed in the significance of the experimental group results.
A big difference between the therapies that were given in this study is that the experimental therapy presumes a biopsychosocial approach, while the control therapy starts from a biomedical view. The latter conceptualizes mind and body as two separate things that function independently from each other, while the former acknowledges that psychosocial factors (e.g. emotions, stress etc.) can impact symptoms, medical disorders and treatment response [8]. To date, the biopsychosocial approach has
25
been globally accepted and many chronic pain studies implement this theory by using PNE as part of the treatment [39,40,41,42,43]. Despite the global use and promising results, Cuenda-Gago et al. (2017) [44] stated that, although there is a positive effect for pain relief, normalizing pain cognitions, fear avoidance and self-care, the effect on more technical measures (e.g. algometry) remains inconclusive. This supports the findings of the experimental treatment in this study: significant results for the NDI, the CSI, the PCS and both types of NRS, which are evaluated in questionnaires and can thus be interpreted as more subjective, while there is a lack of general significance in the results of the technical and more objective QST. This raises a question: what should be of bigger importance to clinicians, subjective or objective pain measurements? And furthermore, what is more beneficial for the patient, subjective or objective pain improvements? This again questions the relevance of statistically significant results [38].
Practical Implications and Recommendations for Research
To our knowledge, there is no study to this date that examines whether subjective or objective pain measurements are of more clinical value. Given the divergent results in our study, investigating whether there is a difference in clinical value, may be of high importance. After all, pain is a subjective experience and therefore, subjective pain measurements may be of higher value than objective ones. This would result in a different interpretation of the results obtained in this study.
Catastrophizing has been a predictor of poor pain-related outcome [45]. A treatment approach like PNE does not directly focus on minimizing pain catastrophizing although it could be an improvement for a specific group of patients. From the baseline outcome, a wide range of pain catastrophizing scores can be noticed. This indicates a big diversity in catastrophizing levels. In order to obtain a better therapy outcome, is it necessary to divide patients in groups according to their PCS? It is proven that fear-related pain, maintained by catastrophizing and other cognitive factors [46,47] and indicated by the PCS, shows more improvement when graded exposure is implemented in therapy compared to graded activity [48,49]. Graded exposure has already been explored in several populations like chronic low back pain [50], complex regional pain syndrome [51], work related upper extremity pain [52] and acute traumatic neck pain [48]. These populations are all chronic, except one, the acute post-traumatic neck pain. Given the positive results in other chronic pain patient groups, it might be interesting to include cWAD in further research surrounding graded exposure and not graded activity like the current study.
26
A last suggestion regarding further research can be made concerning longer follow-up. More significant results were found after six and 12 months than after three months in the study of Malfliet et al. (2018) [19]. Behavioural changes take time as the patients go through all phases of change multiple times [53]. A model of this behavioural change was conducted by Prochaska and DiClemente as an integrative theory of therapy [54]. Therefore, when a study questions behavioural change, a longer follow-up time with three or more measurements is recommended.
Limitations and Strengths
This study comes with some limitations. First, the baseline overall CSI score was significantly higher in the control group for which no correction was made in the analysis. This may be a possible explanation as to why there were no significant between-group differences. Moreover, an a priori power analysis was not included to determine an adequate sample size. A comparable study conducted by Malfliet et. al. 2018 [19] calculated a sample size based on the effects on pain in a pilot study [55], resulting in a sample size of 117 individuals. In contrast, this trial included only 53 patients. To objectify this studies’ sample size, a post hoc power calculation was executed. Researchers are generally satisfied with a statistical power of 0.80, comparable with an 80% chance of a real effect. As previously mentioned, the statistical power of this study is 0.66 which is insufficient as to the standard objective of power. To achieve this objective, an increased sample size or using larger significance criteria should be considered. However, current literature criticizes post-hoc power calculation because a significant result can always be found when the sample size is large enough [38,56].
This study comes with several strengths as well. To the researchers’ knowledge, this is the first randomized controlled trial to compare current best-evidence physiotherapy with PNE combined with cognition-targeted exercise therapy in chronic whiplash patients. Furthermore, current best-evidence physiotherapy was adopted as a control intervention [30,57]. Another strength is that the study was double-blind, meaning that both the researchers who examined the patients, as well as the patients themselves did not know which therapy was applied. For the interpretation of the results, a correction for multiple comparisons was applied by setting the significance level to 0.01.
27
Conclusions
This study concludes that, in cWAD, current best-evidence physiotherapy and the modern neuroscience approach have similar short-term effects. However, significant within group differences were found, as patients who received the modern neuroscience treatment showed improvements on functionality in daily life, somatic and emotional symptoms linked to CS, pain intensity, handling pain and improvement of the negative assumptions towards harmful stimuli. In comparison, patients who received current best-evidence physiotherapy only showed significant progression for pain sensitivity of the right arm and somatic and emotional symptoms linked with CS.
28
References
1. Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R et al. A classification of chronic pain for ICD-11. Pain. 2015;156:1003-1007
2. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life and treatment. Eur J Pain. 2006;10:287-333
3. Van Oosterwijck J, Nijs J, Meeus M, Paul L. Evidence for central sensitization in chronic whiplash: a systematic literature review. Eur J Pain. 2013;17:299-312
4. Carroll LJ, Holm LW, Hogg-Johnson S, Cote P, Cassidy JD, Haldeman S et al. Course and prognostic factors for neck pain in whiplash-associated disorders (WAD): results of the bone and joint decade 2000-2010 Task force on neck pain and its associated disorders. Spine. 2008;33(4):83-92
5. den Boer C, Dries L, Terluin B, van der Wouden JC, Blankenstein AH, van Wilgen CP, Lucassen P, van der Horst HE. Central sensitization in chronic pain and medically unexplained symptom research: a systematic review of definitions, operationalisations and measurement instruments. Journal of psychosomatic research. 2019;117:32-40
6. Rodriquez AA, Barr KP, Burns SP. Whiplash: pathophysiology, diagnosis, treatment and prognosis. Muscle Nerve. 2004;29:768-781
7. Sarrami P, Armstrong E, Naylor JM, Harris IA. Factors predicting outcome in whiplash injury: a systematic meta-review of prognostic factors. J Orthop Traumatol. 2017;18(1):9-16
8. Nijs J, Meeus M, Van Oosterwijck J, Roussel N, De Kooning M, Ickmans K, Matic M. Treatment of central sensitization in patients with ‘unexplained’ chronic pain: what options do we have? Expert Opin Pharmacother. 2011;12(7):1087-1098
9. Lluch E, Torres R, Nijs J, Van Oosterwijck J. Evidence for central sensitization in patients with osteoarthritis pain: A systematic literature review. Eur J Pain. 2014;18(10):1367-1375
10. Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686-688
11. Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. The journal of pain. 2009;10(9):895-926
12. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2010;152(3):2-15
13. Nijs J, Meeus M, Cagnie B et al. A modern neuroscience approach to chronic spinal pain: combining pain neuroscience education with cognition-targeted motor control training. Phys Ther. 2014;94(5):730-738
29
14. Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, Treede RD. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain. 2006;10:77-88
15. Verberne W, Snijders TJ, Liem SK, Baakman AC, Veldhuijzen J. Applications of ‘quantitative sensory testing’. Nederlands tijdschrift voor geneeskunde. 2013;157:A5434
16. Arendt-Nielsen L, Morlion B, Perrot S, Dahan A, Dickenson A, Kress HG, Wells C, Bouhassira D, Drewes MA. Assessment and manifestation of central sensitization across different chronic pain conditions. Eur J Pain. 2018;22(2):216-241
17. Mayer TG, Neblett R, Cohen H, Howard KJ, Choi YH, Williams MJ, Perez Y, Gatchel RJ. The development and psychometric validation of the central sensitization inventory (CSI). Pain Practice. 2012;12(4):276-285
18. Scerbo T, Colasurdo J, Dunn S, Unger J, Nijs J, Cook C. Measurement properties of the central sensitization inventory: a systematic review. Pain Practice. 2018;18(4):544-554
19. Malfliet A, Kregel J, Coppieters I. Effect of pain neuroscience education combined with cognition-targeted motor control training on chronic spinal pain: a randomized controlled trial. Jama Neurol. 2018;75(7):808-817
20. Jorritsma W, de Vries GE, Dijkstra PU, Geertzen JH, Reneman MF. Neck pain and disability scale and neck disability index: validity of Dutch language versions. Eur Spine J. 2012;21(1):93-100
21. Vernon H. The neck disability index: state of the art 1991-2008. Journal of Manipulative and physiological therapeutics. 2008;31(7):491-502
22. Jorritsma W, de Vries GE, Geertzen JH, Dijkstra PU, Reneman MF. Neck pain and disability scale and neck disability index: reproducibility of Dutch language versions. Eur Spine J. 2012;19(10):1695-1701 23. Ferraz MB, Quaresma MR, Aquino LR, Atra E, Tugwell P, Goldsmith CH. Reliability of pain scales in the assessment of literate and illiterate patients with rheumatoid arthritis. J. Rheumatol. 1990;17(8):1022-4
24. Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. 2004;8(4):283-291
25. Kregel J, Vuijk PJ, Descheemaeker F, Keizer D, van der Noord R, Nijs J, Cagnie B, Meeus M, van Wilgen CP. The Dutch central sensitization inventory (CSI): Factor analysis, discriminative power and test-retest reliability. Clin J Pain. 2016;32(7):624-630
26. Darnall BD, Sturgeon JA, Cook KF, Taub CJ, Roy A, Burns JW, Sullivan M, Mackey SC. Development and validation of a daily pain catastrophizing scale. J Pain. 2017;18(9):1139-1149
27. Wheeler C, de Williams AC, Morley S. Meta-analysis of the psychometric properties of the pain catastrophizing scale and associations with participant characteristics. Pain. 2019;160(9):1946-1953
30
28. Verberne WR, Snijders TJ, Liem KS, Baakman AC, Veldhuijzen DS. Toepassingen van sensibiliteitsonderzoek met ‘quantitative sensory testing’. Nederlands tijdschrift voor geneeskunde. 2013;157:A5434
29. Lane E, Fritz JM, Green T, Maddox D. The effectiveness of training physical therapists in pain neuroscience education on patient reported outcomes for patients with chronic spinal pain: a study protocol for a cluster randomized controlled trial. BMC Musculoskeletal disord. 2018;19:386
30. Malfliet A, Kregel J, Meeus M, Cagnie B, Roussel N, Dolphens M, Danneels L, Nijs J. Applying contemporary neuroscience in exercise interventions for chronic spinal pain: treatment protocol. Brazilian Journal of Physical Therapy. 2017;21(5):378-387
31. Louw A, Diener I, Butler DS, Puentedura EJ. The effect of neuroscience education on pain, disability, anxiety, and stress in chronic musculoskeletal pain. Archives of physical medicine and rehabilitation. 2011;92:2041-2056
32. Nijs J, van Wilgen CP, Van Oosterwijck J, van Ittersum M, Meeus M. How to explain central sensitization to patients with ‘unexplained’ chronic musculoskeletal pain: practice guidelines. Manual Therapy. 2011;16(5):413-418
33. Aasvang EK, Werner MU, Kehlet H. Assessment of deep tissue hyperalgesia in the groin - a method comparison of electrical vs. pressure stimulation. Acta Anaesthesiol Scand. 2014;58:986-96.
34. Beissner F, Brandau A, Henke C, Felden L, Baumgärtner U, Treede R D, Oertel B G & Lötsch J. Quick discrimination of A(delta) and C fiber mediated pain based on three verbal descriptors. PloS one. 2010. 5:e12944
35. Zylbergold R, Piper M. Lumbar disc disease: comparative analysis of physical therapy. Arch Phys Med Rehabil. 1981;62:176-179
36. Risch SV, Norvell NK, Pollock ML, Risch ED, Langer H, Fulton M, Graves JE, Leggett SH. Lumbar strengthening in chronic low back pain patients. Physiologic and psychological benefits. Spine. 1993;18:232-238
37. Kuukkanen T, Malkia E. Effects of a three-month therapeutic exercise programme on flexibility in subjects with low back pain. Physiother Res Int. 2000;5:46-61
38. Luus HG, Muller FO, Meyer BH. Statistical significance vs. clinical relevance. Part I. The essential role of the power of a statistical test. S Afr Med J. 1989;76(10):586-570
39. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychological Bulletin. 2007;133(4):581-624
40. Louw A, Zimney K, Puentedura EJ, Diener I. The efficacy of pain neuroscience education on musculoskeletal pain: a systematic review of the literature. Physiother Theory Pract. 2016;32(5):332-355
31
41. Wijma AJ, van Wilgen CP, Meeus M, Nijs J. Clinical biopsychosocial physiotherapy assessment of patients with chronic pain: the first step in pain neuroscience education. Physiother Theory Pract. 2016;32(5):368-384
42. Cheatle MD. Biopsychosocial approach to assessing and managing patients with chronic pain. Medical Clinics. 2016;100(1)43-53
43. Kamper SJ, Apeldoorn AT, Chiarotto A, Smeets RJ, Ostelo RW, Guzman J, van Tulder MW. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain. Cochrane Database Syst Rev. 2014:2(9)
44. Cuenda-Gago JD, Espejo-Antunez L. Effectiveness of education based on neuroscience in the treatment of musculoskeletal chronic pain. Rev Neurol. 2017;65(1):1-12
45. Thorn BE, Boothby JL, Sullivan MJL. Targeted treatment of catastrophizing for the management of chronic pain. Cognitive and behavioural practice. 2002;9:127-138
46. Vlaeyen JWS, Kole-Snijders AM, Boeren RG, et al. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain. 1995;62:363-72.
47. Crombez G, Vlaeyen JWS, Heuts PHTG, et al. Pain-related fear is more disabling than pain itself: Evidence on the role of pain-related fear in chronic back pain disability. Pain. 1999;80:329-339. 48. de Jong JR, Vangronsveld K, Peters ML, Goossens MEJB, Onghena P, Bulté I, Vlaeyen JWS. Reduction of pain related fear and disability in post-traumatic neck pain: a replicated single-case experimental study of exposure in vivo. The Journal of Pain. 2008;9(12):1123-1134
49. Lopez-de-Uralde-Villanueva I, Munoz-Garcia D, Gil-Martinez A, Pardo-Montero J, Munoz-Plata R et al. A systematic review and meta-analysis on the effectiveness of graded activity and graded exposure for chronic nonspecific low back pain. Pain Medicine. 2016;17(1):172-188
50. Woods MP, Asmundson GJ. Evaluating the efficacy of graded in vivo exposure for the treatment of fear in patients with chronic back pain: a randomized controlled clinical trial. PAIN. 2008;136:271-280 51. de Jong JR, Vlaeyen JW, Onghena P, Cuypers C, den Hollander M, Ruijgrok J. Reduction of pain-related fear in complex regional pain syndrome type I: the application of graded exposure in vivo. PAIN. 2005;116:264-275
52. de Jong JR, Vlaeyen JWS, van Eijsden M, Loo C, Onghena A. Reduction of pain-related fear and increased function and participation in work-related upper extremity pain (WRUEP): effects of exposure in vivo. PAIN. 2012;153(10):2109-2118
53. Thijs G, Van Nuland M, Govaerts F. Op de grens tussen ‘cure’ en ‘care’: begeleiding van gedragsverandering door de huisarts. Huisarts Nu. 2005;34(4):186-191
54. Prochaska J, DiClemente C. Stages and processes of self-change in smoking: toward an integrative model of change. Journal of Consulting and Clinical Psychology. 1983;5:390–395.
32
55. Moseley L. Combined physiotherapy and education is efficacious for chronic low back pain. Aust J Physiother. 2002;48(4):297-302.
56. Zhang Y, Hedo R, Rivera A, et al. Post hoc power analysis: is it an informative and meaningful analysis? General Psychiatry. 2019;32:e100069.
57. Dolphens M, Nijs J, Cagnie B, et al. Efficacy of a modern neuroscience approach versus usual care evidence-based physiotherapy on pain, disability and brain characteristics in chronic spinal pain patients: protocol of a randomized clinical trial. BMC Musculoskelet Disord. 2014;15(1):149.
ABSTRACT IN LEKENTAAL
Chronische pijn na whiplash komt steeds vaker voor in onze maatschappij en kan leiden tot langdurige uitval van patiënten, zowel op sociaal als op professioneel vlak. Centrale sensitisatie wordt gezien als een van de mogelijke mechanismen die deze aandoening in stand houden. Bij centrale sensitisatie is het zenuwstelsel als het ware hypergevoelig geworden voor verschillende soorten prikkels waardoor patiënten sneller pijn ervaren en een algemeen slechter welbevinden hebben. Het is niet eenvoudig om chronische aandoeningen te behandelen aangezien de patiënten zich vaak in een aanslepende vicieuze cirkel bevinden die versterkt wordt door negatieve gevoelens en gedragingen zoals angst en vermijdingsgedrag. De laatste decennia worden deze negatieve gevoelens en gedragingen steeds vaker meegenomen als een van de aangrijpingspunten van de therapie. Dit wordt ook wel een biopsychosociale aanpak genoemd waarbij iedere patiënt individueel benaderd wordt in zijn eigen psychische en sociale context. In deze studie worden twee therapieën bij chronische whiplashpatiënten met elkaar vergelijken waarbij de ene uitgaat van een biopsychosociale aanpak en de andere van de beste huidige wetenschappelijke bewezen resultaten. Er zal onder andere bestudeerd worden of symptomen van centrale sensitisatie verbeteren, de patiënt functioneler is geworden en indien pijn gemeten op een objectieve manier is verbeterd.