Fenestrated and Branched Endovascular Repair
for Juxtarenal and Thoracoabdominal Aortic
Aneurysms: Analysis of the first 100 cases
Gilles Uijtterhaegen
Stamnummer: 00702670
Promotor 1: Prof. dr. Frank Vermassen Promotor 2: Dr. Nathalie Moreels
Masterproef master in de specialistische geneeskunde
Contents
Abstract 4
Introduction 5
1. General considerations 5
2. Open surgical repair 9
3. Endovascular treatment 11
4. Complex endovascular aneurysm repair 13
5. Scope of the study 19
Methods 19
Results 22
1. Patient and aneurysm characteristics 22
2. Stent graft design and device implantation 24
3. Early morbidity and mortality (< 30 days) 26
4. All-cause mortality and morbidity 31
Discussion 38
References 42
Nederlandstalige samenvatting 48
Abstract
Introduction: Fenestrated and branched endovascular aortic aneurysm repair (F/BEVAR) is an upcoming minimally invasive technique to treat juxtarenal abdominal aortic aneurysms (AAA) and thoracoabdominal aortic aneurysms (TAAA). The objective of this study is to report our results with this technique and to evaluate its safety and efficacy.
Methods: This is a retrospective single center cohort study analyzing all consecutive patients undergoing a complex endovascular abdominal aortic repair (FEVAR or BEVAR).
Results: One hundred patients underwent a FEVAR or BEVAR between June 2012 and December 2019. Mean age was 73,3 ± 6,9 years and 94% of the patients were male. Fifty-nine patients were treated because of a juxtarenal AAA and 41 because of a TAAA. Thirty-one had a history of aortic repair. Mean follow-up was 33,6 ± 22,4 months. Freedom from all-cause mortality was 93,9% ± 2,4% at 30 days, 89,3% ± 3,2% at one year, 74,4% ± 5,2% at three years and 66,4% ± 7,6% at five years. Freedom from aneurysm-related mortality was 93,9% ± 2,4% at one year and 92,5% ± 2,7% at three and five years. Subgroup analysis shows a trend towards a higher mortality in the TAAA group and in the group of patients who have had previous aortic surgery. On a total of 350 targeted vessels 14 (4%) early and late target vessels events were registered. Thirty-two patients developed 50 major adverse events. At the first computed tomography angiography a total of 32 endoleaks were visualized. A total of ten procedure related secondary interventions were observed in eight patients, including six interventions for an endoleak. Freedom from secondary intervention was 93,6% ± 2,8% at one year, 92,0% ± 3,1% at two years and 89,9% ± 3,8% at three and five years.
Conclusion: This study demonstrates that fenestrated and branched endovascular repair is a safe and feasible treatment for juxtarenal and thoracoabdominal aortic aneurysms with acceptable early complication rates and low long term aneurysm related mortality. However, non-aneurysm-related mortality remains important in this population.
Introduction
1. General considerations
The term aneurysm is used to describe a dilatation of any blood vessel. Extracranial arterial aneurysms are most prevalent in the infrarenal abdominal aorta. An abdominal aorta with a diameter larger than 3,0 cm is considered as aneurysmal. The incidence of abdominal aortic aneurysms (AAA) increases with age and before the age of 55-60 the prevalence is negligible.1,2 In 2010 global prevalence in 75-79 year olds was 2275 per 100,000 population and this prevalence is declining over the last 20 years.2 Thoracoabdominal aortic aneurysms (TAAA) are less common than AAAs and have an estimated incidence of 6-10,4 cases per 100,000 person-year. This number seems to be increasing over time and is higher in men.11 Most important risk factor for aortic aneurysms is smoking. Other risk factors are atherosclerosis, hypertension, ethnicity and family history of AAA. Patients with diabetes (especially type 2) are less at risk for developing AAA.1,2 In the majority of the patients the natural history of small AAA is growth, with an average growth rate of 2.2 mm/year for aneurysms between 3,0 and 5,5 cm. There is an exponential increase in average growth rate with the diameter of the aneurysm and when the diameter exceeds 5,0 cm the growth rate is 3,6 mm/year. Smoking is associated with an increased growth rate and diabetes is associated with a decreased growth rate. 2
1.1. Etiology, morphology and anatomic classification
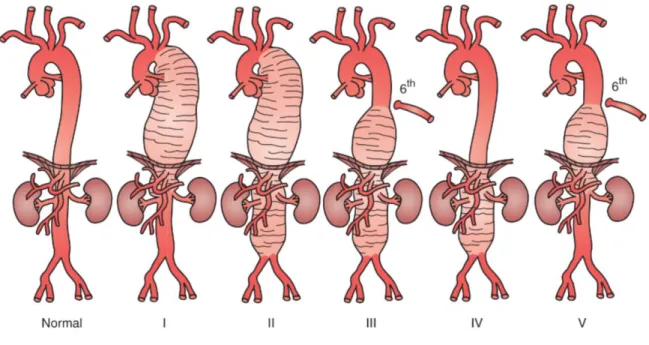
Most aneurysms are the result of degeneration of the aortic wall due to inflammation, infection, trauma or genetic conditions. The shape of an aneurysm is described as fusiform or saccular. True degenerative aneurysms have typically a fusiform shape. Saccular aneurysms can be concentric or eccentric. Eccentric defects arise from a focal ulcer or weakness in the arterial wall, often due to infection or trauma.1 In TAAA 80% of the aneurysms are caused by degeneration and 20% are sequelae from chronic aortic dissection. In 40% of these cases there is a dilatation of the outer wall of the false lumen necessitating aortic repair. Other causes of TAAA are connective tissue disorders such as Marfan syndrome, Ehlers-Danlos and Loeys-Dietz syndrome. The Crawford classification is used to classify the TAAA by their anatomic characteristics (Figure 1). A type I TAAA begins distal to the left subclavian artery and ends above the renal arteries. A type II starts distal to the left subclavian artery and ends below the renal arteries. A type III extends from the sixth intercostal space to the renal arteries and a type IV encompasses the entire abdominal aorta. A type V TAAA starts below the sixth intercostal space and ends just above the renal arteries.1,3
Figure 1. Crawford classification of thoracoabdominal aortic aneurysms.1
There is no consensus on the definition of AAA with short necks or involving visceral arteries, however juxtarenal AAAs are defined as aneurysms extending up to but not involving the renal arteries necessitating suprarenal clamping in open surgical repair (OSR), i.e. a short neck (< 10mm). When the aneurysm reaches up to the superior mesenteric artery (SMA) this is called a suprarenal- or paravisceral AAA. The difference between a suprarenal AAA and a Crawford type IV aneurysm is not clear. 3
1.2. Rupture risk
Primary danger of AAA is rupture with uncontrolled hemorrhage. The risk of rupture is related to the absolute and relative diameter of the aneurysm (Table 1). Other factors influencing rupture risk are location, etiology, growth rate, morphology, female sex, larger initial diameter, smoking, lower FEV1 and higher mean blood pressure.1,2
Table 1. Annual rupture risk based on AAA diameter 1
AAA Diameter (cm) Rupture Risk (%)
3.0-3.9 0,3
4.0-4.9 0.5-1.5
5.0-5.9 1-11
6.0-6.9 11-22
1.3. Diagnosis
1.3.1. Symptoms and physical examination
Patients with AAAs are usually asymptomatic. When developing symptoms patients can feel diffuse nonspecific abdominal and/or lower back pain. Thin patients can feel pulsations or see a pulsatile mass in their abdomen. Physical examination can reveal a pulsatile abdominal mass, depending on size and location of the AAA.1,2 For TAAA the guidelines of the American Heart Association suggest to “perform a focused physical examination, including careful and complete search for arterial perfusion differentials in both upper and lower extremities, evidence of visceral ischemia, focal neurologic deficits, a murmur of aortic regurgitation, bruits, and findings compatible with possible cardiac tamponade.” 4
1.3.2. Imaging
Ultrasound (US) is recommended as the first line imaging tool for diagnosis and surveillance of small AAAs.2 It is inexpensive and a reliable technique with a high sensitivity and specificity.4 Ultrasound imaging is limited by patients habitus, excessive bowel gas, variation of diameters of the aorta with the cardiac cycle, methodological differences in measurement and the difficulty to visualize the suprarenal aorta.The antero-posterior diameter in a plane oblique to the center line of the aorta with a consistent caliper placement should be considered the preferred method.2
Computed Tomography Angiography (CTA) provides excellent imaging of AAAs and has great reproducibility and allows diameter measurements.1 CTA is recommended for therapeutic decision making, treatment planning, and for the diagnosis of rupture.2 It has limitations because of the use of nephrotoxic agents and exposure to radiation, particularly in serial imaging.1,2 CTA allows accurate anatomic measurements and is invaluable for EVAR. It visualizes angulation, tortuosity, calcification and thrombus which is useful for preoperative planning and evaluation of possible clamping and landing sites. 5
There is little data concerning the use of magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) for routine AAA management in clinical practice.2
On chest x-ray a widened mediastinum, enlargement of the aortic knob, tracheal deviation, or left main stem bronchus displacement may be suggestive for a TAAA. Primary method to visualize the thoracoabdominal aorta for determining aneurysm diameter and extent is cross-sectional imaging, mainly by CTA.1
1.4. Screening and surveillance
Screening and surveillance guidelines have been produced by various professional organizations. There is evidence that performing a single ultrasound scan in male patients over 65 years old, significantly reduces the mortality from AAA. Population screening in women is not recommended.2,6 Surveillance should be performed with ultrasound. It is recommended to perform this every 3 years for aneurysms with a diameter between 3.0 and 3.9 cm, annually for aneurysms with a diameter between 4.0 and 4.9 cm and every 3-6 month for aneurysms larger than 5.0 cm.7
1.5. Medical management
Medical management focusses on strategies to reduce aneurysm growth rate and cardiovascular risk. The only act reducing growth rate is smoking cessation, no other actions and no specific medical therapy has been proven to slow the AAA expansion rate. 1,2 Patients with small AAAs have an increased cardiovascular risk, with an annual risk of cardiovascular death of 3,0%.8 Healthy lifestyle strategies including smoking cessation, exercise, and diet are recommended for any patient with cardiovascular disease. The European guidelines on cardiovascular disease prevention recommend anti-platelet therapy and statins for all patients symptomatic peripheral arterial disease, antihypertensive therapy for patients with a systolic blood pressure higher than 140 mmHg, and low density lipoprotein cholesterol should be reduced to less than 70 mg/dL.9 1.6. Indication for intervention
The decision about the size at which an aneurysm should be repaired is a balance between operative risk and risk of rupture. In men with degenerative fusiform abdominal aortic aneurysms it is recommended to consider elective abdominal aortic aneurysm repair when the diameter is ≥ 5.5 cm. 10 In women there is an increased risk of rupture and there is no good evidence on the diameter threshold, however it is recommended to consider aneurysm repair when the diameter is ≥ 5 cm. In rapid growing aneurysms (i.e. ≥ 1cm/year) rapid referral to a vascular surgeon is advised and new imaging can be considered. Unruptured symptomatic aneurysms should be urgently referred to a vascular surgeon and scheduled for semi-elective repair. 1,2
In juxtarenal AAA the same cutoff diameter as for AAA are used. However, because of the lack of evidence, a patient tailored approach is appropriate as the surgical risk may be higher in this subgroup. This results in a minimum threshold of 5.5 cm for elective juxtarenal AAA
repair in patients with acceptable surgical risk, whereas a larger threshold may be used in patients with a higher surgical risk.2
For thoracoabdominal aneurysms size criteria are not as clearly defined as for infrarenal AAA. Guidelines advise to consider aortic repair in patients with low to moderate surgical risk and a TAAA with a diameter larger than 6,0 cm. In patients with connective tissue disorder the threshold should be lower. 11 Other authors suggest that the size for repair, independent of etiology, might be a diameter between 5,2 and 5,6 cm. 12
2. Open surgical repair
Since the introduction of the endovascular aortic repair (EVAR) there has been an important transformation in the management of infrarenal AAA repair. Open surgical repair (OSR) seems to be reserved for patients who cannot undergo EVAR and patients with a long life expectancy. Nonetheless, elective open abdominal aortic aneurysm repair has excellent outcomes, with perioperative mortality rates between 1% and 7%. 1
Main indication for open repair are anatomic constraints that make endovascular repair impossible. The leading indication for open repair is the absence of an aortic neck or a hostile aortic neck. Aortic necks that are short, angled, trapezoidal, with a lot of thrombus or heavily calcified have the risk to compromise the proximal sealing zone. This makes that patients with no or hostile necks are better served with an open repair. There are also distal anatomic constraints such as chronic iliac occlusion and iliac aneurysms, however these contra-indications are relative. Other contra-indications for open repair are conversion because of failed EVAR, infected aneurysms, infected endografts or patients in whom preservation of the inferior mesenteric artery (IMA) is indicated.1
There are two possible approaches for an open surgical repair, transperitoneal and retroperitoneal. In the transperitoneal approach a midline incision is used, whereas a transverse flank incision is used for a retroperitoneal approach. The retroperitoneal approach allows good access to the juxtarenal, suprarenal and celiac portion of the aorta. This approach may be associated with fewer post-operative ileus, pneumonia, and incisional hernias than the transperitoneal approach. However a Cochrane review found no clinically important difference between both approaches13, which leaves the choice of approach to the surgeon.2
The proximal anastomosis should be performed as close as possible to the renal arteries. When the risk of colonic ischemia is high, reimplantation of the IMA should be considered. To
prevent buttock claudication and colonic ischemia at least one internal iliac artery should be preserved. Prophylactic use of mesh reinforcement can be considered in the transperitoneal approach. 2
Juxtarenal abdominal aortic aneurysms
Traditionally juxtarenal AAAs are treated by open surgical repair that can be performed by transperitoneal or retroperitoneal approach. Because juxtarenal AAA repair necessitates suprarenal clamping the mortality and morbidity is higher than in open surgical repair of infrarenal AAA. A recent systematic review shows a 30 day or in hospital mortality of 4,1%, 13,9% of the patients had post-operative renal dysfunction and permanent dialysis was necessary in 2,8% of patients. Long term survival was 89%, 80%, and 73% at respectively 1, 3 and 5 years after surgery.14
Thoracoabdominal aortic aneurysms
The open surgical repair of thoracoabdominal aortic aneurysms remains a major challenge and is associated with post-operative complications including myocardial infarction, respiratory failure, renal insufficiency, stroke, paraplegia, and death. Open repair requires proximal aortic clamping resulting in exclusion of organs from perfusion. Extracorporeal circulation with distal aortic perfusion should be considered to reduce ischemic complications, especially in type I, II and III TAAA. To protect the spinal cord cerebrospinal fluid drainage, moderate hypothermia, optimization of arterial pressure, neuromonitoring and reimplantation of intercostal arteries should be considered.11 Mortality rates of open repair in experienced centers ranges from 5 to 15%, and is almost double outside these highly specialized centers. Major complications arise frequently. Respiratory failure occurs in up to 60% of the cases, neurological deficits in 3– 18%, and renal failure in 3–15%.15,16
3. Endovascular treatment
3.1. Endovascular aneurysm repair - EVAR
In endovascular repair the aneurysm is sealed from the inside, while the aneurysm sac is left untouched. This is called exclusion of the aneurysm. Endografts should provide sufficient sealing and fixation. Most devices rely on a degree of oversizing (10-25%) to guarantee this. Other features are specific for individual types of device.2
Figure 2. A: Zenith Flex AAA Main Body with Spiral-Z legs in Anatomy. B: Zenith Flex AAA Endovascular
Graft Main Body17
Modern version stent-grafts are bifurcated grafts, adopting a modular design with two or three components, allowing flexibility in planning.
Anatomical requirements differ between stent-grafts and include: neck length (>10-15 mm), neck diameter (19-32), suprarenal neck angulation (α-angle < 45°), infrarenal neck angulation (β-angle < 60°), distal fixation site length (>10-15mm) and distal fixation site diameter (8-25mm). Other additional criteria are: no significant calcification or thrombus in proximal and distal landing zones, no conical neck shape (<2–3 mm increase in neck diameter for each cm of length) and adequate access. 2
There are several RCT’s comparing open and endovascular treatment of infrarenal AAA in patients with suitable anatomy (EVAR 1 Trial18, DREAM19, OVER20 and ACE21 trials). They have shown a significant early survival benefit for EVAR. However, the benefit is lost during mid-term follow up. Additionally, there were more reinterventions in the EVAR group. 22 On long term follow-up there was a lower aneurysm-related mortality in the EVAR group, but overall mortality did not differ. 23,24 Based on these the European Society of Vascular Surgery guidelines state that “In most patients with suitable anatomy and reasonable life expectancy,
EVAR should be considered as the preferred treatment modality; in patients with long life expectancy, open repair should be considered as the preferred treatment modality; and in patients with limited life expectancy, elective aneurysm repair is not recommended.”2
3.2. Complications after endovascular aneurysm repair
Endoleak
An endoleak is defined as the persistent blood flow in the space between the endograft and the aneurysm wall. Primary endoleaks are endoleaks present at the time of initial repair and secondary endoleaks are endoleaks occurring after prior negative imaging. Endoleaks are categorized in five types differing from each other in etiology and treatment.
Figure 3. Endoleak classification: Type I to IV1,39
Endoleak type I is defined as blood entering the aneurysm sac from around the graft at the level of the proximal sealing zone (IA) or the distal sealing zone (IB). Type II is defined as aneurysm sac filling due to backbleeding from side branches. Type III endoleak is the result of graft erosion or leak between two graft components. Type IV endoleak is defined as blood leakage related to porosity of the fabric, noted within the first 30 days after EVAR procedure. And type V endoleak, also called ‘endotension’, means continued sac enlargement without visualization of an endoleak.1,2,25 In type I and type III endoleak reintervention is recommended, whereas is type II endoleak intervention is only recommended in the presence of significant aneurysm growth.Type IV endoleak is rarely seen with the new type of endografts. When aneurysm sac growth is seen without visualization of an endoleak further imaging for identifying an underlying endoleak is recommended. 2
Migration and neck dilatation
Stent-graft migration refers to the caudal displacement of the graft, which can cause a type IA endoleak. Neck anatomy is important for a good sealing. Stent migration can occur with important neck angulation, short necks, large necks and necks with important thrombus load. 26 The juxtarenal portion of the aorta can dilate after EVAR, resulting in stent migration and a type IA endoleak, with the risk of rupture. 27
Graft limb occlusion and renal artery occlusion
Kinking and occlusion of a limb after an EVAR procedure occurs in 3-7% of the cases, most frequently within the first 6 months. Risk factors are aorto-iliac occlusive disease, small distal aorta and tortuous iliac arteries. 28 Renal artery occlusion can occur after its origin has been covered by the endograft or by embolization.
Stent-graft infection, aortic rupture and pelvic ischemia
Stent-graft infection is a rather rare complication occurring in 0,2-0,7% of the cases. Development of an aorto-enteric fistula has been described. 29 Aortic rupture after EVAR occurs in 1-5% of the cases. Colorectal ischemia, erectile dysfunction and buttock claudication are different forms of pelvic ischemia, which can develop after unilateral or bilateral internal iliac occlusion.30
4. Complex endovascular aneurysm repair
‘Classic’ EVAR has its limitations due to anatomical constraints such as suboptimal necks, angulation and involvement of side branches in the aneurysm. To overcome these limitations technical development has focused on incorporating aortic branches in stent grafts. Fenestrations, branches and parallel stent grafts are possible techniques.
Figure 4. Fenestrated repair (a), fenestrated branched repair (b) and directional branched repair. By
permission of G. Oderich. All rights reserved 31
Figure 5. (A) scallop, (B) fenestration, (C) reinforced fenestration, (D) stented fenestration, (E) fenestrated
branch, and (F) side arm branch.1
In fenestrated and branched endovascular aortic aneurysm (F/BEVAR) repair customized stent-grafts are used to perform a total endovascular aortic repair of aneurysms maintaining perfusion to side branches of the aorta. The goal of this technique is to reduce morbidity and mortality associated with complex open surgical repair. F/BEVAR has been used to treat juxtarenal, suprarenal, thoracoabdominal and aortic arch aneurysms. In the early experience, first reported in 1999, short-neck infrarenal AAA were treated with stent-grafts with two fenestrations for perfusion of the renal arteries. Initially uncovered stents were used to target
arteries, however to achieve adequate seal these stents were quickly changed for covered stents. In treatment of aneurysms with a large aortic diameter at the level of its side branches, the use of directional branches can be more optimal compared to fenestrations. Fenestrations and branches are bridged with covered stents to the target arteries.
Devices for complex endovascular repair can be physician-modified endografts (PMEGs), custom-made patient-specific devices (CMDs), or off-the-shelf endografts. In PMEG the surgeon creates modifications to the graft based on patients anatomy, this implicates a detailed knowledge of the graft and leaves the surgeon with an important responsibility. Custom-made devices with fenestrations and or branches are the type of devices which are most frequently used. Sometimes preloaded wires/catheters are used to facilitate the procedure. The disadvantage of custom made devices is the manufacturing time. Off-the shelf devices do not have this disadvantage, unfortunately these devices are not able to accommodate extreme anatomy.1,31
4.1. Planning and sizing
Precise planning is crucial to perform a successful complex endovascular repair. Planning includes accurate measuring of patients anatomy, the choice of a device (diameter, length, tapering,…), the choice of an artery preservation strategy (scallop, branch or fenestration) and the choice of an access strategy (femoral, axillary, conduit,…). A detailed CTA with 3D reconstruction and adequate post-processing software is necessary to make these decisions.
4.2. Proximal sealing zone
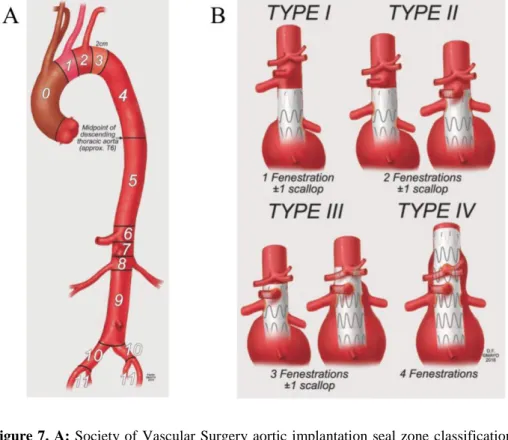
When planning a F/BEVAR procedure choosing the proximal sealing zone is one the most important decisions to make. In ‘classic EVAR’ a seal zone of 10 to 15 mm may be enough, however in F/BEVAR a healthy seal zone of 20 to 40 mm, without important thrombus or calcifications, is advised. The SVS classification system describes the different sealing zones of the aorta. Zone 0-4 are the zones of the aortic arch. Zone 4 starts 2 cm distally from the left subclavian artery and ends at the midpoint of the descending aorta (T6). Zone 5 extends form midpoint descending aorta up to the proximal edge of the celiac artery, zone 6 from the proximal edge of the celiac artery (CA) to the proximal edge of the superior mesenteric artery (SMA), zone 7 from the proximal edge of the SMA to the proximal edge of the highest renal artery and zone 8 from the proximal edge of the highest renal artery to the distal edge of the lowest renal artery. Zone 9 involves the infrarenal aorta to the aortic bifurcation, zone 10 extends to the common iliac artery bifurcation, and zone 11 extends to the external iliac artery.
To classify the types of endovascular repair, a classification system based on the minimal number of fenestrations or branches has been proposed by the Mayo Clinic (figure 6). 31,32
Figure 7. A: Society of Vascular Surgery aortic implantation seal zone classification system. B: Mayo Clinic
endovascular classification system of endovascular repair of complex abdominal aortic aneurysms. By permission of G. Oderich. All rights reserved. 31
Figure 8. Mayo Clinic supra coeliac sealing zones for fenestrated and branched endografts. By permission of G.
4.3. Aortic angulation, mural thrombus and target vessels characteristics
To achieve a predictable deployment of the endograft and avoiding a misalignment with the target arteries, the angulation between the distal thoracic aorta and the visceral aorta, the suprarenal and infrarenal aorta and the infrarenal aorta and the iliac arteries should be less than 45°. The amount of thrombus and degree of calcification should be carefully evaluated preoperatively. Due to the need to position stiff wires across the aortic arch up to the ascending aorta, and the potential need to rotate the fenestrated or branched endograft during the procedure there is an important risk of embolization. Target vessel characteristics influence the technical success rate of the procedure. Highly stenotic or tortuous segments in the proximal part of the target vessels are more difficult to cannulate, resulting in an increased risk of loss of perfusion of the target vessel and an increased risk for an endoleak when cannulation of the target vessel is not possible.1
4.4. Access
Iliac arteries should at least have a diameter of 7 mm. The custom made devices are delivered through a 18-26 French delivery system, and a 20-24 French sheath is necessary at the contralateral side to introduce multiple 6-7 French sheaths to extend the fenestrated graft into the target vessels. When access from above is needed, a 7-12 French sheath is introduced in the axillary artery, and when multiple sheaths should be introduced an 8-10 mm conduit can be sewn on the axillary artery.The risk for lower extremity ischemia due to a long procedure with large sheaths in the femoral vessels should be evaluated and if necessary lower extremity perfusion techniques should be considered.1
4.5. Spinal perfusion
There are three major arteries supplying blood to the spinal cord: the anterior spinal artery and two posterior spinal arteries. Blood supply to the spinal arteries arises from the aorta, the intercostal arteries and the lumbar arteries. Traditionally it was thought that there was one dominant artery feeding the spinal cord, the artery of Adamkiewicz, however this theory seems to have been omitted.33 Spinal cord ischemia (SCI) resulting in paraplegia is a devastating complication after thoracoabdominal aortic aneurysm repair. The risk of SCI resulting in paraplegia is 1-5% after complex endovascular repair of abdominal aortic aneurysms. The risk of SCI is associated with the extent of aortic coverage, the need to cover the aorta up to 4 cm above the celiac artery and preoperative impaired renal function.34 In the prevention of spinal
cord ischemia a staged repair (type I and II TAAA), preoperative cerebrospinal fluid drainage, avoidance of hypotension and avoidance of pelvic and limb ischemia are recommended.35
4.6. Outcomes and complications after complex endovascular repair
There is no level I evidence regarding the results of F/BEVAR and there are no prospective trials comparing complex endovascular aortic aneurysm repair with open surgical repair. Multiple centers have reported their results in observational cohort studies and there are limited meta-analyses.
Juxtarenal aortic aneurysms
High technical success rate, low morbidity and low mortality have been reported for complex endovascular repair of juxtarenal and pararenal abdominal aortic aneurysm repair. In a recent meta-analysis analyzing results of complex endovascular repair of juxtarenal aortic aneurysms, there was a perioperative mortality of 3,3%, a postoperative renal insufficiency rate of 16,2% and a postoperative permanent need for dialysis in 0,8%. There was a postoperative reintervention rate of 11,2%. Main indications for reintervention after F/BEVAR are an endoleak or a vessel occlusion/stenosis. Incidence of type I/III endoleak was 4,9%, and target vessel occlusion rate was 0-3,9%. 36 Data concerning long term survival after F/BEVAR is even more limited. In an earlier meta-analysis the long term survival after F/BEVAR was 93%, 74%, and 55% at respectively 1, 3 and 5 years.14
Thoracoabdominal aortic aneurysms
Perioperative mortality data shows widely distributed results with a range of 0% to 25%. A pooled perioperative mortality of 3,7% was calculated based on 11 retrospective studies.1 Survival also depends on type of TAAA. In 2016 Eagleton et al. reported repair on type II and type III TAAA. Perioperative mortality was 7% for type II and and 3.5% for type III TAAA repair. Permanent spinal cord ischemia occurred in 4% of patients and renal failure requiring hemodialysis was reported 2.8% of patients.37 In a prospective study on 127 patients, performed by the group of Oderich et al. there was no perioperative mortality, no dialysis, nor conversions and 1 year survival was 96%.38
5. Scope of the study
In 2012 complex endovascular repair has been introduced for the treatment of juxtarenal and thoracoabdominal aneurysms in Ghent University Hospital. The goal of this study is to evaluate the results with this technique in our center. The hypothesis of this study is that the perioperative mortality after complex endovascular repair performed in our center is similar to that found in literature. All consecutive complex endovascular aortic aneurysm repairs performed for juxtarenal and thoracoabdominal aneurysms will be analyzed in this study.
Methods
Study design and setting
This is a single center retrospective cohort study. The first one hundred consecutive patients that underwent a complex endovascular abdominal aortic aneurysm repair between June 2012 and December 2019 at Ghent University Hospital are included. All patient data, information on the aneurysm morphology, perioperative data and device characteristics were collected from the electronic patient file in the hospital information system. Follow-up was performed one month, six months and one year after surgery. After one year there a yearly follow-up was performed.
Participants
Every patient undergoing a complex endovascular repair with a fenestrated or branched endograft.
Variables
Patient characteristics that were collected include: age, gender, cardiac history, low left ventricular function (less than 40%), arrhythmia (atrial fibrillation), arterial hypertension with need for medication, diabetes mellitus (insulin dependent or non-insulin dependent), history of stroke, preoperative kidney function (serum creatinine and glomerular filtration rate), preoperative need for dialysis, preoperative peripheral arterial disease, preoperative open or endovascular aortic surgery.
Classification of aneurysms based on anatomy: definition based on the radiologists findings and controlled by a single supervising surgeon. A difference is made between juxtarenal, pararenal and suprarenal aneurysms. A juxtarenal AAA is an aneurysm reaching up to but not involving the renal arteries, a pararenal AAA is reaching up to and involving the renal arteries,
and a suprarenal AAA is an aneurysm involving the renal arteries and extending up to the inferior border of the superior mesenteric artery (SMA). Thoracoabdominal aortic aneurysms are defined by the Crawford classification. A type II TAAA starts distal from the left subclavian artery and runs to below the renal arteries; a type III from the sixth intercostal space to the renal arteries. and a type IV embodies the entire abdominal aorta.3The classification is based on the radiologists findings and controlled by a single supervising surgeon.
Device characteristics
Cook® custom made endografts have been used in all cases. Devices were designed with 1 up to 4 fenestrations and/or branches, with or without scallop. Device and procedural details that were collected include: operation time, type of anesthesia, cerebrospinal fluid drainage, number of target vessels, size and type of stent used for the target vessels. Fluoroscopy time, blood loss and device implantation time were not retrievable.
Outcome
Primary outcome is survival. Early survival is defined as survival during the first 30 days after the procedure, short term as survival after 1 year and mid-term as survival after 3 years. Cause of death is noted. Results are retrospectively retrieved from the medical file.
Secondary outcomes are technical success, target vessel patency, major adverse events, renal function impairment, dialysis, endoleak and need for a secondary intervention. These results are obtained from the medical file and the laboratory results.
Primary technical success is defined as successful arterial access using a remote site (femoral, iliac, or brachiocephalic arteries), successful deployment of the graft with secure fixation, aneurysm exclusion without endoleak type I or III and patent endograft without significant twist, kinking or obstruction. When an additional procedure was necessary the term secondary technical success is used. 39 For this purpose we used the final angiogram and not the imaging performed at 30 days postoperatively.
Target vessel patency is defined as a non-occluded target vessel. In case of occlusion, the timing, the target vessel and the need for reintervention is noted.
Renal function is analyzed. Renal morbidity is defined as a diminishment of the glomerular filtration rate (GFR) of at least 30%. Need for dialysis is noted.
Endoleak type I is defined as blood entering the aneurysm sac from the proximal sealing zone (IA) or the distal sealing zone (IB). Type II is defined as aneurysm sac filling due to backbleeding from side branches. Type III endoleak is the result from graft erosion or leak between two graft components. Type IV endoleak is defined as blood leakage related to porosity of the fabric, and is noted within the first 30 days after EVAR procedure. And type V endoleak, also called ‘endotension’, means continued sac enlargement without visualization of an endoleak40 Time of diagnosis, need for secondary procedure and description of secondary procedure is noted.
A secondary intervention was defined as an intervention performed in the early or late postoperative period, being directly related to the complex endovascular repair (i.e. intervention for endoleak, intervention for target vessel occlusion, intervention for limb occlusion). Other vascular interventions, not directly related to the graft are not counted in this group.
Ethical approval
Ethical approval was obtained for conducting this study (BC-1264-LBG).
Bias
Inclusion bias was excluded by including all consecutive patients who have undergone a complex endovascular aortic aneurysm repair in the study period. Variables such as type of aneurysm and outcomes such as endoleak and target vessel patency are at risk for information bias and measurement bias by misclassification and by inaccurate imaging interpretation respectively. This bias is partially prevented by having a single supervising surgeon.
Statistical methods
Data was managed and analyzed by the investigators with Apple Numbers® and with IBM SPSS Statistics 26®. To analyze categorical variables the Pearson Chi2-test was used. Differences between continuous variables were tested using 2-sided Student t-test or One-way ANOVA when comparing more than two groups. The Kaplan-Meier survival analysis was performed to analyze mortality, freedom from major adverse events and freedom from secondary interventions. The Log rank test was used to evaluate any difference in survival between subgroups. Statistical significance vas defined as a p-value of < 0,05.
Results
1. Patient and aneurysm characteristics
The first 100 patients treated with a fenestrated or branched endograft in our center were included in this study. No patients were excluded for this study. The number of procedures performed each year are shown in figure 1. There were 94 male patients and six female patients with a mean age of 73 ± 7 years. Seventy-nine patients had a history of smoking, 47 patients had a history of coronary artery disease, and 44 had chronic kidney disease stage 3, however no patients had preoperative need for dialysis. Thirty-one patients had previous aortic surgery, of which 13 were endovascular repairs, 10 were open repairs and eight patients had a previous endovascular and open repair. Forty-seven patients were treated for a juxtarenal AAA, 12 for a pararenal AAA, 10 for a suprarenal AAA, 13 for a type II TAAA, 10 for a type III TAAA and 13 for a type IV TAAA. The mean aneurysm diameter was 61,6 ± 8,1mm (range 29 - 86mm). Patient characteristics and anatomical details of the aneurysms are listed in Table 1.
Figure 1. Complex Endovascular Aortic Aneurysm Repair
Table 1. Patient characteristics and anatomical aneurysm details
Factors No. or mean ± SD Percentage or range
Demographics Age, years 73,3 ± 6,9 49-85 Male 94 94 BMI (missing 25) 26,5 ± 4,8 18-38 History of smoking 79 79 Active smoker 45 45 Former smoker 34 34 Arterial hypertension 64 64 4 3 7 12 18 17 19 20 0 5 10 15 20 25 2012 2013 2014 2015 2016 2017 2018 2019
Factors No. or mean ± SD Percentage or range
Diabetes mellitus type 2 17 17
History of heart disease 47 47
PCI 24 24
CABG 16 16
CAD 7 7
Heart failure or recent myocardial infarction 8 8
Atrial fibriliation 24 24
Chronic obstructive pulmonary disease 26 26
History of stroke 10 10
Chronic kidney disease stage 3a 44 44
Preoperative need for dialysis 0 0
Peripheral arterial disease 16 16
History of aortic surgery 31 31
Endovascular repair 13 13
Open repair 10 10
Both 8 8
Previous abdominal surgery 38 38
Laparoscopic 14 14
Open surgery 24 24
ASA physical status classification
ASA 1 0 0
ASA 2 16 17,7
ASA 3 70 77,7
ASA 4 4 4,4
Anatomical aneurysm classification
Juxtarenal AAA 47 47 Pararenal AAA 12 12 Suprarenal AAA 10 10 Type 2 TAAA 13 13 Type 3 TAAA 5 5 Type 4 TAAA 13 13
Maximum aneurysm diameter, mm 61,6 ± 8,1 29 - 86
ASA = American Society of Anesthesiologists Physical Status, CABG = coronary artery bypass grafting, CAD = coronary artery disease, PCI = percutaneous coronary intervention
2. Stent graft design and device implantation
Complex endovascular repair was performed under general anesthesia in all patients, with a mean operating time of 267 ± 94,7 min (range 110 - 540 min). Cerebrospinal fluid drainage was performed in 17 patients. A fenestrated endograft was used in 90 patients, a branched endograft in five patients and a graft with a combination of fenestrations and branches was used in five patients. A type I endograft was used in four patients, a type II in six patients, a type III in 26 patients and a type IV endograft was used in 64 patients. A total of 369 visceral arteries were incorporated in the graft design. Large fenestrations were used for 63 celiac arteries (CA) and 85 superior mesenteric arteries (SMA), and small fenestration for 179 renal arteries (RA). Branches were used for nine CAs, five SMAs and nine RAs, and scallops were used for sixteen CAs and for three SMAs. Stent graft design, type of fenestrations/branches and type of stents are summarized in table 2.
Table 2. Stent graft design, type of fenestration/branch and type of stents
No. and % Type of endograft
Fenestrations 90
Branches 5
Combination 5
Mayo Clinic endovascular classification system 32
Type I 4
Type II 6
Type III 26
Type IV 64
Design of proximal stent graft
1 fenestration 4
2 fenestrations 5
2 fenestrations and 1 scallop 1
3 fenestrations 16
3 branches 2
3 fenestrations and 1 scallop 8
4 fenestrations 53
4 branches 3
2 fenestrations and 2 branches 1
3 fenestrations and 1 branch 4
No. % Type of fenestration/branch used for target vessel
Celiac artery
Large fenestration 63 17,1
Branch 9 2,4
Scallop 16 4,3
Superior mesenteric artery
Large fenestration 85 23,0 Branch 5 1,4 Scallop 3 0,8 Renal artery Small fenestration 179 48,5 Branch 9 2,4
Total targeted vessel 369 100
Allignment stent
Advanta V12 (Getinge) 214 61,0
Lifestream® stent (BARD) 50 14,2
BeGraft stent (Bentley) 77 22
Fluency™ stent graft (BARD) 7 1,8
Viabahn® endoprsthesis (GORE®) 3 0,9
Total number of stents 351 100
The intention was to align 350 target vessels with a bridging stent. In 346 vessels the bridging was successful (98,8%). Four vessels, targeted by a fenestration, could not be stented. One renal artery could not be targeted due to a too small lumen, one renal artery could not be targeted due to the intimal flap in an aortic dissection patient prohibiting cannulation and two fenestrations designed for the CA could not be cannulated and were left open but unstented. In five vessels a supplementary stent was needed to bridge the target vessel with the endograft, two times in a directional branch and three times in a small fenestration. This results in a total of 351 balloon expandable stents that were used (table 2).
Technical success. Primary technical success was achieved in 87 patients and secondary technical success in 89 patients. Laparotomy was necessary in two patients; in one patient to remove an incorrectly positioned bridging stent in the SMA and in one other patient to control a bleeding from a side branch of the SMA. One iliac limb kinking was treated with stent
placement and in one iliac limb an additional stent graft extension was necessary to obtain distal sealing.
In four patients the implantation of the fenestrated graft was combined with an iliac branched device and in nine patients with a thoracic stent graft. In one patient an aorto-uni-iliac endograft was used in combination with a femoro-femoral crossover bypass and in two patients a conduit was necessary to introduce the stent graft. A unilateral common femoral artery reconstruction was performed in eleven patients, a bilateral common femoral artery reconstruction in four patients, and a subclavian artery reconstruction in two patients. Six target vessel events were noted (1,7%). Out of the 72 targeted CAs one event was registered (1,3%), out of the 90 targeted SMAs two events were registered (2,2%) and out of the 188 targeted renal arteries three events were registered (1,6%). An overview of the intraoperative target vessel events is shown in Table 3.
Table 3. Intraoperative target vessel event
Target vessel Event Fensestration Reintervention Result
CA Occlusion Directional branch No Discharge POD 6,
Death on POD 15
SMA Wrongly placed stent Large fenestration Conversion and stent
removal by arteriotomy No implication
SMA Bleeding Large fenestration Conversion and control of
bleeding No implication
RA Dissection Small fenestration Additional stent
placement
GFR drop > 15 ml/min
RA Unable to catheterize Small fenestration Amplatzer plug
RA occlusion Permanent dialysis,
death on POD 17
RA Occlusion Small fenestration Dilatation GFR drop > 30
ml/min CA = celiac artery, SMA = superior mesenteric artery, RRA = right renal artery, LRA = left renal artery, GFR = glomerular filtration rate, POD = postoperative day
3. Early morbidity and mortality (< 30 days)
Median length of hospital stay was 7 days (IQR = 5 - 10), and median length of stay at the intensive care unit was 1 day (IQR = 1 - 3). Overall early postoperative survival was 94%. There were five early in hospital deaths and one early out of hospital death (Additional Table1). The first early death was caused by a myocardial infarction. The second early death was due to multiple organ failure caused by embolization, in a patient treated with a stent graft with three fenestrations for a type IV TAAA. A scallop was used to target the celiac artery, because of a chronic high grade stenosis. Postoperative imaging showed liver, bowel and kidney
ischemia, however no stent or vessel occlusion was observed. A laparotomy with bowel resection was performed and dialysis was started, but therapy was stopped due to further deterioration. The third early death was on postoperative day 17 from bowel ischemia, in a patient treated with a stent graft with three fenestrations for a type IV TAAA. An infrarenal AAA was treated earlier with a straight tube graft. Patient had a pre-operative GFR of 15ml/min and the right renal artery was intentionally not incorporated in the graft because of chronic occlusion. Postoperative course was complicated with an acute coronary syndrome, need for dialysis and bowel ischemia. However, after several laparotomies, therapy was stopped because of too extensive small bowel ischemia. No stent occlusion was observed on imaging. The fourth early death was on postoperative day 16 from an acute myocardial infarction with cardiogenic shock. The patient was treated with a four fenestrations stent graft combined with a thoracic stent graft extension for a type IV TAAA. The fifth in hospital early death was on postoperative day six due to multiple organ failure after coronary artery bypass grafting (CABG) because of acute myocardial infarction on postoperative day two. Imaging on postoperative day one did not show any target vessel occlusion. There was one out of hospital death on postoperative day 15, the cause of death is unknown. The patient was treated with a stent graft with 3 directional branches. The procedure was complicated with an occlusion of the branch targeting the celiac artery and was closed with a plug. Nonetheless, initial postoperative course was uneventful and patient was discharged on postoperative day six.
Table 4. Early postoperative death
Day Cause of death Type of aneurysm Type of graft Additional Complication
2 Cardiac arrest Crawford type 4 TAAA 4 fenestrations No
3 Embolization, Multiple
Organ Failure Crawford type 4 TAAA 3 fenestrations Stroke
6 Multiple Organ Failure
after CABG Juxtarenal AAA 3 fenestrations No
15 Unknown Crawford type 2 TAAA 4 fenestrations + TEVAR CA occlusion
16
Acute coronary syndrome and cariogenic shock
Crawford type 4 TAAA 4 fenestrations Bowel ischemia
17 Embolization, Bowel
ischemia Crawford type 4 TAAA 3 branches No
Cardiac complications were observed in fifteen patients. In three patients this was a major adverse event resulting in death. A cardiac complication with severe hemodynamic dysfunction occurred in two patients who eventually died from a different cause. Nine other patients had a
minor cardiac complication, not influencing postoperative course. Digestive complications occurred in nine patients. Bowel ischemia was observed in four patients and it was the cause of death in two of them. In one patient bowel ischemia was treated with antibiotics and did not necessitate surgery. Two patients developed an acute cholecystitis necessitating cholecystectomy and two patients developed a postoperative gastro-intestinal bleeding necessitating blood transfusion. Neurological complications were observed in eight patients. Three patients had a stroke, of which two patients died in the early postoperative period due to another cause. Four patients developed spinal cord ischemia. In one patient a resolution was seen within 24 hours, however in the three other patients partial resolution was seen but major deficit remained. Respiratory complications occurred in thirteen patients. Two patients developed respiratory insufficiency necessitating ventilatory support, acute respiratory distress syndrome was seen in one patient and seven patients suffered from pneumonia. One postoperative COPD exacerbation was seen. This patient was dismissed on postoperative day six and died from an unknown cause on postoperative day fifteen. Vascular complications were seen in six patients. One embolectomy and stent placement in the external iliac artery in combination with femoral artery reconstruction was performed. In two patients a drainage of a hematoma at the access site was performed, and there were three pseudoaneurysms of which two necessitated an intervention.
Table 5. Early (<30d) postoperative complications
Complications No. and %
Cardiac complications
Cardiac arrest 2
Coronary artery bypass grafting 1
ACS treated with PCI 3
Acute decompensated heart failure 3
Atrial fibrillation 6
Total cardiac complications 15
Digestive complications
Bowel resection for ischemia 4
Cholecystectomy 2
Mucosal bowel ischemia, treated with antibiotics 1
Gastro-intestinal bleeding 2
Total digestive complications 9
Complications No. and %
Ischemic cerebrovascular accident 3
Spinal cord ischemia (SCI) - paraplegia / major deficit 3
SCI, spontaneous resolved 1
Total neurologic complications 7
Respiratory complications
Respiratory insufficiency 2
ARDS 1
Pneumonia 7
COPD exacerbation 1
Pleural effusion, no need for drainage 1
Bronchitis 1
Total respiratory complications 13
Renal complications
Postoperative need for dialysis 4
Serum creatinine level rise to > 2 mg/dL and GFR-drop of more
than 30% 4
Kidney bleeding, drainage 2
Kidney bleeding, transfusion, no reintervention 1
Renal artery stenting 2
Renal artery occlusion, open retrograde stenting 1
Total renal complications (patients) 8
Vascular complications
Embolectomy, stenting external iliac artery and common femoral
artery reconstruction 1
Drainage hematoma subclavian incision 2
Resection pseudoaneurysm left groin, Trombin injection
pseudoaneurysm right groin 1
Pseudoaneurysm groin, no reintervention 1
Trashing in both feet, no reintervention 1
Total vascular complication 7
ACS = acute coronary syndrome, ARDS = acute respiratory distress syndrome, COPD = chronic obstructive pulmonary disease, GFR = glomerular filtration rate, PCI = percutaneous coronary intervention, SCI = spinal cord ischemia, TEVAR = thoracic endovascular aortic aneurysm repair
Renal function, renal complications and other target vessel events. Mean postoperative serum creatinine level was 1,21 ± 0,649 mg/dL (range 0,6 - 5 mg/dL). Forty patients had a glomerular filtration rate (GFR) lower than 60 ml/min. Four patients needed dialysis and four other patients showed a drop of more than 30 % compared to their preoperative GFR, resulting
in a serum creatinine level of more than 2 mg/dL (Table 5). Four more patients had renal function decline of more than 15%. Kidney perforation was observed in three patients, leading to the need for a drainage procedure in two and the placement of an ureter stent in one. Renal artery stent placement was performed in two patients and in one patient an open retrograde stent placement was performed because of a crushed stent. A total of 10 intraoperative and early postoperative renal artery events occurred in 188 targeted renal arteries (5,3%) in eight different patients. No other early postoperative target vessel events were recorded.
Table 5. Early postoperative impaired renal function
Preoperative GFR (ml/min)
Postoperative renal
function or GFR (ml/min) Cause Evoltuion
45 Permanent dialysis Embolization Death (MOF)
15 Permanent dialysis Embolization Death (cardiac)
37 Permanent dialysis RA occlusion Death (cardiac)
> 60 Temporary dialysis RA occlusion, crushed stent,
retrograde stent placement
GFR > 60 ml/min, therapy stop POD 148
(SCI)
> 60 25 RA bleeding, drainage performed GFR 3y postoperatively:
35 ml/min
50 28 RA bleeding, ureter stent
placement
GFR 2y postoperatively: 45 ml/min
58 12 RA bleeding, drainage performed GFR 1y postoperatively:
35 ml/min
>60 20 Embolization Death (MOF)
GFR = glomerular filtration rate, MOF = multiple organ failure, RA = renal artery, SCI = spinal cord ischemia,
Postoperative need for dialysis. Four patients needed temporary or permanent dialysis. The
first patient had a preoperative serum creatinine level of 1,49 mg/dL (GFR 45 ml/min), and
was treated with a stent graft with three fenestrations and one scallop for a type IV TAAA. No peroperative complications were noted. Postoperative course was complicated with multiple organ failure due to embolization, necessitating bowel resection and dialysis. The patient died on postoperative day three. The second patient was a patient with a preoperative serum creatinine level of 3,85 mg/dL (GFR 15 ml/min). He already had an open surgical repair of an infrarenal AAA with a straight tube graft. There was a known chronic occlusion of the right renal artery and dialysis was prepared with creation of an AV fistula. Patient was treated with a stent graft with three fenestrations and the right renal artery was intentionally not incorporated in the stent graft. Postoperative course was complicated with an acute coronary syndrome, need for dialysis and bowel ischemia. However, after several laparotomies, therapy was stopped
because of too extensive small bowel ischemia. No stent occlusion was observed on imaging. The third patient had a preoperative serum creatinine level of 1,76 mg/dL (GFR 37 ml/min) and was treated with a thoracic stent graft and a stent graft with 4 fenestrations for a type IV TAAA. Left renal artery could not be catheterized. Postoperative course was complicated with bowel ischemia treated with resection and an acute myocardial infarction on day 13 necessitating percutaneous coronary intervention (PCI), however resulting in death due to cardiogenic shock on postoperative day 17. The fourth patient was a patient with a normal preoperative kidney function, treated for a pararenal AAA with a stent graft with 4 fenestrations. On postoperative day one an occlusion of the left renal artery was diagnosed as a result of a crushed stent and abdominal ischemia was suspected. A laparotomy was performed to exclude abdominal ischemia and the left renal artery occlusion was treated with a dilatation and stent placement via retrograde puncture. This resulted in a complete recuperation of the kidney function. Nonetheless, therapy was stopped on postoperative day 148 after a prolonged stay with respiratory insufficiency and spinal cord ischemia.
4. All-cause mortality and morbidity
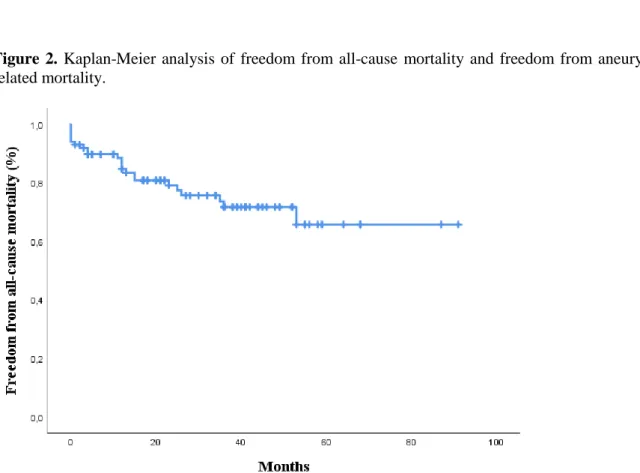
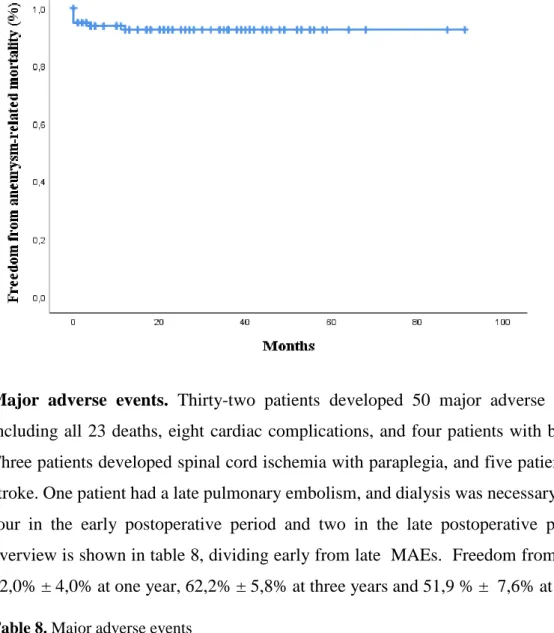
Mean follow-up was 33,6 ± 22,4 months and estimated overall survival was 67,5% ± 4,4%. Freedom from all-cause mortality was 89,3% ± 3,2% at one year, 74,4% ± 5,2% at three years and 66,4% ± 7,6% at five years (Figure 2). Seventeen deaths were observed in the period after the first 30 days, of which two deaths were related to the primary procedure. In one patient therapy was stopped on day 148 after a long hospitalization due to paralysis, general weakness and respiratory insufficiency. In the other patient therapy was stopped on day 384, 22 days after open surgical repair (infrarenal banding) for rupture due to a type Ib endoleak with concomitant infection. Seven deaths were not related to the primary procedure and eight deaths had an unknown cause (Table 7). Freedom from aneurysm-related mortality in the total study population was 95,0% ± 2,2% at 30 days, 93,9% ± 2,4% at one year and 92,5% ± 2,7% at three and five years. Freedom from all-cause mortality and freedom from aneurysm-related mortality shows no statistical difference between patients treated for a juxtarenal AAA and patients treated for TAAA, nor between patients with or without history of aortic surgery, nor between the first 50 treated patients and the last 50 treated patients. However, there seem to be a trend towards a higher mortality in the TAAA group and in the group of patients who have had previous aortic surgery (Additional figure 1).
Table 6. All-cause mortality
Days Cause of death Relation to procedure
2 Cardiac arrest Indirectly related
3 Embolization, Multiple Organ Failure Procedure related
6 Multiple Organ Failure after CABG Indirectly related
15 Unknown Unknown
16 Acute coronary syndrome and cariogenic shock Indirectly related
17 Embolization, Bowel ischemia Procedure related
119 Stroke Unrelated
148 Therapy withdrawal after spinal cord ischemia with general
weakness and respiratory insufficiency Procedure related
149 Unknown Unknown
359 Unknown Unknown
365 Pneumonia Unrelated
367 Unknown Unknown
384 Therapy withdrawal after open surgical repair for rupture Procedure related
400 Prostate cancer Unrelated
461 Unknown Unknown
476 Intracranial bleeding Unrelated
722 Unknown Unknown
775 Lungcancer Unrelated
822 Oncologic, primary unknown Unrelated
1073 Dementia, general weakness Unrelated
1097 Unknown Unknown
1641 Unknown Unknown
Unknown Unknown Unknown
Table 7. Freedom from all-cause mortality and freedom from aneurysm-related mortality
Estimated survival (%) ± standard deviation (%)
30 days 1 year 3 years 5 years Log Rank
Freedom from all-cause mortality
Total study population 93,9 ± 2,4 89,3 ± 3,2 74,4 ± 5,2 66,4 ± 7,6
Juxtarenal AAA 98,3 ± 1,7 90,9 ± 3,9 79,9 ± 6,2 76,7 ± 6,8
p = 0,079
Estimated survival (%) ± standard deviation (%)
30 days 1 year 3 years 5 years Log Rank No history of aortic surgery 97,1 ± 2,0 90,9 ± 3,6 78,2 ± 5,7 75,7 ± 6,1
p = 0,051
History of aortic surgery 86,7 ± 6,2 82,3 ± 7,3 65,0 ± 9,6 48,8 ± 15,8
First 50 cases 94,0 ± 3,4 90,0 ± 4,2 77,9 ± 5,9 69,5 ± 8,2
p = 0,231
Last 50 cases 93,9 ± 3,4 86,0 ± 5,5 62,9 ± 1,3 52,2 ± 13,5
Freedom from aneurysm-related mortality
Total study population 95,0 ± 2,2 93,9 ± 2,4 92,5 ± 2,7 92,5 ± 2,7
Juxtarenal AAA 98,3 ± 1,7 96,3 ± 2,5 96,3 ± 2,5 96,3 ± 2,5
p = 0,094
TAAA 90,2 ± 4,6 90,2 ± 4,6 87,0 ± 5,5 87,0 ± 5,5
No history of aortic surgery 97,1 ± 2,0 95,4 ± 2,6 95,4 ± 2,6 95,4 ± 2,6
p = 0,119
History of aortic surgery 90,3 ± 5,3 90,3 ± 5,3 85,6 ± 6,8 85,6 ± 6,8
First 50 cases 94,0 ± 3,4 94,0 ± 3,4 94,0 ± 3,4 94,0 ± 3,4
p = 0,594
Last 50 cases 96,0 ± 2,8 93,5 ± 3,7 89,7 ± 5,1
-Figure 2. Kaplan-Meier analysis of freedom from all-cause mortality and freedom from
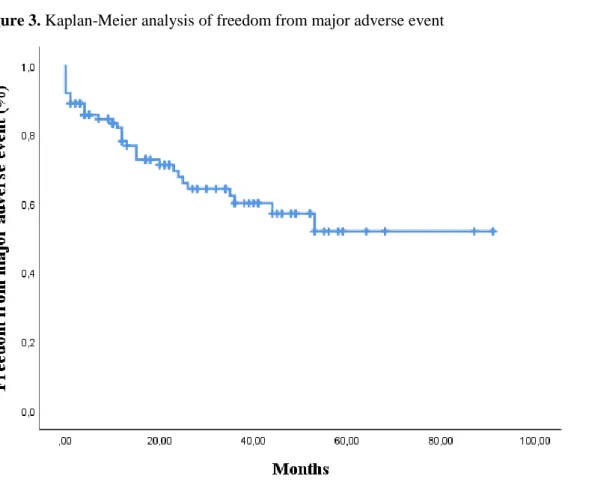
Major adverse events. Thirty-two patients developed 50 major adverse events (MAE), including all 23 deaths, eight cardiac complications, and four patients with bowel ischemia. Three patients developed spinal cord ischemia with paraplegia, and five patients developed a stroke. One patient had a late pulmonary embolism, and dialysis was necessary in six patients, four in the early postoperative period and two in the late postoperative period. Detailed overview is shown in table 8, dividing early from late MAEs. Freedom from any MAE was 82,0% ± 4,0% at one year, 62,2% ± 5,8% at three years and 51,9 % ± 7,6% at five years.
Table 8. Major adverse events
Major adverse events MAE Early Late
Death 6 17
Cardiac complications
Cardiac arrest 2 0
Coronary artery bypass grafting 1 0
PCI 3 2
Digestive complications
Bowel resection for ischemia 4 0
Neurologic complications
Stroke 3 2
Major adverse events MAE Early Late
Respiratory complications
Pulmonary Embolism 0 1
Renal complications
Need for permanent dialysis 3 1
Need for temporary dialysis 1 1
PCI = percutaneous coronary intervention, SCI = spinal cord ischemia
Figure 3. Kaplan-Meier analysis of freedom from major adverse event
Target vessel events. Three late postoperative target vessel events were diagnosed. In one patient a bilateral renal artery occlusion occurred, which was successfully treated with a bilateral iliorenal bypass. In one patient a celiac artery occlusion was diagnosed, but did not necessitate any treatment and one patient developed a renal artery occlusion resulting in permanent dialysis. A total of 14 target vessels events were registered of the 350 targeted vessels (4%), six intraoperative, five early postoperative and three late postoperative. Table 9 shows an overview of the late target vessel events and table 10 shows the estimated freedom from target vessel events.
Table 9. Late target vessel events
Days Artery Event Reintervention Result
135 Bilateral RA Occlusion Bilateral ileorenal bypass Temporary dialysis,
full recuperation
394 CA Occlusion No No influence
457 RA Stent fracture RA No Permanent dialysis
CA = celiac artery, RA = renal artery
Table 10. Freedom from target vessel event
Estimated survival (%) ± standard deviation (%)
30 days 1 year 3 years 5 years
Freedom from all target vessel event 89,8 ± 3,1 89,8 ± 3,1 86,9 ± 3,6 86,9 ± 3,6
Freedom from celiac artery event 99,0 ± 1,0 99,0 ± 1,0 97,5 ± 1,8 97,5 ± 1,8
Freedom from superior mesenteric
artery event 98,0 ± 1,4 98,0 ± 1,4 98,0 ± 1,4 98,0 ± 1,4
Freedom from renal artery event 93,9 ± 2,4 93,9 ± 2,4 92,4 ± 2,8 92,4 ± 2,8
Endoleak. At the final intraoperative angiogram 42 endoleaks were observed. There were two type 1a endoleaks, 18 type 2, nine type 3 and five endoleaks of unknown origin. At the 30-day computed tomography angiography (CTA), a total of 32 endoleaks were observed consisting of three type 1a endoleaks, two type 1b endoleaks, 14 type 2 endoleaks, eight type 3 endoleaks and in five cases the type of the endoleak was unclear. In the total follow-up period four endoleaks resolved spontaneously and four new endoleaks were observed. A total of six interventions were performed to treat endoleaks; including three embolizations for a type 2 endoleak, two percutaneous transluminal angioplasties for a type 3 endoleak and one open surgical repair (aortic banding) for a rupture based on a type 1b endoleak.
Table 11. Interventions for endoleak
Days Endoleak Procedure
239 Type 2 Embolization IMA
245 Type 3 PTA SMA and RA
362 Type 1b Banding
801 Type 2 Embolization IMA
789 Type 3 PTA RA
1262 Type 2 Embolization lumbar artery
IMA = inferior mesenteric artery, PTA = percutaneous transluminal angioplasty, RA = renal artery, SMA = superior mesenteric artery
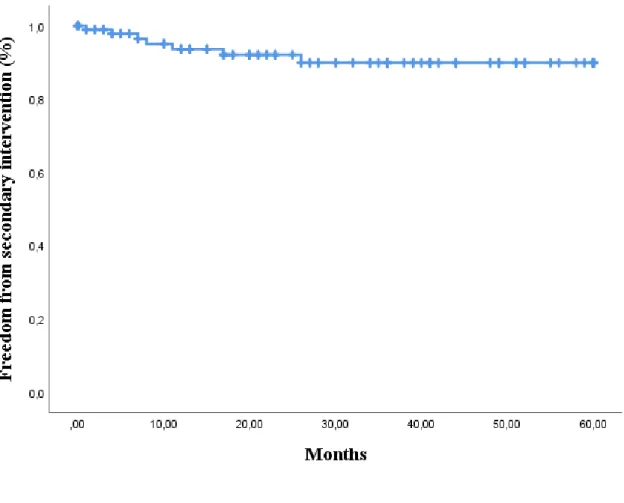
Secondary intervention. A total of ten procedure related secondary interventions were observed in eight patients. Freedom from secondary intervention was 93,6% ± 2,8% at one year, 92,0% ± 3,1% at two years and 89,9% ± 3,8% at three and five years. Six interventions were performed because of endoleak. In one patient a bilateral iliorenal bypass was necessary for a bilateral renal artery occlusion. There was one rupture because of infection resulting in an open surgical repair with banding of the aorta. In one patient a reconstructive procedure was necessary to treat a sacral pressure ulcer, in a patient with paralysis due to spinal cord ischemia. And one femoro-femoral crossover bypass was performed because of a stent graft limb occlusion.
Discussion
Juxtarenal abdominal aortic aneurysms and thoracoabdominal aortic aneurysms used to be treated by open surgical repair. Since the introduction of fenestrated and branched endovascular aortic repair there is an alternative manner to treat these patients. Initially endografts with one or two fenestrations were used to treat juxtarenal aortic aneurysms, later endografts with three or four fenestrations or branches were used to treat juxtarenal aortic aneurysms and thoracoabdominal aortic aneurysms. High technical success rate, low morbidity and low mortality have been reported for complex endovascular repair.36-38,41-45 In a recent meta-analysis analyzing results of complex endovascular repair of juxtarenal aortic aneurysms, there was a perioperative mortality of 3,3%.36 Perioperative mortality for complex endovascular TAAA repair shows widely distributed results with a range of 0% to 25% and a pooled perioperative survival of 3,7%.1 Since fenestrated/branched EVAR procedures are performed in the university hospital of Ghent, no studies have been implemented to evaluate the results. The aim of this retrospective study was to evaluate our results. Primary outcome was early (< 30 days) mortality.
In our study population early postoperative mortality is 6%, which is in line with the results found in literature. In patients treated for a juxtarenal AAA early mortality is 1,6% compared to 12,1% in patients treated for a TAAA. In patients without history of aortic surgery early mortality is 2,9% compared to 12,9% in patients with a history of aortic surgery. Most frequent cause of early mortality is cardiac death or death due to embolization. Kaplan-Meier Log Rank survival analysis shows a nearly statistical significant difference in freedom from all-cause mortality between patients treated for a juxtarenal AAA compared to patients treated for a TAAA (p =0,079), and between patient with a history of aortic surgery compared to patients without a history of aortic surgery (p = 0,051). The difference in survival is less pronounced when comparing aneurysm-related mortality. This highlights the importance of non-aneurysm-related mortality and the high risk population treated in our cohort. Nonetheless, there is an important number of late deaths of which the cause is unknown, making it more difficult to substantiate any statement.
Technical success might appear to be low in our study population, 87% primary technical success and 89% secondary technical success. However, the definition of technical success differs substantially in literature. In this study we used the definition of Chaikof et al. 39 Primary technical success was defined as successful arterial access using a remote site, successful