CHRONIC PULMONARY
ASPERGILLOSIS IN A TERTIARY
CARE CENTRE IN BELGIUM
THE GHENT COHORT
Van Acker Lander
Stamnummer: 00704980Promotor 1: Prof. dr. Eva. Van Braeckel Promotor 2: Prof. dr. Jerina Boelens
Masterproef master in de specialistische geneeskunde
Content
0. Abstract ... 1 1. Introduction ... 2 1.1. General ... 2 1.2. Epidemiology ... 3 1.3. Definition ... 4 1.4. Spectrum of CPA ... 5 1.5. Diagnostic work-up ... 8 1.5.1. Symptoms ... 8 1.5.2. Microbiology ... 8 1.5.3. Serology ... 10 1.5.4. Radiology ... 11 1.5.5. Differential diagnosis ... 12 1.6. Treatment ... 13 1.6.1. General principles ... 13 1.6.2. Antifungal therapy ... 13 1.6.3. Antifungal resistance ... 15 1.6.4. Surgery ... 17 1.6.5. Treatment of complications ... 181.6.6. Other treatment modalities ... 18
1.7. Specific comorbidities ... 19
1.7.1. Sarcoidosis ... 19
1.7.2. Non-tuberculous mycobacteria ... 20
1.8. Follow-up and response monitoring ... 20
1.9. Prognosis ... 21
1.10 Aim of the master thesis ... 21
2.1. Literature review ... 21
2.2. Data collection ... 22
2.3. Data processing issues ... 23
2.4. Statistical analysis ... 23 3. Results ... 23 3.1. Demographics ... 23 3.2. Diagnosis ... 25 3.3. Treatment ... 28 3.4. Follow-up ... 31 4. Discussion ... 33 4.1. Diagnosis ... 33
4.2. Treatment and follow up ... 36
4.3. Limitations ... 37
4.4. Conclusion ... 38
5. References: ... 39
1
0. Abstract
Introduction:
Chronic pulmonary aspergillosis (CPA) is an uncommon severe fungal infection, generally presenting in immunocompetent patients with underlying structural lung disease. Tuberculosis is the leading cause worldwide, but in Europe more people with COPD are affected. Since the publication of two international guidelines in the last five years, there is a growing interest and awareness for CPA.
Methods:
A descriptive data analysis was performed in 25 patients, who were treated in a tertiary care hospital in Ghent, in the context of the CPAnet registry. The latter is a multinational multicentre web-based questionnaire. Over a period of 1 year (December 2018 - November 2019) 23 prospective and 2 retrospective cases were included, based on the ERS diagnostic criteria for CPA.
Results:
Most patients were male (78.3%) and several had COPD (48%) or a history of smoking (76%). Some kind of immunosuppression was observed in 14 patients (56%) of which eight ((57%) had a positive BAL Aspergillus antigen. A cut-off level of 50 mg/L instead of 75mg/l for
Aspergillus IgG Immunocaps lead to a higher sensitivity, without taking specificity into
account. Voriconazole was the preferred first-line therapy (15/25), particularly in case of high disease burden.
Conclusions:
To our knowledge this small observational study was the first to specifically assess Belgian CPA patients. This study population resembled other European cohorts, but a remarkable higher incidence of immunosuppression was found, what has to be further investigated in larger groups. CPA remains a tough diagnosis with several incompletely explored areas on microbiology, prognosis and treatment. A better insight by means of the CPAnet registry can lead to standardisation of clinical and radiological follow-up criteria. This will be helpful in performing randomised controlled trials to evaluate and compare therapeutic efficiency of several established (voriconazole, itraconazole) and newer (posaconazole, isavuconazole) antifungal treatments.
2
1. Introduction
1.1. General
Chronic pulmonary aspergillosis (CPA) is an uncommon severe fungal infection, caused by
Aspergillus species (spp.). It is an indolent destructive disease with high morbidity and
mortality, mostly seen in non- or mildly immunocompromised patients with underlying pulmonary disease (1–3).
Aspergillus is an aerobic mould, named after the holy water sprinkler, “aspergillum”, because
of its similar appearance. It grows ubiquitously and can be found in rainwater, soil, or decaying vegetation. It exists of a conidial head with branching hyphae, which can be visualised by calcofluor staining. Aspergillus is spread by inhalation, but healthy subjects get seldom infected. Aspergillus fumigatus is the principal disease-causing subspecies, less frequent pathogens are A. flavus, A. niger, and A. terreus (4).
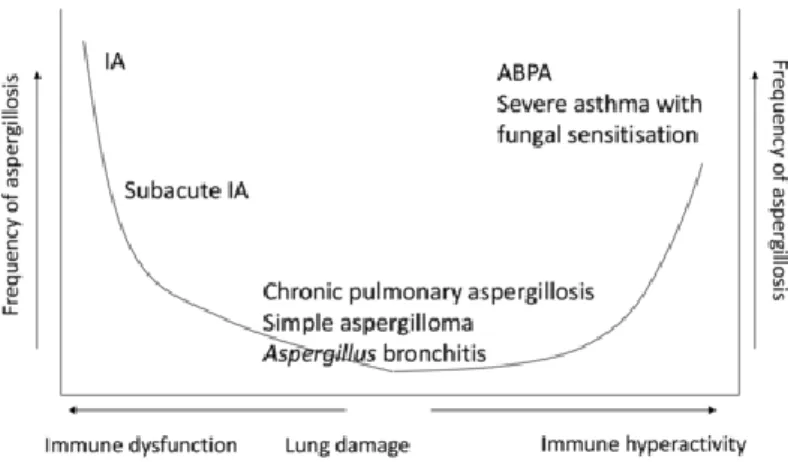
There exists a large spectrum of Aspergillus disease (figure 1), depending on the underlying immune defect or structural lung disease. Saphrophytic colonisation without disease is common in pre-existing lung disease. However, in patients with asthma or cystic fibrosis it can provoke a type 2 immune response, leading to an allergic bronchopulmonary aspergillosis (ABPA) or severe fungal-sensitised asthma. On the other side of the spectrum, severely immunocompromised patients (prolonged neutropenia, T-cell dysfunction) are at high risk of developing an invasive pulmonary aspergillosis (IPA), characterised by a high mortality rate (2). In the milder immunosuppressed, the infection evolves more slowly, indicated as a subacute invasive aspergillosis (SAIA) (1). Finally, in some patients with pre-existing lung disease but a normal or mildly impaired immune system, colonisation will lead to a chronic pulmonary aspergillosis (CPA). The exact underlying mechanisms remain elusive, but the pathophysiology of CPA is presumably defined by a combination of epithelial destruction and mild impairment of the immune system with fungal infiltration and, subsequently, infection (1).
3
Figure 1 The clinical spectrum of pulmonary aspergillosis (5)
A complex network of innate and adaptive immune systems interacts with fungi. Phagocytic cells and cytokine-mediated recruitment of natural killer effector cells play a prominent role. Severely impaired immune responses are mostly seen in IPA, but milder immune defects (e.g. lymphopenia, interferon-gamma deficiency) are also found in CPA (1,2,4).
The most frequent cause of structural lung disease worldwide is a history of cavernous tuberculosis (TB), present in 30-81% of the CPA patients depending on the study population. Other causes are for example non-tuberculous mycobacterial (NTM) infection, bronchiectasis, chronic obstructive pulmonary disease (COPD), asthma, ABPA, prior pneumothorax, lung cancer, sarcoidosis, ankylosing spondylitis, rheumatoid arthritis, and hyper-IgE syndrome. In European countries, COPD is a more prevalent cause than tuberculosis (1,2,6,7).
1.2. Epidemiology
Because of the different definitions used in studies and the heterogeneity of the disease (see below), specific epidemiologic data are lacking. The prevalence of CPA is estimated at 240.000 people in Europe and 3 million worldwide. Most patients are older (58-60 year) males with a history of smoking. Almost all have an underlying respiratory disorder and one third also has a non-respiratory comorbidity (1,8,9).
Data from Leading International Fungal Education (LIFE) have shown differences in worldwide prevalence with a high prevalence in the Philippines, Pakistan and Romania. In the United Kingdom (UK), the prevalence was estimated on 5.7/100.000, but Belgian data are lacking (9). There is only one study about serious fungal infections, mentioning a CPA prevalence of 662 in Belgium. This figure is estimated on the incidence of tuberculosis in Belgium and the assumption of an underlying tuberculosis in 20% of the Belgian CPA patients. This illustrates the lack of available data in Belgium, but also abroad (10).
4
1.3. Definition
CPA was already recognised in the 19th century in a case report, but the first treated patient was reported in the 1950s. In 1959, aspergillosis was initially classified as a “mycetoma” and later in the 1980s the terms of semi-invasive and chronic necrotising pulmonary aspergillosis were introduced. Since then, several criteria were proposed and refined, based on new findings on diagnosis and treatment outcomes (1). Still diagnosis and treatment of CPA remained a challenge for the clinician, because diagnostic criteria were inconsistent and outdated (6). Some of the difficulties of defining CPA are the heterogeneity and complexity of the disease. First, CPA is seen in different lung diseases with their own specific presentation. Secondly, there are different types of CPA, not always clearly to define because they can evolve into one another. In addition, there is an overlapping spectrum with ABPA and IPA, as mentioned above (5,7). As a consequence, little data are available about diagnosis, treatment and prognosis of CPA over the past 10 years. To overcome these problems, the European Respiratory Society (ERS) published in December 2015 the ESCMID/ERS/ECMM guideline on CPA (1). A diagnosis of CPA was defined as a combination of the following characteristics: one or more cavities with or without a fungal ball present or nodules on thoracic imaging, either direct evidence of
Aspergillus infection (e.g. culture or microscopy from biopsy) or indirect by an IgG antibody
response to Aspergillus spp. and exclusion of alternative diagnoses (especially mycobacterial infection), all present for at least 3 months (1).
In 2016 the Infection Diseases Society of America (IDSA) published a guideline on this topic as well, but with some important differences in definition criteria (2). Both guidelines recommend a diagnosis based on a combination of characteristics, present for more than three months. In the same way the two classifications demand direct or indirect evidence for
Aspergillus species with an important role for Aspergillus-specific IgG. The mycological
evidence in the IDSA guidelines however is less strict; e.g. serum galactomannan and positive microscopy or fungal culture on sputum are allowed. Secondly, the radiological criterion is less important: theoretically patients with chronic pulmonary symptoms, but without radiological findings can be diagnosed with CPA, according to the IDSA guidelines. Radiological findings have to be progressive according to the IDSA, but not to the European guideline. On the other hand, they mention no or minimal immunodeficiency as one of the key diagnostic criteria. In the ERS guidelines this was not an obligatory diagnostic criterion, although stated that CPA usually was found in non-immunocompromised patients (6).
5 ESCMID/ERS/ECMM guidelines IDSA guidelines
1. One or more cavities with or without a fungal ball present or nodules on thoracic imaging all present for ≥3 months.
2. Direct evidence of Aspergillus infection or an immunological response to Aspergillus spp. 3. Exclusion of alternative diagnosis.
1. Three months of chronic pulmonary symptoms or chronic illness or progressive radiographic abnormalities, with cavitation, pleural thickening, pericavitary infiltrates, and sometimes a fungus ball.
2. Elevated Aspergillus IgG antibody or other microbiological data.
3. No or minimal immunocompromised, usually with one or more underlying pulmonary disorders.
Table 1 Adapted from Salzer et al. (4)
Salzer et al. applied both guidelines to a real-life population and found that in 70% of the cases the diagnostic criteria of both guidelines were fulfilled. Thirteen percent of the patients could not be diagnosed with CPA, based on both guidelines, but for none of them specific IgG testing was available. Only 80 and 78% of the patients respectively, met the ERS and IDSA guidelines, suggesting that there is still room for improvement in the diagnostic criteria. This finding highlights again the difficulty to compare future study data when different classifications are used (6). In this master thesis patient inclusion was only based on the ERS criteria (see below).
1.4. Spectrum of CPA
The clinical spectrum of CPA consists of 5 forms: simple aspergilloma, chronic cavitary pulmonary aspergillosis (CCPA), chronic fibrosing pulmonary aspergillosis (CFPA),
Aspergillus nodule(s) and subacute invasive aspergillosis (SAIA). These forms can overlap by
diagnosis or evolve into another over time (figure 2).
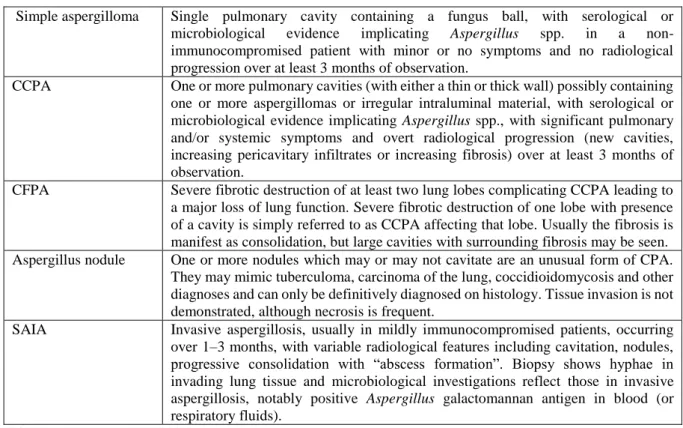
Except for the Aspergillus nodule, the most characteristic feature of CPA is the aspergilloma, a fungus ball formed by hyphae, visualised in a pulmonary of pleural cavity or bronchiectasis. Definitions of the different forms are listed in the table below (1).
6 Simple aspergilloma Single pulmonary cavity containing a fungus ball, with serological or
microbiological evidence implicating Aspergillus spp. in a non-immunocompromised patient with minor or no symptoms and no radiological progression over at least 3 months of observation.
CCPA One or more pulmonary cavities (with either a thin or thick wall) possibly containing one or more aspergillomas or irregular intraluminal material, with serological or microbiological evidence implicating Aspergillus spp., with significant pulmonary and/or systemic symptoms and overt radiological progression (new cavities, increasing pericavitary infiltrates or increasing fibrosis) over at least 3 months of observation.
CFPA Severe fibrotic destruction of at least two lung lobes complicating CCPA leading to a major loss of lung function. Severe fibrotic destruction of one lobe with presence of a cavity is simply referred to as CCPA affecting that lobe. Usually the fibrosis is manifest as consolidation, but large cavities with surrounding fibrosis may be seen. Aspergillus nodule One or more nodules which may or may not cavitate are an unusual form of CPA.
They may mimic tuberculoma, carcinoma of the lung, coccidioidomycosis and other diagnoses and can only be definitively diagnosed on histology. Tissue invasion is not demonstrated, although necrosis is frequent.
SAIA Invasive aspergillosis, usually in mildly immunocompromised patients, occurring over 1–3 months, with variable radiological features including cavitation, nodules, progressive consolidation with “abscess formation”. Biopsy shows hyphae in invading lung tissue and microbiological investigations reflect those in invasive aspergillosis, notably positive Aspergillus galactomannan antigen in blood (or respiratory fluids).
Table 2 Definitions of CPA (1)
A simple aspergilloma is an isolated fungus ball in a single cavity without signs of progression over more than 3 months. Most of the time there are no or few pulmonary/systemic symptoms. Without biopsy it can be difficult to obtain serological or microbiological evidence to establish the diagnosis of a simple aspergilloma (1).
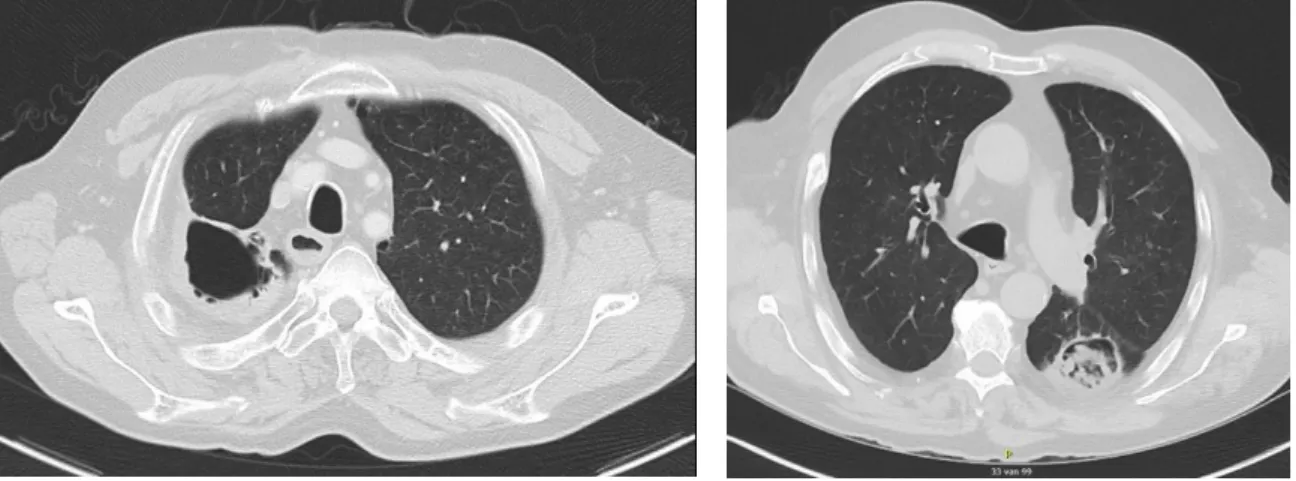
Figure 3 Simple aspergilloma post-TBC (left, G013)/ in a COPD patient (right, G023)
Chronic cavitary pulmonary aspergillosis (CCPA) or formerly called “complex aspergilloma” consists of one or more pulmonary cavities, containing one/several aspergilloma(s) or irregular intraluminal debris, with developing pericavitary infiltrates or fibrosis over more than three months. It is the most common form of CPA and, as stated in the definition, serological or microbiological evidence has to be present. Most patients are symptomatic.
7 If left untreated, CCPA can evolve into a chronic fibrosing pulmonary aspergillosis (CFPA). A diagnosis of CFPA implies a fibrotic destruction (pleural fibrosis or large cavities) of at least two lobes (figure 5B) (1).
Figure 4 CCPA without fungal strands (left, G005)/with fungal strands (right, G019)
Figure 5 A. CCPA with extrathoracic fistulisation and subcutaneous cavity (left, G025)/ B. CFPA (right, G02)
The fourth manifestation of CPA, an Aspergillus nodule, is a rather unusual and earlier-stage form. These single or multiple round nodules are smaller than 3 cm (otherwise they are called mass lesions caused by Aspergillus spp.) and are difficult to differentiate from lung carcinoma, infectious or underlying lung disease in CPA (e.g. rheumatoid nodules). The nodules can cavitate and evolve to a real aspergilloma (1).
Figure 6 Aspergillus nodule (G001)
8 The last form is a subacute invasive aspergillosis (SAIA), formerly known as a chronic necrotising aspergillosis. Although it has similar clinical and radiologic features to CCPA, it is an invasive form of aspergillosis, but with a slower progression over 1-3 months. Instead of a CCPA, which mainly affects immunocompetent patients, most individuals with SAIA are at least mildly immunocompromised (prolonged corticosteroid use, diabetes, alcoholism, COPD, connective tissue disorder). The absence of an existing cavity is usual. First a single area of consolidation develops in an upper lobe, which will cavitate further over weeks. Beside pleural thickening and an aspergilloma, pleural effusion and pneumothorax can be present at diagnosis. Invasive features are a positive Aspergillus antigen in blood and/or bronchoalveolar lavage (BAL) fluid and invading hyphae in lung tissue. The combined characteristics of CPA and IPA commonly result in detection of a positive Aspergillus antibody and antigen (1).
Figure 7 Different presentations of SAIA (left, G019/ right, G017)
1.5. Diagnostic work-up
Because of the variable radiologic presentation and lack of well-defined diagnostic criteria, CPA has often been underdetected in the past. As implied in the definitions, diagnosis is based on a combination of clinical, radiologic and microbiological data (1,2).
1.5.1. Symptoms
Characteristic symptoms include not only respiratory symptoms like dyspnoea or haemoptysis, but also constitutional symptoms like weight loss, fatigue and night sweats. Chronic cough is most prevalent and haemoptysis is present in one third of the patients at diagnosis. Chest pain is rather uncommon. Weight loss and fatigue are the most common general symptoms, while fever is rather infrequent (2,6,7).
1.5.2. Microbiology
A microbiological diagnosis can be based on direct evidence like a positive culture, microscopy (or histology), Aspergillus antigen or PCR, mostly tested on respiratory fluids or biopsy. Most
9 evidence is based on a positive fungal culture, but the role of direct microscopic examination on sputum or BAL fluid is less studied (1).
Albeit rather seldom available, a lung biopsy showing hyphae invading the lung parenchyma, with a surrounding acute inflammatory reaction, remains the gold standard for diagnosis, for instance in subacute invasive aspergillosis (1).
Because of its ubiquitous presence, a positive respiratory sample often reflects colonisation by
Aspergillus spp. Therefore, the ESCMID/ERS guidelines exclude a single positive sputum
sample as diagnostic criterion. On the other hand, sputum cultures in CPA are often negative, but the diagnostic yield increases with higher volume samples (e.g. entire undiluted sputum), the amount of samples, or when specific fungal culture media instead of bacterial plates are used. Even fungal cultures obtained by BAL, have at best a moderate sensitivity (1,9). A broad difference in culture sensitivity for Aspergillus is reported in the literature (11-81%) (11). A.
fumigatus is the main causative agent of CPA (31-100%), but other species like A. flavus, niger
and terreus were variably isolated from respiratory samples in case series, depending on the study population (9). In a Belgian registry, Aspergillus fumigatus and flavus were respectively found in 75% and 20% of cases (n = 20) (12). Positive samples under treatment are suspicious for therapy resistance. Of note, co-infections with e.g. Pseudomonas aeruginosa or non-tuberculous mycobacteria (NTM) are common (1,2,9).
DNA detection by PCR is more sensitive than culture. According to the assay used, a sensitivity and specificity of 71.4 and 63.6% respectively, were found (13). A high signal reflects a high fungal load, which is more compatible with infection. Rapid diagnostic tests for mutations are also performed by PCR (see below) (1).
Galactomannan (GM) is a component of the cell wall of Aspergillus, which is released during growth. The cut-off level for GM antigen detection in BAL fluid is a matter of debate. Sensitivity and specificity levels differ among studies with a sensitivity often below 50%. The specificity depends largely on the used cut-off level. The positive predictive value remains rather low (63.6%), in comparison with IPA, even when using a higher cut-off (>1) (1,9,14). A combination of an Aspergillus PCR and BAL-GM has a high negative predictive value (> 90%) and can increase the positive predictive value as well (13).
10 Serum GM and beta-D-glucan testing are not used as a diagnostic tool in CPA, because both have a low sensitivity (resp. 23% and 20%). GM testing on sputum also has a low sensitivity and specificity, even if a cut-off of 6.5 is used (1,9,11).
Recent advances have led to the development of fungal point-of-care tests by making use of lateral flow assays. For invasive pulmonary aspergillosis there is a high sensitivity for
Aspergillus antigen detection in neutropenic and non-neutropenic patients, respectively for
serum and BAL specimens (14,15). However, the results for CPA are much lower, in line with the diagnostic value of GM testing in CPA.
1.5.3. Serology
Indirect evidence for CPA implies an immunological response, presented by a raised
Aspergillus IgG titre. Both the IDSA and ESCMID/ERS guidelines highlight the use of specific Aspergillus antibodies, because it is a key diagnostic feature in differentiating infection and
colonisation with a positive predictive value of 100% (1,2).
Historically the presence of Aspergillus IgG was based on the detection of Aspergillus precipitins, by use of immunodiffusion or counter-immunoelectrophoresis. Later, several commercial immune-assays came available (e.g. ImmunoCAP®), but the given studies do not recommend one specific assay (1). There is some evidence for better performance of ImmunoCAP® and Immulite®, with cut-off levels of respectively 50 mg/L and 25 mg/L (9). False negative results are probably related to a hypogammaglobulinemia or a selective antibody deficiency for A. fumigatus, or in rare cases for non-fumigatus CPA. If clinical suspicion is high, another IgG assay and IgE (in rare cases also IgM, IgA) testing can be performed (1,15). Often (>50%) there is an increase in the total and Aspergillus-specific IgE titre and eosinophilia is observed (2). The role of IgG in Aspergillus nodules is unclear. In other CPA forms there seems to be little relationship to the extent or severity of the disease, however very high titres are only detected in the presence of an aspergilloma (1). The role of a raised anti-Aspergillus IgG in asymptomatic persons with Aspergillus airway colonisation is unclear, possibly they indicate a preclinical stadium in some cases (15).
A new lateral flow assay, using immunochromatographic technology (ICT) has also been developed for Aspergillus IgG (and IgM) testing, with a high sensitivity (91.6%) and specificity (91.6%). It probably has its use as diagnostic screening tool in low-income countries or as quick test during follow-up (16).
11 Unfortunately all microbiological and serological tests remain characterized by suboptimal sensitivity and specificity, leading to the necessity to apply diagnostic algorithms, such as the one proposed in Figure 8 (9,11).
Figure 2 Diagnostic algorithm, proposed by Takazono et al. (11)
1.5.4. Radiology
The recommended imaging modality is a computed tomography (CT) of the chest, preferably performed with contrast injection, to evaluate the vasculature as well. Because of the complex imaging findings, X-ray is only useful during follow-up. There is no place for positron emission tomography (PET); if performed, PET-avidity is to expect and therefore does not facilitate any narrowing of the differential diagnosis (1).
A combination of imaging findings due to the underlying respiratory disorder and the
Aspergillus infection itself contribute to the radiologic appearance of CPA. Most commonly,
CPA develops in a pre-existing pulmonary (or pleural) cavity, but disease progression will further lead to formation and expansion of new cavities and nodules. These expanding cavities with variable wall thickness, pleural thickening and intracavitary fungal ball formation will
12 cause a complex pleuroparenchymal destruction and/or fibrosis. Sometimes this is complicated by an Aspergillus empyema, pneumothorax or vascular abnormalities (aberrant (non-)bronchial arteries, pseudo-aneurysm), causing haemoptysis (1).
Fungal growth in a bronchiectasis or lung cavity is suspected when the interior surface receives an irregular appearance, that becomes thicker and starts to develop a network of fungal strands. As a late manifestation, an aspergilloma is formed, when the fungal strands coalesce to form a round intracavitary mass. An aspergilloma doesn’t always grow in an existing cavity. The collapse of the inner surface of a fungal mass/nodule will first form an air crescent sign and subsequently give the typical appearance of the fungus ball (1). As a consequence, in the majority of CCPA, there is no intracavitary fungal ball present, but the cavities stay empty or have an irregular internal wall with pleural thickening and pericavitary infiltrates (2).
The fungus ball typically presents in the upper lobes, partially surrounded by an air crescent and with adjacent pleural thickening. An aspergilloma can calcify, but normally doesn’t enhance after intravenous contrast injection (1).
The imaging findings of a CCPA, CFPA and SAIA are already described above. The different pathogenesis of an air crescent sign in a SAIA stresses again its invasive character. The air crescent here is caused by necrosis of the lung parenchyma, instead of collapse of the fungal surface into a cavity (1).
1.5.5. Differential diagnosis
The most important differential diagnosis of CPA is mycobacterial infection (tuberculous or non-tuberculous), which can also precede, follow or coexist with CPA. Other diseases to differentiate from are necrotising lung cancer, pulmonary infarction, nodules caused by auto-immune disorders and Actinomyces infection. They can not only have a similar clinical or radiologic appearance, but can also predispose to an Aspergillus infection, what makes an early diagnosis of CPA difficult to achieve (1,2). Other fungal infections like (para)coccidioidomycosis and histoplasmosis have to be kept in mind, taking travel history or country of origin into account (1).
13
1.6. Treatment
1.6.1. General principles
Treatment of CPA mainly consists of (oral) antifungal therapy. There are some indications for other modalities (surgery, embolization), primarily to treat complications. Therapy is guided by the subtype of CPA, patient’s symptoms and operability.
The treatment goal for SAIA is curation. In inoperable patients and in other subtypes, improvement in quality of life on the one hand and avoiding progression and complications by infection control on the other hand are the primary intention. Even after surgery relapses are common, so the majority of the patients will need chronic treatment with a focus on stabilising symptoms and lung function (1,2).
There is a broad clinical spectrum with at one side asymptomatic patients who do not progress without therapy and at the other side highly progressive symptomatic patients. In case of a single aspergilloma in asymptomatic patients without progression over 6-12 months, further observation is recommended during 6 to 24 months, following the IDSA guidelines. There is no need for immediate therapy, because an aspergilloma is generally not rapidly progressive. The IDSA guidelines also suggest observation in patients without symptoms or major lung function impairment for other forms of CPA, however this is a weak recommendation (2). Diagnostic and response criteria or treatment outcomes are not standardized, what complicates the comparison of the available evidence about therapy. A systematic review of the literature reveals for example only two prospective randomised trials of antifungal treatment regimens. Consequently, guidelines are based on cohort studies, case reports and expert opinion. Despite the differences in classification, the IDSA and ERS treatment guidelines are similar (1,7,8).
1.6.2. Antifungal therapy
SAIA is treated as an acute invasive aspergillosis with voriconazole as the treatment of choice. Treatment duration is 6 months, or longer if immunosuppression is continued. The efficacy of voriconazole for SAIA seems better than for CCPA (1,3).
For CCPA, the only published randomised clinical trial to our knowledge, was performed with oral itraconazole, which was superior to conservative treatment in clinical and radiologic evolution (17). Oral voriconazole has also been evaluated as primary therapy or after itraconazole in case of failure or intolerance (1). Both guidelines recommend itraconazole and voriconazole as first-line treatments (1,2). However, in most studies itraconazole has been used
14 as first-line treatment because of lower costs. The efficacy of itraconazole is in the range of 30-93% with a 16-33% prevalence of side effects (18). The most frequent adverse events are ankle swelling, gastrointestinal complaints, peripheral neuropathy and dyspnoea (9). The IDSA guidelines are slightly in favour of voriconazole in case of a high fungal load (clinical, radiological or higher baseline Aspergillus IgG), because resistance to itraconazole is more frequent (2). Its success rate ranges from 13-65% and 6-21% of the patients experienced side effects. Most common side effects are visual disturbance, photosensitivity, fluorosis and hallucinations (18). Oral posaconazole remains a second-line treatment, because of the higher cost and less experience (1,2,9). It is better tolerated than the former azoles, but lacks evaluation in studies for this indication. There are no data in CPA with isavuconazole, but it has fewer side effects than the other triazoles, and is associated with a shortening instead of prolongation of the QTc interval (19).
Because of a high intra- and interpatient variability, therapeutic drug monitoring (TDM) is recommended for voriconazole, itraconazole and – to a lesser extent – posaconazole. Itraconazole and voriconazole are well known for their drug interactions. In contrast TDM is not required for isavuconazole and it has fewer drug-drug interactions (3,19).
Before considering second- or third-line therapy, it is important to evaluate the serum concentrations of the used antifungal and to search for antifungal resistance. In case of toxicity or clinical or radiological progression, switching to another triazole (itraconazole, voriconazole or secondly to posaconazole or finally isavuconazole) is recommended (19).
Treatment takes at least 4-6 months. Deterioration in this period is synonym to treatment failure and recommends switch of regimen. In responders, indefinite long-term treatment translates into better outcomes. If no or minimal response, the treatment period should be extended to 9 months (1,2). There is some recent evidence to prolong standard duration to 12 months for all patients (18). In case of stable disease, according to the European guidelines, continuation of suppression therapy has to be evaluated, taking disease severity, medication tolerability or interactions and costs into account. Even in stable patients preventing complications and fibrosis can be more important than overt clinical/radiologic improvement (1). Also the IDSA guidelines advocate for long-term treatment, moreover because of the irreversible pulmonary damage and underlying immunological defects (2). After treatment discontinuation relapse is common (almost 1/3), but long-term data are lacking (1,19).
15 Intravenous therapy (amphotericin B, micafungin, caspofungin) is reserved for patients with progressive disease, treatment failure, triazole intolerance or resistance. Some authors suggest an intravenous induction phase followed by oral maintenance therapy in case of acutely ill patients (1,2). A short course (mean 17 days) of liposomal amphotericin B after triazole use, resulted in a clinical response in 65%, but was complicated with acute renal failure in 32% of cases (1).
Amphotericin has also T-cell helper 1 (Th1) upregulating effects, which are often deficient in CPA (2). The response rate of intravenous echinocandins and voriconazole as initiation therapy during 2-4 weeks were in the same range (resp. 60% versus 53%) (1,9). In some cases, the lipid-based formulations, mainly liposomal amphotericin B, have been used through nebulization to treat pulmonary aspergillosis (19).
1.6.3. Antifungal resistance
Azole resistance is frequently underrecognized, because cost and technical problems decrease the routine performance of fungal susceptibility testing in clinical laboratories. The main problem is the low sensitivity of a fungal culture in CPA and even positive cultures are in poor conditions with attenuated growth activity in vitro as a consequence (9,11). Based on the knowledge in IPA, specific mycology laboratories can provide fungal susceptibility testing, using MIC values, established by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) and the Clinical Laboratory Standards Institute (CLSI). However no commercial tests to determine the minimal inhibitory concentration (MIC) have been officially approved and these techniques remain technically demanding (20). In general, a positive culture during treatment suggest resistance (19).
The most common screening method defines resistance by detection of some resistance mutations. Point mutations in the Cyp51A gene are most common, but when using this technique, knowledge of regional differences in mutation prevalence is essential (20). A multiplex polymerase chain reaction (PCR), named AsperGenius©, is commercially available, detecting not only the presence of Aspergillus but also several resistance mutations (L98H, T289A, Y121F, and TR34) (11). An alternative method consists of an azole-containing agar screening multiwell plate, as validated in a study of Arendrup et al. This screening test had a sensitivity of 99% and a specificity of 99% (20). This kind of method is commercialized as VIPcheck©.
16 Azole-resistant strains are obtained by either the “environmental route” (e.g. induced by agricultural use of fungicides) or the “patient-acquired route” (induced by long-term use of azoles) (11). Non-azole resistance has been seldom reported (9,19). Antifungal resistance is more likely to develop in patients with higher fungal loads, prolonged exposure to azoles, low serum concentrations of itraconazole and the presence of an aspergilloma (1,9,18). Rates of azole resistance range from 2 to 54% in literature (9). There is some evidence that itraconazole resistance is more frequent than voriconazole, but rates differ geographically and according to the study population (18). However Belgian data are scarce. In a Belgian multicentre analysis, overall azole resistance in Aspergillus infections was 5.5%, but in none of the 20 isolates of CPA patients resistance was found (12).
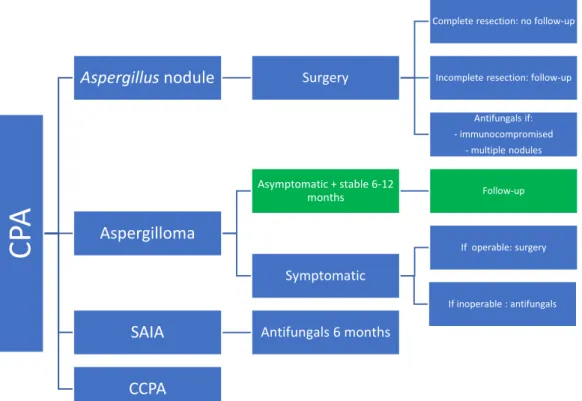
Figure 9 Overview of antifungal treatment in CPA: blue ERS guidelines (1), green IDSA guidelines (2)
CP
A
Aspergillus nodule Surgery
Complete resection: no follow-up
Incomplete resection: follow-up
Antifungals if: - immunocompromised - multiple nodules Aspergilloma Asymptomatic + stable 6-12 months Follow-up Symptomatic If operable: surgery If inoperable : antifungals
SAIA Antifungals 6 months
17
Figure 10 Antifungal treatment CCPA: blue: ERS guidelines (1); green: IDSA guidelines (2); in brackets: weak recommendations; red: clinical practise/research gaps; vorico: voriconazole; itra: itraconazole; posa: posaconazole, isavu: isavuconazole; ampho B: amphotericin B; m: months; CPA: chronic pulmonary aspergillosis; CCPA: chronic cavitary pulmonary aspergillosis; SAIA: subacute invasive aspergillosis; CFPA: chronic fibrotic pulmonary aspergillosis;
1.6.4. Surgery
Surgical resection (lobectomy, segmentectomy, bullectomy) is indicated in localised and refractory disease (azole resistance, destroyed lung) or in case of complications. The success rate mainly depends on the resectability of the aspergilloma and the risk of fungal spillage. Multicavitary disease also increases the peri- or post-operative risk of complications, which explains the better results in simple aspergilloma as compared to CCPA. A relapse rate up to 25% has been reported (1,2). For simple aspergilloma, primary surgery will maximize cure rate and prevent complications in operable patients. Even a bilobectomy can be considered if respiratory function is sufficient. In CCPA, surgery remains an option in refractory disease (e.g. multi-azole resistance) or to treat complications (1).
There is no evidence about perioperative prophylactic or adjuvant antifungal therapy. Guidelines however recommend prophylactic treatment from weeks before the surgery to avoid spillage of fungal spores. In cases of perioperative spillage, pleural instillation with amphotericin B and postoperative antifungal treatment can be considered. In suboptimal surgery, if positive cultures or pericavitary hyphae in the lung parenchyma are found,
CCP
A
Asymptomatic Follow-up Disease specific IV induction(Ampho B, Vorico, echinocandine) Oral continuation
Oral Itra/Vorico
high fungal load: Vorico
Response after 6
months Indefinite suppression Minimal
response/stable 6 m: extend to 9 m
Continuation depending on: -medication tolerance,
interaction, cost -Disease severity, underlying disease (immunosuppression)
Deterioration during 4-6 months/intolerance
Switch regimen: Vorico, Itra, Posa
Switch IV IV: - intolerance - antifungal resistance -progression Surgery Switch Isavu
18 continuing antifungal treatment is indicated. The duration of treatment is unknown, but the IDSA guidelines suggest at least 4 weeks (other rapports 8 weeks) (1,2,19).
A special entity is the Aspergillus nodule, mainly diagnosed after surgery. No postoperative antifungal treatment is required, unless immunocompromised or multiple nodules are present. For incompletely resected nodules, close monitoring every 3 months is preferred over antifungal treatment (1).
1.6.5. Treatment of complications
One third of patients have haemoptysis at diagnosis. There are no data about the overall incidence of (severe) haemoptysis, nor of pneumothorax, another important complication. Antifungal treatment is the principal treatment to prevent recurrent haemoptysis. In mild or moderate haemoptysis, treatment with tranexamic acid is sufficient. For moderate to severe haemoptysis, embolization may be performed as definitive or temporal measure before surgery. The bronchial arteries are most frequently affected. The success rate in experienced centres is 50-90%, with recurrence of 30-50% over 3 years. In very severe haemoptysis surgery will be needed, if no cardiopulmonary contra-indication (1,2).
In inoperable patients with recurrent haemoptysis, local injection of an antifungal drug by an endobronchial catheter or percutaneous transthoracic catheter can be considered. Amphotericin B is the drug of choice in these cases. Complications are chest pain, pneumothorax and endobronchial reflux. Clinical reports have reported cessation of haemoptysis, culture conversion, fall in antibody titre and even regression of an aspergilloma (1).
1.6.6. Other treatment modalities
Optimizing the nutritional state is an important treatment strategy, mainly for those being evaluated for surgery.
Two clinical cases showed a good response, using interferon-gamma (IFN-gamma) immunotherapy, because IFN-gamma deficiency was identified as one of the immune defects predisposing for CPA (21). Because of lack of solid evidence, this is however not generally recommended (1).
In several comorbidities like sarcoidosis or rheumatoid arthritis, treatment with immunosuppressive drugs, including steroids, are required. However, the dose of the inhaled and oral corticosteroids has to be maintained as low as possible and initiation of corticosteroids
19 is generally discouraged. The intake of immunosuppressive therapy requires continuation of antifungals (1).
Intercurrent bacterial infections, mainly with Streptococcus pneumoniae, Haemophilus
influenzae, Pseudomonas aeruginosa and Staphylococcus aureus, are a common cause of death.
Pneumococcal vaccination is recommended. In case of Pseudomonas colonisation, eradication is proposed (1).
1.7. Specific comorbidities 1.7.1. Sarcoidosis
The past 20 years, increasing evidence has highlighted the importance of CPA in cavitary sarcoidosis. Studies found an incidence of about 2.6% in patients with sarcoidosis, in only 10% already present at diagnosis. In 7.1% of the CPA patients, sarcoidosis was the predisposing condition. CCPA was the most common manifestation (49%), followed by simple aspergilloma (31%) and CFPA (15%). In relation to other underlying diseases, simple aspergilloma and CFPA are more common, while Aspergillus nodules are rare (22,23).
CPA is one of the serious complications of sarcoidosis, because it holds the risk of evolving to fibrosis and inducing severe haemoptysis with a high mortality rate. In cavitary sarcoidosis, screening with Aspergillus IgG might be indicated. Individuals with sarcoidosis are mildly immunocompromised, even unrelated to the use of corticosteroids. Because in both CPA and sarcoidosis genetic variants in immunological pathways are found, genetic testing may help in the risk assessment of CPA in sarcoidosis if common genetic variants could be revealed in the future. Moreover, immunotherapy could have its benefits. Not only an immunological cause can explain the frequent co-existence of CPA and sarcoidosis. There is also an association between environmental fungal exposure and the development of sarcoidosis (22).
A specific treatment regimen for CPA in sarcoidosis is not available. Cyclic courses of caspofungin in combination with oral triazoles as maintenance therapy between infusions, have been investigated (24). There is a very high relapse rate after surgery (91% vs 25%), so surgery should be considered as salvage therapy (1).
It is still a matter of debate whether CPA patients with sarcoidosis have a worse prognosis than other underlying diseases. Mortality rates are according to the studies 62-74% survival at 5 years, 47-61% at 10 years. The highest mortality was seen in patients with advanced sarcoidosis, not influenced by the presence of CPA (1,22).
20
1.7.2. Non-tuberculous mycobacteria
Because of the increasing incidence of NTM infections, CPA occurs more in non-tuberculous cavities. The incidence of CPA in studies is reported between 3.9 and 11% of patients with non-tuberculous mycobacterium pulmonary disease (NTM-PD) (25,26). The mean time in a study of Jhun et al. was about 18 months after treatment initiation. Older male, current smokers, a low body mass index (BMI) (or hypoalbuminemia), history of tuberculosis or COPD and the use of steroids are significant risk factors. In one series, 40% of the CPA patients coinfected with NTM, Mycobacterium abscessus was the underlying pathogen, most common in the fibrocavitary form (25). In another study, M. avium complex (59.7%) and M. kansasii (32.3%) were most prevalent (27). A specific risk scoring system for CPA has been developed for M.
avium pulmonary disease (26). CPA increases the mortality in NTM disease (19.5% vs. 1.7%).
Diagnosis and treatment are often delayed because of the difficult differentiation with progression of NTM-disease. At diagnosis of NTM infection or in case of an unsatisfactory treatment response, CPA has to be considered. Moreover, drug-drug interactions can complicate concomitant therapy (e.g. itraconazole and rifabutin/rifampicin). However in the given studies, people received mainly itraconazole as initial treatment (25).
1.8. Follow-up and response monitoring
Treatment regimens are guided by clinical and radiological response, although the reported clinical, mycological and radiological criteria are not standardized (7). The IDSA guidelines recommend monitoring by low-dose CT or X ray (every 3-12 months), inflammatory markers,
Aspergillus IgG titres and annual lung function tests for CCPA (2).
Clinical response is assessed by improvement in quality of life and daily life activities, evaluated by using the St. George’s Respiratory Questionnaire (SGRQ). Screening for adverse events during follow-up is also very important. Clinical deterioration (weight loss or new respiratory symptoms) indicate treatment failure (1).
There is no consensus about the recommended respiratory sample to evaluate definitive eradication of the Aspergillus species (7). In case of treatment failure, azole resistance should be sought. Serial measurement of Aspergillus IgG levels is useful. A slow fall after treatment initiation, is followed by a low titre (mostly only undetectable if therapy is given for years) in stable disease or a raise in case of therapy failure/relapse (1).
Radiological improvement is slow and not visible before 3 months of treatment (1). Variation (at least 20% or 2 mm) in cavity and pleural wall thickness (including pleural fibrosis) seemed
21 the most significant response indicator in the study of Godet et al. Moreover, loss of fungus ball had the strongest association with clinical and radiological improvement, however changes in fungus ball volume (at least 30%) were not statistically significant. Decrease in cavity size (at least 30%) was not included as a response criterion, because paradoxically growth happened, even with clinical improvement. This was explained by a fibrotic process of scarring and distortion, increasing the cavity volume (7). Although postulated in another study of Agarwal
et al., attenuation of pericavitary infiltrates was not withheld as statistical significant (7,17).
Indicators of failure remain the formation of new cavities, nodules or aspergilloma (1). Follow-up imaging is recommended 3-6 months after treatment initiation and then less often, depending on the clinical evolution. Both low-dose CT and X-ray are useful during follow-up (1).
1.9. Prognosis
Without intervention, the 5-year mortality of CPA is 75-80%. Even on treatment, a 5-year case fatality rate of 20-50% is found in older literature (6). More recent results revealed a survival of 86%, 62% and 47% at 1, 5, and 10 years respectively (28). Negative prognostic markers are NTM-PD, COPD, pleural involvement, cavitary disease, presence of aspergilloma (especially bilateral), dyspnoea, low physical activity and low body mass index. Although not statistically significant, the 10-year survival was also higher for azole-susceptible than azole-resistant disease (68% vs. 46%) (28). Twelve months of therapy with itraconazole or voriconazole lead to an improvement of 5 points in SGRQ in a study of Bongomin et al. (18).
1.10 Aim of the master thesis
The higher life expectancy of patients with chronic pulmonary disease (CF, COPD, bronchiectasis), the frequent use of immunosuppressive therapies and the remaining high incidence of tuberculosis are raising the prevalence of CPA. Many aspects of CPA are still unknown, so there is huge need for answers on several research questions. The goal of this master thesis is the collection of clinical, radiological, microbiological and treatment data of patients followed in Ghent University Hospital. Moreover, the role of the Aspergillus antigen in immunocompromised patients is evaluated.
2. Methods
2.1. Literature review
A review of the relevant literature was performed by using the Medline database. The following head terms were used: “chronic pulmonary aspergillosis, “chronic cavitary aspergillosis”,
22 “chronic necrotizing aspergillosis” and aspergilloma. There were no Mesh terms available. Search strings were narrowed through subheadings like “rheumatic disease” (Mesh term), “antirheumatic drugs”, “GPA” and “rheumatoid arthritis”. There was no defined time frame, but the latest literature search dates from October 2019. Only articles with English abstracts were included.
2.2. Data collection
Data collection was performed in the context of participation in the CPAnet registry, approved by the local independent Ethics Committee of Ghent University Hospital, Belgium (project 2018/1230). This is a multinational and multicentre web-based questionnaire, established in March 2017, promoting research about several research topics for diagnosis, microbiology, treatment and outcomes of CPA (8). Written informed consent was obtained from patients who were still in follow up at our centre; informed consent was waived for deceased patients. The patient data were pseudonymized and inserted through the online platform www.clinicalsurveys.net. A separate databank was created to analyse these data in our own population. The design of our study was only observational with the aim of getting more epidemiologic information about CPA. Patients were included prospectively and retrospectively. Patient inclusion was based on the ERS criteria, not the IDSA criteria. Other inclusion criteria were age (> 18 years on the moment of inclusion) and the absence of invasive pulmonary aspergillosis by diagnosis. The inclusion age deviated from the CPAnet registry criteria (age >18 by diagnosis).
In total we included 23 prospective and 2 retrospective cases over a time course of 1 year. Informed consent was obtained, except for the 2 deceased patients. No financial compensation nor costs were related to participation, because the study course did not differ from daily clinical practice. Patient data were pseudonymized at the end of the data collection. Beside clinical data, questionnaires like CAT (COPD Assessment Test), mMRC (modified Medical Research Council) and SGRQ were recorded after patient inclusion, but these were often not present at the time of diagnosis. Radiological response was evaluated, based on the criteria of Godet et al., as described in the introduction (7). Patient enrolment started in December 2018 when the first informed consent was given. Enrolment ended in November 2019, when data analysis started.
23 2.3. Data processing issues
The inclusion date was defined at the time that criteria for CPA diagnosis were fulfilled (mostly when microbiological data were present), not when the probable diagnosis was mentioned in the patient files. Often there was already a suspicion of CPA before definite diagnosis, but in other cases the diagnosis was made later, although theoretically diagnostic criteria were present earlier. This time lag was indicated by “diagnostic uncertainty”. In some cases, the results of the galactomannan assay were already present before the date of formal diagnosis, but these values were imputed on the time point of diagnosis.
2.4. Statistical analysis
Because most information was collected retrospectively, a principally descriptive data analysis was performed, making use of SPSS Statistics software version 26.0. Statistical testing of categorical variables was performed making use of Chi-square tests with statistical significance taken as p= 0.05.
3. Results
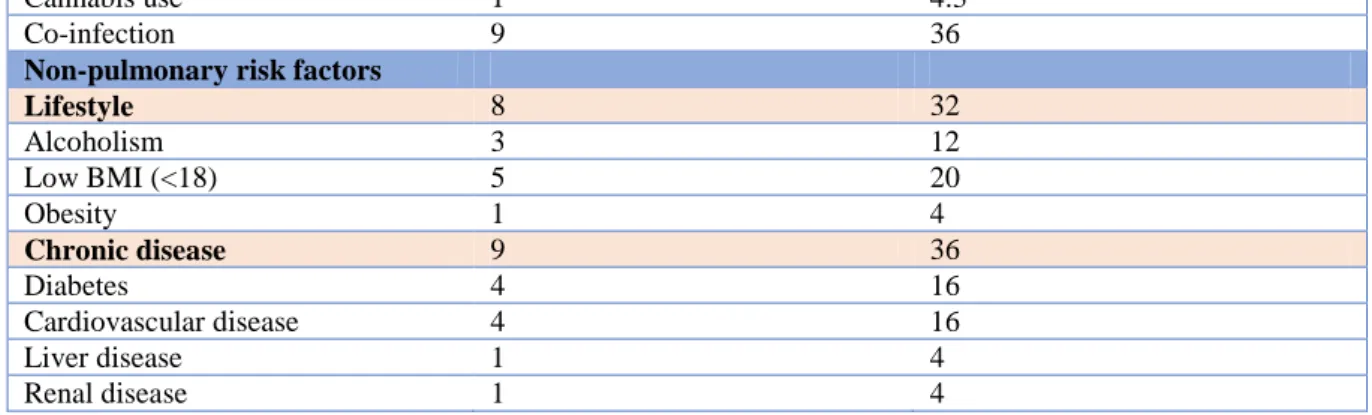
3.1. Demographics Number (n=25) Percent (%) Demographics Age (years) 54 (16-88) Male 18 78,3 Female 5 21.7 BMI 23 (16-33) Type of CPA Simple aspergilloma 3 12 CCPA 16 64 SAIA 6 24 CFPA 0 0 Aspergillus nodule 0 0Pulmonary risk factors 25 100
Familial history 0 0 Active tuberculosis 1 4 History of tuberculosis 3 12 Active/history of NTM-PD 1 (M. xenopi) 4 Sarcoidosis 1 4 ABPA 4 16 COPD 12 48 Asthma 9 36 Idiopathic bronchiectasis 1 4 Pneumothorax 3 12 Thoracic surgery 5 20 History of IPA 1 4,3 Thoracic radiotherapy 1 4 Oxygen use 1 4 Previous/active smoking 19 76
24
Cannabis use 1 4.3
Co-infection 9 36
Non-pulmonary risk factors
Lifestyle 8 32 Alcoholism 3 12 Low BMI (<18) 5 20 Obesity 1 4 Chronic disease 9 36 Diabetes 4 16 Cardiovascular disease 4 16 Liver disease 1 4 Renal disease 1 4
Table 3 Demographics: CPA: chronic pulmonary aspergillosis; CCPA: chronic cavitary pulmonary aspergillosis; SAIA: subacute invasive aspergillosis; CFPA: chronic fibrotic pulmonary aspergillosis; NTM-PD: non-tuberculous mycobacterial pulmonary disease; IPA: invasive pulmonary aspergillosis; BMI: body mass index.
The mean population age was 54 year with a male predominance. CCPA was the most frequently diagnosed phenotype. In three patients, SAIA evolved to a CCPA during follow-up, 2 patients with CCPA to SAIA and 1 CCPA to CFPA, despite treatment.
All patients had predisposing pulmonary risk factors. There often was a history of (current) smoking (76%). COPD (48%) and asthma (36%) were the most frequent underlying lung diseases. There were only 16% patients with active or previous pulmonary tuberculosis. Many patients (36%) also had non-pulmonary chronic comorbidities, such as diabetes or cardiovascular disease. Co-infections with other respiratory pathogens were frequent (36%). Three cases were coinfected with Pseudomonas aeruginosa and 2 with Mucorales. One patient had mucormycosis following allogeneic stem cell transplantation in the past and developed CCPA 8 years after in a remaining cavity. Two patients had active mycobacterial co-infections, respectively with M. tuberculosis and M. xenopi.
A remarkable observation was the high prevalence of some degree of immunosuppression (56%), with 7 (28%) patients having an auto-immune disease and 11 (44%) taking immunosuppressive drugs, mainly oral steroids (36%) and methotrexate (16%). Some patients took more than one drug.
25
Immunosuppression Number (n=25) Percent (total)
Immunosuppression 14 56
HIV 1 4
Allogeneic stem cell Tx 1 4
Oral corticosteroids 9 36 Hyper-IgE syndrome 2 8 Auto-immune disease 7 28 GPA 2 8 RA 3 12 Sarcoidosis 1 4 Psoriasis 1 4 Immunosuppressive drugs 11 44 Methotrexate 4 16 Leflunomide 3 12 Adalimumab 1 4 Azathioprine/cyclophosphamide /rituximab 1 4 Oral corticosteroids 9 36
Table 4 Immunosuppression: HIV: human immunodeficiency virus; Tx: transplantation; IgE: immunoglobulin E; GPA: granulomatosis with polyangiitis; RA: rheumatoid arthritis.
3.2. Diagnosis
Diagnosis Number (n=25) Percent (total)
Presenting symptoms 23 92 Respiratory Cough 18 72 Dyspnoea 20 80 Sputum 15 60 Haemoptysis 9 36 Chest pain 6 24 General Weight loss 13 52 Fatigue 11 44 Fever 10 40 Night sweats 8 32 Radiology
Specific fungal signs
Intracavitary Aspergillus 19 76
Cavity without fungus 7 28
Aspergilloma 2 8
Single Aspergillus nodule 1 4
Aspergillus nodules 10 40
Localisation
Right upper lobe 16 64
Middle lobe 1 4
Right lower lobe 7 28
Left upper lobe 15 60
Lingula 3 12
Left lower lobe 6 24
Other signs Pleural disease 14 56 Bronchiectasis 17 68 Emphysema 9 36 Fibrosis 1 4 Consolidation 16 64 Lymphadenopathy 12 48 Pneumothorax 2 8
26 Microbiology (Total) Aspergillus IgG Performed by diagnosis Positive result 17 9 53 Ever performed Positive result 23 15 65
Aspergillus IgE (>0.1kU/l)
Ever performed Positive result
21
16 76
Sputum culture Sputum ever performed Positive result 13 9 69 BAL Ever PCR performed Positive result 7 4 57
Ever culture performed Positive result 23 3 13 Galactomannan by diagnosis Positive result 17 10 59
Ever Galactomannan performed Positive result 22 14 64 Pathology/histology BAL histology/microscopy 1 4 Transthoracic puncture 2 8 Transbronchial biopsy 1 4 Surgical biopsy 5 20 Diagnostic uncertainty 11 44
Diagnostic delay (months) 25.4 (3-54)
Table 5 Diagnosis: * by diagnosis, **ever performed, if not specified results only indicating positive results by diagnosis or follow-up.
In 44% of the cases there was a diagnostic delay with a mean interval of 25 months. Patients often presented with dyspnoea (80%) and productive cough (60%). Mild or more severe haemoptysis was mentioned in 9 cases (36%). Weight loss was the most prevalent general complaint (52%).
CCPA with an intracavitary fungus ball was a common radiological feature, mostly affecting the upper lobes, both to an equal extent. Surrounding Aspergillus nodules were frequent detected. Other rarely observed radiologic signs were air trapping, bronchiolitis, hydropneumothorax, pulmonary embolism, bullae and bronchopleural fistula.
Microbiological diagnosis was mostly based on Aspergillus-specific IgG and Aspergillus antigen. Aspergillus PCR was positive in 4 cases (sensitivity 57%). Sputum and BAL culture were positive in resp. 69% and 13% when these tests were performed.
In almost all positive cultures, Aspergillus fumigatus was detected. Resistance was examined in 10 of the 24 cases (42%), in 4 cases by means of an antimycogram, in 6 by using the
27
Aspergillus isolate was posaconazole- and itraconazole-resistant, the other was resistant to
amphotericin-B, posaconazole (intermediate resistance) and echinocandins.
A cut-off level of 72 mg/L lead to a positive Aspergillus IgG in 9 of 17 (53%) patients at diagnosis and in 15 of 23 (65%) during follow-up. With a cut-off level of 50 mg/L, this changes into resp. 14/17 (82%) and 19/23 (83%). Aspergillus IgE was increased in 76% of patients.
Aspergillus antigen on BAL was performed in 17 cases at diagnosis, with 10 positive results
(59%). When also including a positive galactomannan during follow-up, a positive test was found in 14 of 22 patients (64%), based on a cut-off level of 1. When increasing the cut-off to 1.5 this was 54.5%. Serum galactomannan was positive in one case with fulminant progressive disease upon assessment during follow-up.
Fifty-seven percent of the patients with an ever-documented positive Aspergillus antigen test were known with an underlying immunosuppressing condition. Eight of the 14 patients with immunosuppression ever had a positive galactomannan test. This was not statistically significant (p= 0.877). All patients (n = 3) with rheumatoid arthritis had a positive Aspergillus antigen. No statistically significant association was found between immunosuppression or oral steroid use and SAIA. The galactomannan (BAL or serum) was positive in 57% percent of patients with SAIA. This was also not significant (p=0.278 ever positive galactomannan in patients with SAIA).
Microbiology Number (n = 25) Percent (total)
Aspergillus IgG* Cut-off 72 mg/L** 15/23 (ever) 9/17 (diagnosis) 65 53 Cut-off 50 mg/L 19/23 (ever) 14/17 (diagnosis) 83 82 Aspergillus antigen By diagnosis 2.64 (0.10-6.64) Aspergillus culture 13/25 52 Aspergillus fumigatus 13/13 100% Aspergillus niger 1/13 8.3
Table 6 Microbiology: *1 sample not with Immunocap, not mentioned here. **The mean was not calculated, because levels above 200mg/l were not reported in the lab.
28
3.3. Treatment
Treatment Number (N = 25) Percent
First-line therapy total
Antifungal
Voriconazole 15 60
Itraconazole 7 28
Posaconazole 1 4
Amphotericin B 1 4
Reason therapy stop total
No stop 6 24
Treatment completion 7 28
Treatment switch 3 12
Drug-related adverse event 8 32
Failure 1 4
Clinical response 1st-line 24 subtotal
Stable 8 33
Improvement 15 63
Progression 1 4
Radiologic response 1st-line 21 subtotal
Stable 6 29
Improvement 14 67
Progression 1 5
Second-line therapy 16 subtotal
Antifungal Voriconazole 4 25 Itraconazole 4 25 Posaconazole 6 38 Amphotericin B 2 13 Caspofungin 1 6
Reason therapy stop subtotal
No stop 6 38
Treatment completion 4 25
Treatment switch 1 6
Drug-related adverse event 4 25
Failure 1 6
Clinical response 2nd-line subtotal
Stable 3 19
Improvement 8 50
Progression 1 4
Radiologic response 2nd-line
Stable 3 19
Improvement 8 50
Progression 1 6
Treatment duration (months)
Follow-up time 25 (0-108) Completed first-line treatment 8 (6-12) First-line treatment CPA
CCPA 16 Subtotal Voriconazole 11 69 Itraconazole 5 31 Simple aspergilloma 3 Itraconazole 3 100 SAIA 6 Voriconazole 4 66.6 Posaconazole 1 16.6 Amphotericin B 1 16.6 Table 7 Treatment
29 Voriconazole was the most frequent administered first-line treatment (60%), followed by itraconazole (28%). Overall treatment failure in first line was rare (4%). The clinical response was 63% and radiological improvement was noted in 67% (14 of 21 cases). The best response was seen for voriconazole, but in 47% treatment was changed because of a drug-related event. Sixty-four percent of the patients received second-line therapy. Most frequently, posaconazole was given in second line (38%), and this was usually continued over the follow up time (66% continued treatment) with a clinical improvement in all cases with a response assessment. Reasons for stopping therapy after first-line treatment were mainly treatment completion (28%) and drug-related adverse events (32%). This also applied for second-line treatment.
Most patients received one or two therapy lines (76% of patients). Only 6 patients had a third-line treatment with clinical and radiological improvement in resp. 50% and 33% of cases. In very small amounts (1-5 patients), additional changes were proposed, up to 7 in 1 patient. In addition, we observed that the clinical and radiological response were markedly lower after 4 treatment changes.
Antifungal therapy Number (n=25) Percent (%)
Itraconazole 13 52 Adverse events 4 31 Generalised oedema 2 Liver dysfunction 2 First-line 8 32 Clinical response 8 improvement 2 25 stable 5 63 progression 1 13 Radiological response 6 improvement 2 33 stable 3 50 progression 1 16 Stop therapy No stop 3 38 Completion 3 38
Switch to another drug 1 13
Drug related event 1 13
Failure 0 0
Overall response 10 (3 unknown)
Overall clinical/radiological improvement 2 20
Overall Stable 5 55.5 Overall progression 3 33.3 Voriconazole 15 60 Adverse events 11 73 Liver dysfunction 6 Visual disturbance 5 Phototoxicity 3
30
First-line 15 60
Clinical response 15
improvement 12 80
stable 3 20
Radiological response 14 (1 unknown)
improvement 11 79
stable 3 21
Stop therapy
No stop 2 13
Completion 3 20
Switch to another drug 2 13
Drug related event 7 47
Failure 1 7
Overall response 15
Overall clinical/radiological improvement 13 87
Overall stable 2 13 Progression 0 0 Posaconazole 9 36 Adverse events 1 11% Alopecia 1 Stomatitis 1 First-line 1 4.3 Second line 6 40
Clinical response 4 (2 unknown)
improvement 4 100
Radiological response 4 (2 unknown)
improvement 4 100
Stop therapy
No stop 4 66.4
Completion 2 33.3
Response 7 (2 unknown)
Overall clinical/radiological improvement 5 71.4
Overall Stable 2 28.6 Progression 0 0 Other antifungals Amphotericin B 5 20 Caspofungin 1 4 Isavuconazole 1 4
Table 8 Antifungal therapy: more than one adverse event was observed in one patient
Itraconazole was used in 52% of patients. Most frequent side effects were generalized oedema and hepatotoxicity. Posaconazole was administered in 36% and only one patient experienced side effects (alopecia, stomatitis). Adverse events were present in 73% of patients receiving voriconazole. In almost half this was due to liver dysfunction or visual disturbances, less frequently (severe) phototoxicity was observed. Rare reported side effects were periostitis, haemorrhagic cystitis, depression and hyperkalaemia.
Isavuconazole, at the time of data acquisition only available through a medical need programme, was only used in one case where a good clinical response and stable radiological response was observed, without side effects.
31
Other treatment Number (n=25) Percent (%)
Surgery (treatment) 5 20
Surgery (diagnosis) 5 20
Surgery (complications) 3 12
Embolization 4 16
Table 9 Other treatment
Surgery was performed in 20% of patients because of several diagnostic or therapeutic reasons (table 9).
3.4. Follow-up
Complications Number (n=25) Percent (%)
Non-lethal 12 48 Haemoptysis 11 44 Without embolization 7 With embolization 4 Pneumothorax 3 12 Extrapulmonary manifestation 1 4 Lethal 2 8 Table 10 Follow-up
The mean follow-up time was 25 months at database closure. Almost 64% of the patients were in follow-up for two years, but 4 patients only less than or just 1 month and 2 patients were included post-mortem, resulting in a very diverse follow-up time. Seven of the 25 patients completed treatment with a mean treatment duration of 8 months (6-12 months).
Complications were reported in twelve patients (48%). Almost half of patients mentioned haemoptysis at diagnosis or during follow-up, but only 36% needed embolization. Pneumothorax was present in two cases at diagnosis. Two patients died during follow-up, both because of respiratory complications. One was directly related to the CPA disease and died because of a pneumococcal septic shock with ARDS. The other one developed a post-procedural aspiration pneumonia, leading to a fulminant ARDS.
32
Figure 11 Evolution of Aspergillus antigen of all patients
Figure 12 Evolution of Aspergillus IgG of all patients
0,0 1,0 2,0 3,0 4,0 5,0 6,0 7,0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 15 22 36 38 42 44 55 58 61 65 68 73 79 98 103
Aspergilus antigen evolution
G001 G003 G002 G007 G019 G018 G011 Months Aspergillus antigen 0,00 50,00 100,00 150,00 200,00 250,00 0 2 4 6 8 10 12 14 16 18 20 22 29 36 38 42 47 53 62 70 76 85 100
Aspergillus IgG evolution
G009 G003 G002 G006 G010 G007
G008 G019 G018 G011 G012 G013
G015 G005 G017 G024
Aspergillus IgG (mg/l)