Biology Department
Research Group of Molecular Entomology and Honeybee Pathology
_____________________________________________________________________________________CHARACTERIZATION OF PHYSICAL
AND BIOLOGICAL PROPERTIES OF A
NEWLY DISCOVERED HONEY BEE
VIRUS
Hanne De Rijcke
Studentnumber: 01508732Supervisor(s):
Prof. Dr. Dirk de Graaf
Scientific tutor:
Peter Demaeght
Master’s dissertation submitted to obtain the degree of Master of Science in Biology
© Faculty of Sciences – research group Molecular Entomology and Honeybee Pathology
All rights reserved. This thesis contains confidential information and confidential research results that are property to the UGent. The contents of this master thesis may under no circumstances be made public, nor complete or partial, without the explicit and preceding permission of the UGent representative, i.e. the supervisor. The thesis may under no circumstances be copied or duplicated in any form, unless permission granted in written form. Any violation of the confidential nature of this thesis may impose irreparable damage to the UGent. In case of a dispute that may arise within the context of this declaration, the Judicial Court of Gent only is competent to be notified.
TABLE OF CONTENTS
1. Introduction ... 1
1.Biology of the honey bee ... 1
2.Honey bee importance and decline ... 2
3.Honey bee viruses ... 3
3.1. Structure ... 3
3.2. Classification ... 3
3.3. Modes of viral transmission ... 4
3.4. Common honey bee viruses ... 4
3.5. Replication ... 6
4.Vectors of honey bee viruses ... 6
4.1. Varroa destructor ... 6
4.2. Other biological vectors ... 7
5.Antiviral responses of honey bees ... 7
6.Novel honey bee viruses ... 9
2. Objective ... 10
3. Material and methods ... 11
1.Sample collection ... 11
2.Homogenisation ... 11
3.RNA extraction ... 11
4.First Strand cDNA synthesis ... 11
5.PCR ... 11
6.Plasmid vector ... 12
7.Multiplex Ligation-dependent Probe Amplification (MLPA) ... 12
8.RT-PCR ... 13
9.Phylogenetic tree ... 13
4. Results ... 15
1.Virus detection via PCR ... 15
2.Negative strand detection via MLPA ... 16
3.Quantification of Thika-like virus via RT-PCR ... 16
4.Detection of common honey bee viruses via MLPA ... 17
5.Phylogenetic tree ... 19
5. Discussion ... 20
1.Metagenomics study ... 20
2.Phylogenetic tree ... 20
3.Thika-like viral genome ... 21
4.Virus detection via PCR ... 21
5.Negative strand detection via MLPA ... 22
7.Detection of common honey bee viruses via MLPA ... 25
8.In vitro infection studies ... 26
9.RNAi responses ... 27 6. Conclusions ... 30 7. Summary ... 31 Summary [ENG] ... 31 Samenvatting [NL] ... 34 8. Acknowledgements ... 37 9. Abbreviations ... 38 10. Reference list ... 39 Appendix ... 47
Appendix A: Thika-like virus screening through PCR ... 47
1. INTRODUCTION
1. Biology of the honey beeThe western or European honey bee (Apis mellifera L.) is part of the large insect order Hymenoptera, together with other bee species, wasps, ants and sawflies. The honey bee belongs within small genus Apis in the family of the Apidae. Most of the current managed and domesticated honey bees have been derived from the western honey bee, leading to a global expansion of their distribution range (van Engelsdorp & Meixner, 2010).
Western honey bees, whom will be just referred to as honey bees from now on, are eusocial insects that live within colonies or hives containing about 50 000 to 80 000 individuals. They belong to the few insects that have social structures or castes. Honey bee colonies contain three different castes: a single fertile female which is called the queen, sterile females called worker bees, and fertile males called drones (Lal, 2009). A honey bee queen is larger than a worker bee and her sole function is to reproduce and lay eggs. Worker bees are the most abundant caste within the hive and only emerge from fertilised eggs. Within the hive worker bees exhibit age-related polyethism; their function depends on their age. The youngest worker bees are responsible for the brood, older worker bees will build the wax combs, manage food stores or guard the entrance of the hive and the oldest worker bees are foragers (Mortensen et al., 2013). Lastly, drones emerge only out of non-fertilised eggs. Their principle function is to mate with a virgin queen, and therefore they are only reared in spring with a peak in numbers around the swarming season (Kilani, 1999).
The purpose of reproduction in honey bees is different compared to most other animals: instead of producing more individuals, honey bees aim to produce more colonies. A sexually mature queen will leave the colony to mate with drones from other colonies. Mating only takes place the first two weeks after the queen has emerged. After mating, the queen stores the sperm of the drones in her spermatheca for several years. In total, only 3 -5% of the spermatozoa will be stored, from which up to 1.7 million fertilised eggs will be produced during the queen’s life span (Baer et al., 2016). The queen then returns to the hive where she lays eggs for the remainder of her life (Falk, 2015; Mortensen et al., 2013). Egg production is initiated by increased activity of neurosecretory cells, but the mechanism that stimulates this increase in activity is not well understood. The production of fertilised or non-fertilised eggs is primarily dependent on the condition of the colony and the season. Unfertilised drone eggs are only produced in spring and summer, if the colony is strong enough. Worker bees then produce drone combs, which consist of drone cells specifically shaped in such a way that fertilised eggs cannot be deposited in the cell. Most likely, the production of unfertilized eggs by a queen is stimulated by the presence of such drone cells in the hive (Koeniger, 1986).
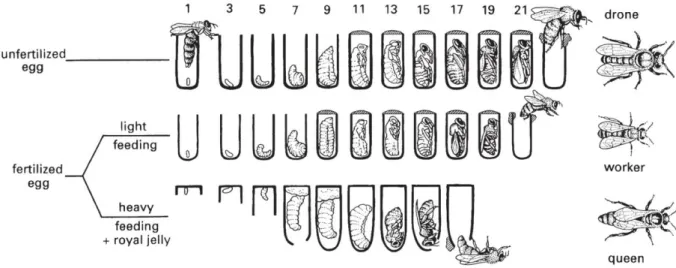
Honey bees are holometabolous insects, meaning that they undergo complete metamorphosis throughout their development. They have three immature stages (eggs, larvae and pupae) and one mature stage (imago or adult). The development of honey bees belonging to different castes is shown in figure 1.1. Eggs are separately deposited in wax comb cells, which are filled with pollen and nectar. The egg stage has a duration of about three days, after which the larvae emerge. Worker bee and drone larvae will be fed royal jelly for the first three days and honey or pollen afterwards, whilst queens are fed royal jelly until they emerge as adults. The length of the larval stage is dependent on the caste of the bee: drones have the longest larval stage and queens the shortest. When the larvae are ready for moulting, the cell will be capped with wax and the larvae will surround themselves with a cocoon. The cocooned larvae then become prepupae, followed by moulting to become pupae. Finally, a last moult is needed for the pupae to become adult bees, which emerge by chewing through the wax (Falk, 2015; Jay, 1963; Mortensen et al., 2013).
The term ‘swarming’ is used for the production of a new honey bee colony. Honey bees swarm in spring or early summer, when food resources are most abundant. The process is initiated by the presence of 10 to 20
new daughter queens in the colony. New queens are only produced when certain factors trigger a need, the main ones being lack of space for eggs in combs due to excessive amounts of honey or if the reigning queen is failing. Right before the new adult queens emerge, the reigning queen will leave the hive together with the majority of her worker bees to find a place where they can create a new colony. After the first daughter queens have emerged in the original colony they will fight and kill each other and their unborn siblings, until only one remains.
Figure 1.1: Honey bee development in different castes. The developmental time in days is indicated at the top of the
figure. Drones develop from unfertilised eggs whilst workers and queens develop from fertilised eggs. The composition of the provided food is one of the factors which determines differentiation into worker or queen. Eggs moult into larvae around day 3, after which the cell is capped. Several moultings then occur through which larvae become prepupae, prepupae become pupae and pupae become adult bees, whom emerge from the capped cell. The developmental period is shortest for queens (circa 16 days) and longest for drones (circa 24 days). Reprinted from The Insects: An Outline of
Entomology, Fifth Edition. By P. J. Gullan and P. S. Cranston. (C) 2014 John Wiley & Sons, Ltd. Published 2014 by John
Wiley & Sons, Ltd. Companion Website: www.wiley.com/go/gullan/insects
2. Honey bee importance and decline
The honey bee is globally recognised as an insect of great importance. It does not only play a vital role in maintaining healthy natural ecosystems, but also in agriculture (Ruiz, 2018). Managed honey bees contribute greatly to agricultural productivity by assisting the pollination of many different food crops. About 35% of the global food production is dependent on pollinating animals and about 90% of this pollination is performed by managed honey bees (Degrandi-Hoffman et al., 2019; Genersch, 2010; Potts et al., 2016). Their pollination services provide us with several different kinds of fruits, nuts and vegetables (Hitaj et al., 2018). In Europe, the contribution of honey bees to the agricultural industry is estimated to be at least 22 billion euro yearly (Erdős, 2018). In the United States, it is estimated that the economic dependence of agricultural sectors on pollination services is worth 14.2 to 23.8 billion US dollars (Chopra et al., 2015). The global economic value of crop pollination is even estimated to be around €153 billion (Alebachew, 2018). In addition to this, honey bees also provide us with products that are not directly obtained through pollination. Honey, beeswax, royal jelly, pollen and propolis are examples of these products and they are used for causes such as consumption, medicine or cosmetics (Ediriweera & Premarathna, 2012; Linskens & Jorde, 1997; Meo et al., 2017). There is no doubt about the usefulness of honey bees in our current economy.
Unfortunately, for the past few decades a decrease in managed honey bee colonies has been observed. Whilst the global numbers of managed honey bee hives have been increasing since the 1960s, high losses of colonies have been reported which are related to a decreased annual survival or Colony Collapse Disorder
(CCD) (Goulson et al., 2015; Staveley et al., 2014). Colony losses are reported nearly worldwide, but the largest part of the losses are reported in Europe, Asia and North America (Neumann & Carreck, 2010; Pettis & Delaplane, 2010; Potts et al., 2010). CCD is a phenomenon that first was described in 2006, when several U.S. beekeepers noticed large but inexplicable colony losses. CCD has been a problem ever since, with an average colony loss of 30% every winter (van Engelsdorp & Meixner, 2010). The main symptoms of CCD include complete absence of adult bees in a colony without presence of dead bees in or around the hive, presence of capped brood, and presence of untouched food stores (Ellis et al., 2010).
Several causes for both the general decline and CCD have been pushed forward and can be divided into two groups: the drivers for the long-term decline appear to be socioeconomic and political pressure on production of bee products, whilst the drivers for the annual losses are mainly considered to be introduced pathogens and pests (Smith et al., 2013). Other debated annual drivers for the bee declines are loss, fragmentation and degradation of habitat, the use of pesticides and climate change (Goulson et al., 2015; Paudel et al., 2015).
A significant variety of diseases exists to which honey bees are susceptible, often caused by bacterial, fungal or viral pathogens. Amongst the bacterial pathogens, the most important bacteria are Melissococcus plutonius and Paenibacillus larvae, which cause European and American Foulbrood respectively. They both only affect larvae and are found globally. When infected, honey bee larvae darken, rot and die (Fünfhaus et al., 2018; Paudel et al., 2015) The Nosema disease or Nosemosis is caused by microsporidian parasites belonging to the genus Nosema. Microsporidia are obligate intracellular pathogens that belong to the Fungi. Infection with Nosema parasites causes damage to the midgut of the honey bees, leading to digestive problems and a shortened lifespan. Whilst bacterial and microsporidian pathogens certainly play a significant role in honey bee health, they only belong to the smallest group that causes disease in honey bees. The majority of pathogens that infect honey bees are of viral origin (Glenny et al., 2017).
3. Honey bee viruses 3.1. Structure
Ever since the discovery of Sacbrood virus in 1913, honey bee viruses have been a well-researched subject (G. F. White, 1913). Since then, a broad range of viruses have been discovered and described (Gisder & Genersch, 2015). By obtaining more information about for instance physiochemical properties, transmission, incidence and pathology of viruses, researchers hope to understand their relation to honey bee decline better. Honey bee infecting viruses are mainly positive sense single stranded RNA viruses (+ssRNA), belonging to the order of Picornavirales (McMenamin & Flenniken, 2018). Only little is known about DNA viruses of honey bees and recently several honey bee associated ssDNA viruses have been discovered, but these still have to be characterised (Kraberger et al., 2019). To date, the only known double stranded DNA virus of honey bees is the Apis mellifera filamentous virus (AmFV), which was discovered in 1981 (Bailey et al., 1981). Most of the bee viruses usually persist as unnoticeable infections that cause no direct signs of disease or clinical symptoms, but under certain conditions they can become very lethal and cause rapid death. Some honey bee viruses are able to attack all different honey bee developmental stages or castes, making them hard to fight.
3.2. Classification
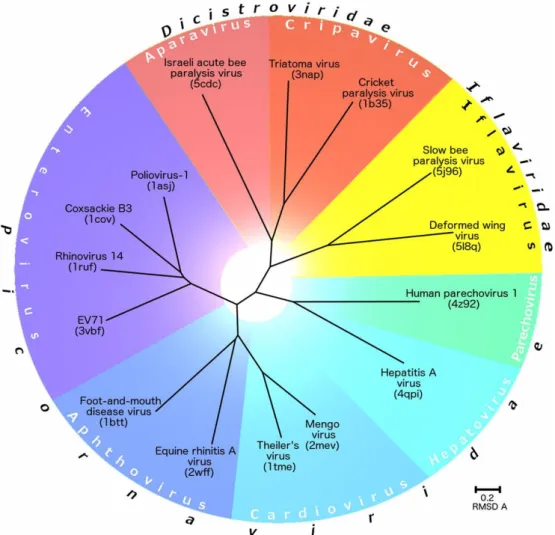
Within the order of the Picornavirales, the ssRNA honey bee viruses either belong to the family of the Dicistroviridae or the Iflaviridae (figure 1.2). The Dicistroviridae family contains small positive sense and non-enveloped RNA viruses of invertebrates, and is expanding rapidly. Members of the family can be distinguished from other members within the Picornavirales by having the structural proteins at the 3’ end of the genome instead of at the 5’ end (Bonning, 2009). The members of the Iflaviridae family are characterised by being +ssRNA viruses that infect arthropod hosts (Valles et al., 2017).
Figure 1.2: Evolutionary relationship among viruses from the families Dicistroviridae, Iflaviridae, and Picornaviridae based
on structural alignment of their capsid proteins. Reprinted from “Structure of deformed wing virus, a major honey bee pathogen” by Škubník K. et al., 2017, PNAS, volume 114 (12), p. 3210-3215.
3.3. Modes of viral transmission
The mode of transmission is one of the most important aspects of viral diseases, it determines how the infection will spread and how it will persist in a population. There are two categories of viral transmission: vertical transmission and horizontal transmission. If infection occurs amongst individuals of the same generation, it is called horizontal transmission. This mode of transmission can be further split up into direct or indirect routes. Air-borne, food-borne or sexual transmissible infections are examples of direct routes, and if an intermediate biological host spreads the infection this is called an indirect route. Vertical transmissions occur if the virus is passed on from a mother to her offspring via the egg (Chen et al., 2006; Chen & Siede, 2007).
3.4. Common honey bee viruses
The most common honey bee infecting viruses belonging to the Dicistroviridae are Black Queen Cell Virus (BQCV), Chronic Bee Paralysis Virus (CBPV) and Acute Bee Paralysis Virus (ABPV). For the Iflaviridae, the most common viruses are Deformed Wing Virus (DWV), Sacbrood Virus (SBV) and Slow Bee Paralysis Virus (SBPV) (Chen & Siede, 2007).
BQCV is one of the most common, yet least understood honey bee viruses. Most of the time it persists chronically but without symptoms in bee colonies. Transmission occurs socially amongst adults and vertically from the queen to her offspring or from adults to larvae through glandular secretions. When the virus is present in high titres, mostly in spring and early summer, it kills developing queen larvae or pupae in the capped-cell stage. Their necrotic remains then stain the pupal cells with a black colour. BQCV can also infect adult worker bees, but no outer symptoms are usually noticeable. The disease primarily is a problem in the honey bee queen-rearing industry, but not as much in wild honey bee populations (Chen & Siede, 2007; Spurny et al., 2017). Many studies indicate an association of BQCV with Nosema spp., which causes an increased mortality if both pathogens are simultaneously present in the honey (Bailey et al., 1983).
CBPV is also a virus that in normal circumstances persists asymptomatically, but becomes very severe if present in high levels in honey bees. The virus causes two forms of chronic paralysis symptoms in bees. Symptoms of the first form include trembling of the bee’s wings and bodies, making bees crawl over the ground rather than flying. Bees also become bloated and potentially have dislocated wings. In the other form of paralysis, bees lose their hair, making them appear darker and thus more susceptible to nibbling attacks of their healthy siblings or being rejected entrance from guard bees of their colony. The virus takes several days to kill diseased bees, but can still cause significant losses in colonies (Chen & Siede, 2007; Olivier et al., 2008).
ABPV was discovered during infectivity tests with CBPV. This virus yet again occurs commonly in healthy looking colonies and rarely causes disease. The transmission most likely happens via salivary gland secretions that are fed to young larvae or end up in pollen. When ABPV is present in honey bees in high titres due to combination with parasitic mites of the genus Varroa, infection can become very severe and cause mortality in both brood and adult bees. Bees then become flightless and start to tremble as they do when infected with CBPV, but they die much more quickly, usually within 1 day (Aubert et al., 2008; Chen & Siede, 2007).
The most heavily investigated honey bee virus is DWV, as it is seen as one of the main causes for colony collapses. Again, the virus will not negatively impact its host in standard conditions, but in high quantities it will induce severe clinical symptoms and eventually cause mortality. The presence of the virus and its high impact on honey bee health is often associated with the occurrence of the parasitic mite Varroa destructor. Typical symptoms induced by the virus include vestigial and crumpled wings, smaller body size, discoloration and a shortened lifespan in emerging worker and drone bees (figure 1.3). The virus develops fairly slowly, allowing honey bee brood to develop throughout the pupal stage. Due to this low virulence DWV is seen as the main virus associated with the Varroa mite, as it kills very slowly and the infected bees are able to reach adulthood (Chen & Siede, 2007; de Miranda & Genersch, 2010; Lanzi et al., 2006).
As mentioned above, the first honey bee virus to be described was SBV, one of the most widely distributed viruses of honey bees. The virus mainly infects the early larval stage and transmission occurs through ingestion of glandular secretions of adult bees. Its name is derived from the sac-like appearance it causes in diseased larvae. This is because they are unable to shed their endocuticle, leading to accumulation of ecdysial fluids containing large amounts of SBV particles between the body and the cuticle. Larvae then die before they are able to reach their pupal stage. Adult bees can get infected with the virus too and will have a shortened life span (Chen & Siede, 2007; J. Li et al., 2019).
Lastly, just like most viruses, SBPV also persist unnoticed in honey bees. The virus can be transmitted by V. destructor amongst adult bees and pupae, enabling it to reach high levels of infection and causing lethal effects. The symptoms of severely infected bees show up quite late compared to most other viruses, typically after 10 days, hence the name of the virus. Two pairs of the anterior legs will suffer paralysis and about two days later the bees eventually die of infection (Bailey & Woods, 1974; de Miranda et al., 2010).
3.5. Replication
Replication of honey bee viruses always occurs within the cytoplasm of the host cell. The virus will invade the host cell by attaching itself to the cell wall and injecting its RNA genome (Chen & Siede, 2007). One of the key components for replication of RNA viruses is RNA-dependent RNA polymerase (RdRp). This protein is part of the replicase complex, which differs in proteins among different virus families. The viral genome will hijack the host cell’s machinery to aide with replication (Marques & Imler, 2016; Payne, 2017). Once enough viral copies have been created within the host cell, the progeny will be released.
4. Vectors of honey bee viruses 4.1. Varroa destructor
The best known and studied biological host which facilitates the spread of viruses amongst honey bees is the ectoparasitic mite Varroa destructor. It is seen as one of the greatest threats for honey bees for several reasons. The first and foremost reason is that V. destructor is a relatively new parasite for the honey bees. The mite originates as a parasite of the Eastern honey bee Apis cerana, where it is called Varroa jacobsoni. Due to honey bee transportation and trade there was a host switch to A. mellifera (Boecking & Genersch, 2008; Rosenkranz et al., 2010). In A. cerana, reproduction of V. jacobsoni mites solely takes place in drone brood, thus reproduction is restricted to certain periods of the year. In A. mellifera, mites can reproduce in both worker and drone brood, leading to a more extensive reproductive period and therefore more beneficial conditions to infest honey bees. After the host switch, the A. mellifera parasitic mite was renamed Varroa destructor (Boecking & Genersch, 2008).
Secondly, the mites are able to reach much larger populations in A. mellifera compared to A. cerana and therefore their range has become nearly worldwide. They are present in almost every honey bee colony and facilitated the transmission of a large number of honey bee viruses (Rosenkranz et al., 2010). Honey bee viruses that occurred naturally at low and asymptotic levels are then able to reach much higher levels, becoming epidemic and increasing the mortality rates of honey bee colonies (Sumpter & Martin, 2004). Even though V. destructor is seen as one of the main causes of CCD, they attributed to colony losses long before CCD was observed (Kang et al., 2016).
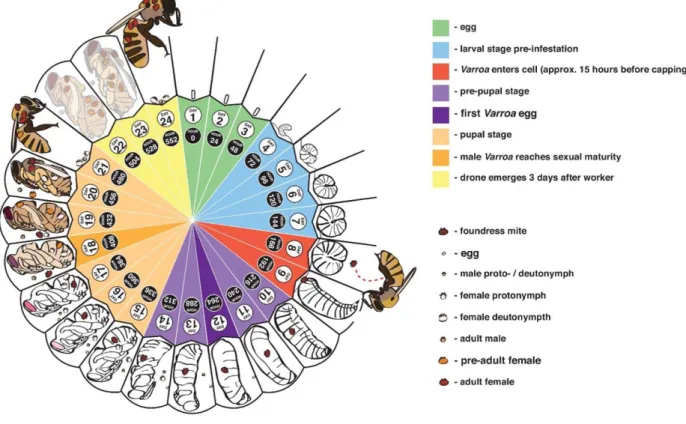
When honey bees are infected with V. destructor, the disease is called ‘varroosis’. It is closely linked to the brood cycle of honey bees, as the mites only reproduce in brood and have no free living stage (Wegener et al., 2016). Figure 1.4 represents the reproduction cycle of V. destructor mites. Reproduction is started by female mites entering brood cells of honey bee larvae prior to the capping of the cells. After capping of the brood cells, the mites will externally feed on fat body tissue of the bee pupa (Ramsey et al., 2019). Several days after cell capping, the mites start laying eggs on the cell wall. Usually, the first egg is male and all other eggs that follow are female. When the eggs hatch, they go through several developmental stages: first a
larval stage, followed by several nymphal stages, after which they emerge as adult mites. Males are not able to survive outside of brood cells, so they mate with their siblings within the brood cell directly after emergence. The new fertilised female mites then wait until the honey bee hatches and leave the cell together with their host. They reside on the honey bee for a variable time before they enter new brood cells where they deposit their eggs and start the cycle again (Calderón et al., 2010; Evans & Cook, 2018).
Figure 1.4: The reproductive cycle of Varroa destructor mites. Mated female mites will enter the honey bee cell just prior
to capping (around day 8). After several days, females start laying eggs sequentially. When the eggs hatch, the offspring will mate with each other within the cell. Finally, they either emerge with worker bees (on day 21 of development) or with drones (on day 24). Reprinted from “Genetics and physiology of Varroa mites” by Jay D. Evans and Steven C. Cook, 2018, Current Opinion in Insect Science, volume 26, p. 130-135.
4.2. Other biological vectors
Infection and replication of DWV has also been observed in the ectoparasitic mite Tropilaelaps mercedesae, which infects honey bee brood similarly to V. destructor. This suggests that these mites also act as biological vectors for honey bee viruses (Dainat et al., 2009; Forsgren et al., 2009). Detection of ABPV in the honey bee louse Braula schmitzi and detection of DWV in both the parasitic phorid fly Apocephalus borealis and the parasitic small hive beetle Aethina tumida indicates that these three species also are potential viral vectors for honey bees. Viral replication was detected in the latter species, A. tumida. For all three species further research has to be conducted to determine their potential of being biological vectors of honey bee viruses (Avalos et al., 2019; Core et al., 2012; Eyer et al., 2009)
5. Antiviral responses of honey bees
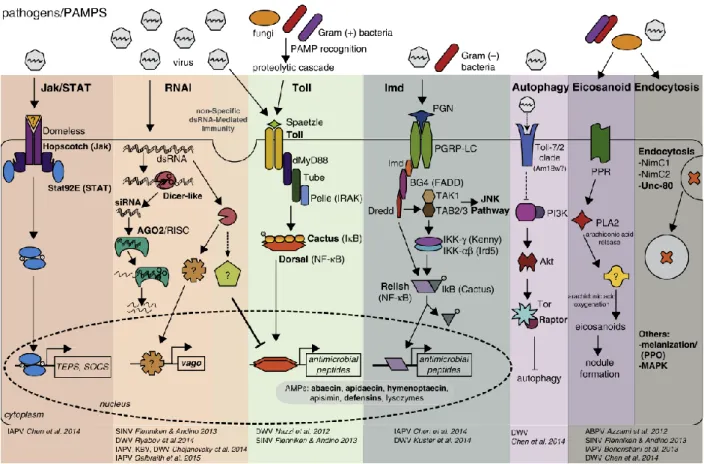
Honey bees evolved several mechanisms in response to infections by pathogens (figure 1.5). Immune activation is first set off by host recognition of pathogen-associated molecular patterns (PAMPs). Honey bees can utilise cellular immune responses as defence reactions, such as phagocytosis, nodule formation,
encapsulation or melanisation. Additionally, honey bees can also utilise humoral immune responses, which include pathways such as Jak/STAT, RNA interference (RNAi), Toll, immune deficiency (Imd), JNK and MAPK. Different pathways can be activated according to the type of pathogen. For example, gram positive bacteria and fungi generally induce the Toll pathway, whilst gram negative bacteria induce the Imd pathway (Chen & Siede, 2007; McMenamin et al., 2018). Recent research has shown that DWV interferes with NF-κB signalling, actively supressing the immune system of the bee, which in turn is favourable for feeding and reproduction of the V. destructor (Di Prisco et al., 2016)
Figure 1.5: Examples of honey bee immune pathways, including key genes that are involved in antiviral immune
responses. Bold text indicates genes or proteins which are differentially expressed in virus-infected honey bees. PAMPS will be recognized by the host, after which specific pathways are activated (depending on the PAMP). Reprinted from “Antiviral defence mechanisms in honey bees” by Laura M. Brutscher et al., 2015, PNAS, volume 10, p. 71-82.
The mechanism of siRNA/RNAi is seen as one of the major antiviral defences in honey bees (Brutscher & Flenniken, 2015). The RNAi pathway is triggered by foreign viral DNA or double stranded RNA. In virus infected honey bees, dsRNA is present as an intermediate of viral replication. The trigger DNA or RNA gets recognized and will be processed into short interfering RNA (siRNA) by two RNase II enzymes: Dicer and Drosha. These siRNA’s then get loaded into an effector complex called the RNA-induced silencing complex (RISC). The double stranded siRNA’s are unwound during the loading process. This way, the single stranded RNA is able to hybridize with the RNA target, which then undergoes nucleolytic degradation by the protein Argonaute (Tijsterman & Plasterk, 2004). Through genome analysis, it was shown that honey bees contain the necessary RNAi machinery genes, such as dicer-like and Argonaute-2 (Brutscher et al., 2015; De Smet et al., 2017; Yang et al., 2018).
6. Novel honey bee viruses
The most prevalent and virulent honey bee viruses have already been described extensively in current literature, but many other, less common viruses also persist in honey bees which still need to be researched or even discovered. Honey bee viruses generally persist asymptotically in their host when they are present in low levels, which is one of the reasons that many viruses remain undiscovered. Nevertheless, this does not mean that they are harmless. If they are able to reach high titres, they can potentially cause very severe symptoms that lead to mortality. Novel honey bee viruses are found rather frequently and are in need for characterisation and identification to determine whether they play a role in honey bee health (McMenamin & Flenniken, 2018; Remnant et al., 2017; Schoonvaere et al., 2018). For the past few years, the discovery of new honey bee viruses has been accelerated by next generation sequencing technologies, which helps tremendously with increasing our knowledge of the impact of these viruses on honey bees (McMenamin & Flenniken, 2018).
In an unpublished metagenomics study by Deboutte et al. (2020), several unknown honey bee viruses were discovered. The main goal of the study (VIROBEE) was to investigate the role of viruses in winter loss of Belgian honey bee hives. Viruses in honey bees were identified using the NetoVIR (Novel enrichment technique of VIRomes) protocol, which is based on viral particle purification techniques combined with Ilumina NGS deep sequencing technology and optimised bio-informatics pipelines. A large number of known, common honey bee viruses was identified, such as DWV, SBV and ABPV, but novel eukaryotic viruses were detected too. For all of the novel viruses, (near) complete viral genomes were obtained using the metagenomics data.
Three novel viruses were deemed as interesting candidates for further research. The first virus is Thika-like virus, a Picorna-like virus that was initially detected in Drosophila melanogaster (Webster et al., 2015). The next virus is Thogoto-like virus and belongs to the Orthomyxoviridae. This virus was first described in ticks and livestock hosts. Lastly, there is the Uncultured virus which had not been described in any other study. Given the high presence of these viruses in samples, it is important that more knowledge about them is obtained so that their role in honey bee health can be understood better. Out of these three novel viruses, Thika-like virus was selected based on its high coverage and prevalence.
2. OBJECTIVE
The goal of this dissertation was to further characterize one of the novel honey bee viruses that was discovered using a next generation sequencing (NGS) method with an enrichment of viral genome (Deboutte et al., 2020). Thika-like virus was chosen as it was the most prevalent virus out of three candidates. By obtaining more information about the virus’ specific characteristics, its impact on honey bee health could be assessed and it could be determined whether the virus plays a significant role in honey bee decline.
First of all, some general information about the virus was examined: prevalence, viral load and virus activity. Seasonal variation was accounted for by sampling honey bees hives at different time frames throughout the year. For all of the samples, the prevalence of the virus was determined. In samples that tested positive for the virus, the viral load was quantified and compared to the viral load of other honey bee viruses. The presence of a complementary negative strand, which is indicative for active infection, was determined as sometimes viruses are merely passively present within a host. As some other honey bee viruses could potentially confound or associate with Thika-like virus, the honey bee samples were also screened for a number of common honey bee viruses. Furthermore, these screenings were performed before and after winter so that a potential impact of winter on virus prevalence could also be assessed. Next, the possibility of the parasitic mite V. destructor being a vector of Thika-like virus was examined. The same general information about the virus - prevalence, viral load and virus activity - was examined in mites which were sampled from honey bees from the same hives.
Honey bees are able to fight viral infection using their antiviral RNAi machinery, but recently it was proven that certain components of the antiviral RNAi machinery of the honey bee can be negatively influenced by infection with viruses (De Smet et al., 2017). To determine whether Thika-like virus could possibly interfere with the antiviral defence mechanism, the expression of a number of RNAi related genes were to be measured and compared between virus free honey bees and honey bees infected with Thika-like virus. Lastly, in vitro infection studies would have been set up, where different honey bee life stages were going to be infected with the virus. This way, characteristics such as the pathogenesis, mode of transmission, or incubation period of Thika-like virus could have been investigated in a safe and controlled environment.
3. MATERIAL AND METHODS
All of the work was performed at the Laboratory of Molecular Entomology and Honeybee Pathology (L-MEB), Faculty of Sciences, UGent, Belgium, which is led by prof. dr. Dirk de Graaf.
1. Sample collection
Honey bees were sampled from different hives at Honeybee Valley (Ghent, Belgium) in spring (March/April 2019), summer (July/August 2019), late-summer or fall (September 2019) and winter (early March 2020). Varroa destructor mites were sampled from honey bees from different hives in fall (October 2019). Samples were stored at -20°C before further processing.
2. Homogenisation
For each time frame, 10 honey bees were selected per hive and homogenized. Bees were pooled in 5 ml tubes containing 0.25 ml zirconia beads (0.1 mm; Biospec products, USA) and 3-5 stainless steel beads (2.3 mm; Biospec products, USA) in 5 ml Phosphate Buffered Saline (PBS). The samples were homogenized using the Bullet Blender Storm 5®/VISUM IDPBW at maximum speed for 10 minutes. Homogenisation of V. destructor samples was performed by selecting a number of mites per hive and manually homogenizing them with a small pestle in 1 ml PBS in a 1.5 ml tube. The number of selected mites varied according to the number of mites that were sampled originally per hive, ranging from 1 to 10 mites.
3. RNA extraction
Total RNA extraction of the samples was performed using the QIAamp Viral RNA Mini Kit (Qiagen, Germany) according to manufacturer’s instructions. Per homogenized sample, 140 µl supernatant was added to 560 µl of Buffer AVL-carrier RNA in a microcentrifuge tube and pulse vortexed for 15 seconds. The solution was left to incubate at room temperature for 10 minutes. After incubation, 560 µL of 100% ethanol was added to the solution and again pulse vortexed for 15 seconds. Next, 630 µl was applied to an QIAamp Mini column in a collection tube and the solution was centrifuged at 8 000 rpm for 1 minute. This step was repeated for the remainder of the solution. Next, 500 µl of Buffer AW1 was added to the column and centrifuged at the same settings. Then, 500 µl of Buffer AW2 was added and centrifuged at 14 000 rpm for 3 minutes. To eliminate remnants of Buffer AW2, the column was centrifuged again at 14 000 rpm for 1 minute. Lastly, 60 µl of Buffer AVE was added to the column, left to incubate for 1 minute and centrifuged at 8000 rpm for 1 minute. The elution with extracted RNA was stored at -80°C until further use.
4. First Strand cDNA synthesis
Synthesis of cDNA was performed using the RevertAid H Minus First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, USA), according to manufacturer’s instructions. For each reaction 5 µl of RNA, 1 µl of random hexamer primer and 6 µl of nuclease free water were added to a PCR tube placed on ice, which was then heated to 65°C for 5 minutes. Next, 4 µl of 5X Reaction Buffer, 1 µl of RiboLock RNase Inhibitor (20 U/µl), 2 µl of 10 mM dNTP mix and 1 µl of RevertAid H Minus M-MuLV ReverseTranscriptase (200 U/µl) were added in the indicated order and heated for 5 minutes at 25°C, 60 minutes at 42°C and 5 minutes at 70°C. The end product was stored at -20°C.
5. PCR
Forward and reverse primers for Thika-like virus screening were designed using primer 3 (http://bioinfo.ut.ee/primer3/) with default settings. The primers were synthesized by Integrated DNA Technologies (Leuven, Belgium) and are listed in table 3.1. PCR was performed using the HotStartTaq Plus DNA Polymerase Kit (Qiagen, Germany). Each reaction consisted of a master mix and 1 µl of cDNA template.
The master mix consisted of 2.5 µl 10X PCR Buffer, 1 µl dNTPs, 1 µl forward primer, 1 µl reverse primer, 1 µl MgCl2, 0.25 µl Taq polymerase and 17.25 µl MQ water per reaction. The PCR program went as following: 5 minutes at 95°C, 35 cycles of 30 seconds at 94°C, 30 seconds at 58°C and 1 minute at 72°C, and 3 minutes at 72°C.
Analysis of PCR products was performed with agarose gel electrophoresis. PCR products loaded on a 2% agarose gel and ran for 40 minutes at 120 V. Visualisation was done using ethidium bromide staining and the UVP High-Performance UV Transilluminator.
6. Plasmid vector
To obtain a positive control for PCR screening for the Thika-like virus, the TOPO TA Cloning® Kit for Sequencing (Invitrogen) was used according to manufacturer’s instructions. Previously identified positive samples for the Thika-like virus (Deboutte et al., 2020) were used to construct the positive control. For eight Thika-positive samples, the same steps for sample preparation (homogenisation, RNA extraction, first strand cDNA synthesis) and PCR were performed as described above.
For the TOPO® Cloning reaction, 1 µl of PCR product was combined with 1 µl salt solution, 3 µl MQ water and 1µ of TOPO® vector. After 5 minutes incubation at room temperature, the construct was transformed into E. coli by adding 2 µl of the reaction to a vial of One Shot® chemically competent E. coli. The vial was then incubated on ice for 30 minutes, followed by a heat-shock for 30 seconds at 42°C. Next, 250 µl of S.O.C. medium was added and incubated for 1 hour at 37°C whilst being shaken horizontally at 200 rpm. After incubation, 50 µl and 200 µl of the transformation was spread out on two 37°C pre-heated lysogeny broth (LB) agar plates, each containing 50 µg/ml kanamycin. The plates were then left to incubate at 37°C overnight. The next day, six colonies were picked out from the 50 µl transformation plate. Six PCR tubes were filled with a PCR master mix and 1 µl MQ water. Each colony was lightly touched with a pipetting tip, which was rinsed of in a PCR tube. PCR was then performed on the samples to verify the insertion in the plasmids. A new pre-heated agar plate was then divided into six parts and each colony was picked from the old plate and transferred to one part of the new plate. This plate was again left to incubate at 37°C overnight. Afterwards, plasmid RNA was isolated of two colonies using the PureLink® Quick Plasmid Miniprep Kit (Thermo Fisher Scientific, USA) according to manufacturer’s instructions.
7. Multiplex Ligation-dependent Probe Amplification (MLPA)
MLPA analysis was performed to determine the presence of the Thika-like virus negative strand and to screen for presence of five common honey bee viruses. For Thika-like virus, MLPA left and right oligo probes were designed as described by De Smet et al. (2012). The RT primer and negative strand as template for positive control were designed using primer 3 (http://bioinfo.ut.ee/primer3/). The probes, RT primer and template were synthesized by Integrated DNA Technologies (Leuven, Belgium) and listed in table 3.1. For screening of five of the most common honey bee viruses, the multiparameter assay ‘BeeDoctor’ was used, as described by De Smet et al. (2012). Probes and primers used for screening for BQCV, SBV, DWV, ABPV and SBPV are also listed in table 3.1.
Negative strand detection in honey bees was performed by dissecting the bees into three different body parts: head, thorax and abdomen. Exactly 10 honey bees were cut up to obtain the head, thorax and abdomen separately. Each of the body parts were then pooled, after which homogenisation and RNA extraction took place. For negative strand detection in V. destructor, three previously made RNA samples were selected.
MLPA analysis was performed in four steps, using the MRC-Holland SALSA MLPA EK1 reagent kit. First, a reverse transcription reaction was performed: 1 µl RNA sample, 1 µl SALSA RT buffer, 1 µl custom made RT primer mix and 1.5 µl MQ water were brought together on ice and incubated for 1 minute at 80°C followed
by 5 minutes at 45°C. The custom made RT primer mix consisted of 1 µl of each RT primer and 10 µl dNTP (10 mM), which was brought to a total volume of 20 µl with TE buffer. Next, 1.5 µl of a reverse transcriptase solution was added to the reaction, which consists of 0.68 µl MQ water, 0.68 µl SALSA Enzyme Dilution buffer and 0.15 µl M-MLV Reverse Transcriptase (Promega). The reaction was incubated for 15 minutes at 37°C and 2 minutes at 98°C. The second step was hybridisation of the probes: 1.5 µl SALSA probe-mix and 1.5 µl MLPA buffer were added to the RT reaction. The probe-mix was prepared by adding 0.8 µl of each 1 µM probe oligo in TE buffer to obtain a final volume of 600 µl. The reaction was then left to hybridize for 16-20 hours at 60°C. Thirdly, a 32 µl Ligase-65 mix was prepared which consists of 3 µl Ligase 65 buffer A, 3 µl Ligase 65 buffer B, 25 µl MQ water and 1 µl Ligase-65. The mix was added to the reaction and incubated for 15 minutes at 54°C and 5 minutes at 98°C. The fourth and last step was the PCR reaction, where per reaction 7.5 µl MQ water, 2 µl SALSA-primers and 0.5 µl SALSA polymerase were added. The reaction was then left to incubate at 35 cycles of 30 seconds at 95°C, 30 seconds at 60°C and 60 seconds at 72°C and finished with 20 minutes at 72°C.
To analyse the MLPA products, agarose gel electrophoresis was performed with a 4% high resolution gel for 2 hours at 75 V. Visualisation was done using ethidium bromide staining and the UVP High-Performance UV Transilluminator.
8. RT-PCR
RT-PCR was performed to quantify the viral load in samples. A standard curve was made using 8 dilutions of the Thika-like plasmid vector (10-1 to 10-8). For each reaction 1 µl of cDNA was added to a master mix, which consists of 12.5 µl Platinum® SYBR® Green qPCR SuperMix-UDG, 0.5 µl forward primer (10 µM), 0.5 µl reverse primer (10 µM) and 10.5 µl DEPC-treated water per reaction. RT-PCR reactions were performed using the CFX96 Real-Time System (Bio-Rad). The primers used here were the same as for PCR (table 3.1). The following program was used: 2 minutes at 50°C, 2 minutes at 95°C and 40 cycles of 15 seconds at 95°C and 30 seconds at 60°C. A melt curve was generated at the end of the program and all data wasanalysed by CFX Manager software (Bio-Rad).
9. Phylogenetic tree
A phylogenetic tree was constructed based upon the protein sequences of RNA dependent RNA-polymerase (RdRp). Viruses that were included in the phylogenetic tree were the Thika-like virus, the Thika virus discovered in Drosophila melanogaster (Webster et al., 2015) and a list of important viruses from different orders. These orders included the Picornavirales, Tymovirales, Sobemo-like viruses, Nege-like viruses and Toti-like viruses. The RdRp protein sequence of the viruses was obtained from the NCBI database (https://www.ncbi.nlm.nih.gov/). The RNA sequence of the Thika-like virus that was obtained through a NGS method (Deboutte et al., 2020) was translated to the protein sequence using ExPASy (https://web.expasy.org/translate/) and the protein sequence for RdRp was extracted using InterPro (https://www.ebi.ac.uk/interpro/). Every RdRp protein sequence was entered in BioEdit7 and saved as a FASTA file. Using Mega7, the sequences were aligned by ClustalW and a phylogenetic tree was constructed using the Maximum Parsimony method (Saitou & Imanishi, 1989).
Virus Strand Function 5’ – 3’ Sequence
Thika-like virus + PCR FP ATA CTG GGA GAT GAC ATG CG
PCR RP CAA GAC CAC ACT CAG CGA AG - MLPA negative
strand
TGT TGC ATT TGT GAC TGA TAG CCG TAG TTT TGA GCA GCC ATC GAC CTG TCA AAA TCC TGT TGG GAA TGT TGA ATG GAC GAC TGC TGA CCA AAC TCG GCT TGT TGC ATT GCT GTC TGT TG
cDNA TGC ATT TGT GAC TGA TAG CCG
MLPA LPO GGG TTC CCT AAG GGT TGG ATG AGC AGC CAT CGA CCT GTC AAA ATC CTG TTG G
MLPA RPO /5Phos/ GAA TGT TGA ATG GAC GAC TGC TGA CCA AAC TCG GTC TAG ATT GGA TCT TGC TGG CAC
BQCV + cDNA CGG GCC TCG GAT AAT TAG A
MLPA LPO GGG TTC CCT AAG GGT TGG ACT TCA TGT TGG AGA CCA GGT TTG TTT GCC GAC TTA CGG AA
MLPA RPO /5Phos/ TGT CGT TAA ACT CTA GGC TTT CCG GAT GGC TTC TTC ATG GTC TAG ATT GGA TCT TGC TGG CAC
SBV + cDNA TGG ACA TTT CGG TGT AGT GG
MLPA LPO GGG TTC CCT AAG GGT TGG ACG TTG ATC CAA TGG TCA GTG GAC TCCT TAT ACC GAT TTG TTT AAT GGT TGG
MLPA RPO /5Phos/ GTT TCT GGT ATG TTT GTT GAC AAG AAC GTC CC CTT CAG CCA TTC AGC TCT AGA TTG GAT CTT GCT GGC AC
DWV + cDNA TCA CAT TGA TCC CAA TAA TCA GA
MLPA LPO GGG TTC CCT AAG GGT TGG ATG ACC GAT TCT TTA TGC AGC GAG CTC T
MLPA RPO /5Phos/ TAC GTG CGA GTC GTA CTC CTG TGA CAT CTA GAT TGG ATC TTG CTG GCA C
ABPV + cDNA CAA TGT GGT CAA TGA GTA CGG
MLPA LPO GGG TTC CCT AAG GGT TGG ACT CAC TTC ATC GGC TCG GAG CAT GGA TGA T
MLPA RPO /5Phos/ ACG CAC AGT ATT ATT CAG TTT TTA CAA CGC CCT CTA GAT TGG ATC TTG CTG GCA C
SBPV + cDNA CGC AAA CAC GAC GAA TTT TA
MLPA LPO GGG TTC CCT AAG GGT TGG ACG TTC AAT GGT CGA GAT AGA AGC CAC AGT AGA AGT ATT ACG CGC T MLPA RPO /5Phos/ TCT TGT GTT TTG GCT TAT GGG CGT GGG CCT GAT CTT CAT TCA GCT CTA GAT TGG ATC TTG
CTG GCA C
Table 3.1: Primers and probes used for PCR and MLPA for detection of the positive (+) and negative (-) strand of the Thika-like virus and for detection of the positive strand
4. RESULTS
1. Virus detection via PCR
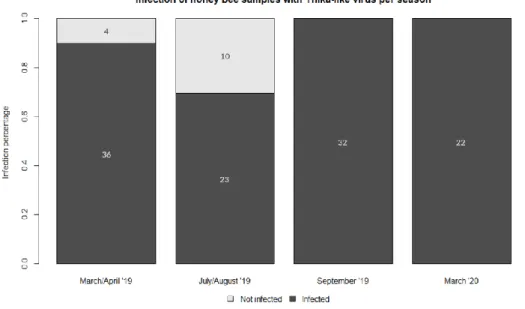
The presence of Thika-like virus was determined in honey bee samples of four different time periods and in V. destructor mites sampled at only one time period, using PCR (Appendix A). Thika-like virus positive hives show a band at 90 bp. Thika-like virus was present in 36 out of 40 honey bee hives sampled in spring, 23 out of 33 hives sampled in mid-summer, and all hives sampled in both late summer and winter (figure 4.1). For the V. destructor mites, the virus was detected in 43 out of 46 hives which had been sampled for mites (figure 4.2).
Figure 4.1: Thika-like virus infected vs. non-infected honey bee hives per sampled time period. Thika-like virus was
detected in 36 out of 40 hives in spring (March/April ’19), 23 out of 33 hives in summer (July/August ’19), all 32 hives in early fall (September ’19) and all 22 hives in winter (March ’20).
Figure 4.2: Thika-like virus infected vs. non-infected V. destructor samples. Thika-like virus was detected in 43 out of
2. Negative strand detection via MLPA
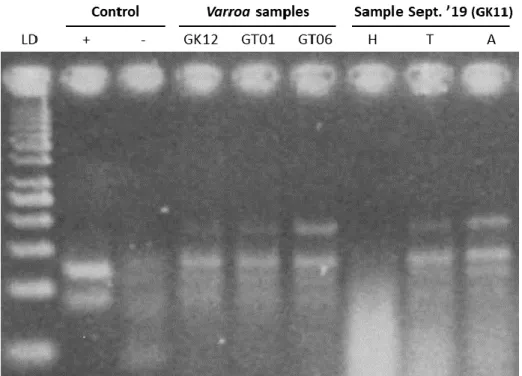
MLPA analysis was performed to determine the presence of the Thika-like virus negative strand in the honey bee and V. destructor samples of September 2019 and March 2020. The band for the negative strand can be seen at 119 bp. Three V. destructor samples were chosen and analysed. In all three samples the negative strand was detected, indicating active replication of the virus (figure 4.3). Honey bees samples were dissected and pooled per head, thorax and abdomen. The negative strand was detected in the thorax and abdomen of honey bees, but not in the head (figure 4.3).
Figure 4.3: High resolution analysis of MLPA for negative strand detection in three V. destructor samples and pooled
head, thorax and abdomen samples from honey bees. From left to right: 50 bp ladder, positive control, negative control, three V. destructor samples (from hives GK12, GT01 and GT06 respectively) and pooled body parts from honey bees
of the GK11 hive sampled in September ’19 (head, thorax and abdomen respectively).
3. Quantification of Thika-like virus via RT-PCR
RT-PCR was performed to quantify the viral particles in honey bee samples from September 2019 and V. destructor samples from October 2019. For the standard curve, the number of copies per dilution was calculated using the concentration of the plasmid vector (301 ng/µl), the length of the amplicon (4046 bp) and the following formula (Prediger, 2013):
𝑛𝑢𝑚𝑏𝑒𝑟 𝑜𝑓 𝑐𝑜𝑝𝑖𝑒𝑠 (𝑚𝑜𝑙𝑒𝑐𝑢𝑙𝑒𝑠) =301 𝑛𝑔 ∗ 6,0221 𝑥 10
23 𝑚𝑜𝑙𝑒𝑐𝑢𝑙𝑒𝑠/𝑚𝑜𝑙𝑒
(4046 ∗ 660𝑚𝑜𝑙𝑒𝑔 ) ∗ 1 𝑥 109 𝑛𝑔/𝑔 = 67 880 439 341,5 𝑚𝑜𝑙𝑒𝑐𝑢𝑙𝑒𝑠
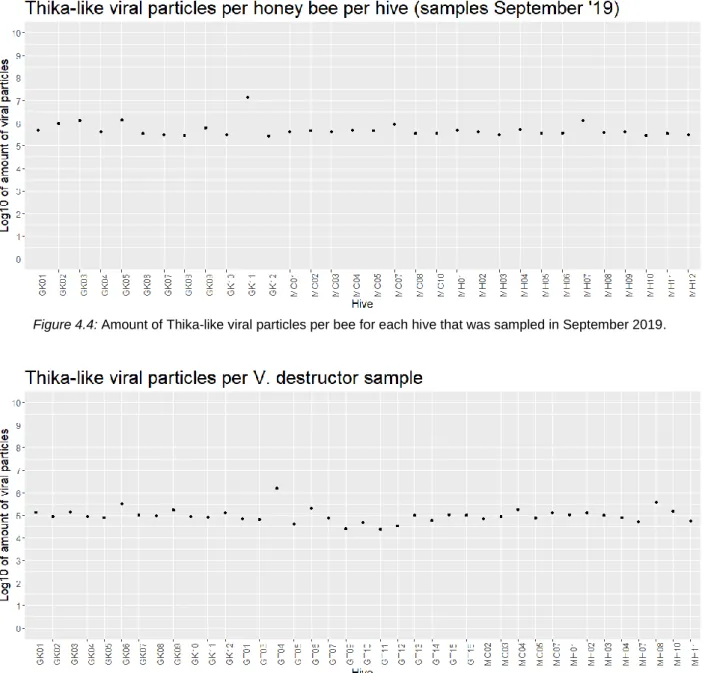
For the samples of September, the viral particles per bee were calculated for each hive sampled. These fluctuated around an average of 5.26e+05 particles per bee, excluding the higher value of hive GK11 (1.46e+07 particles per bee) (figure 4.4). For the V. destructor samples, the viral particles were calculated per sampled hive, not per individual mite. Here, the values fluctuated around an average of 1.07e+05 particles per sample, again excluding the higher value of hive GT04 (1.53e+06 particles) (figure 4.5).
Figure 4.4: Amount of Thika-like viral particles per bee for each hive that was sampled in September 2019.
Figure 4.5: Amount of Thika-like viral particles per V. destructor sample.
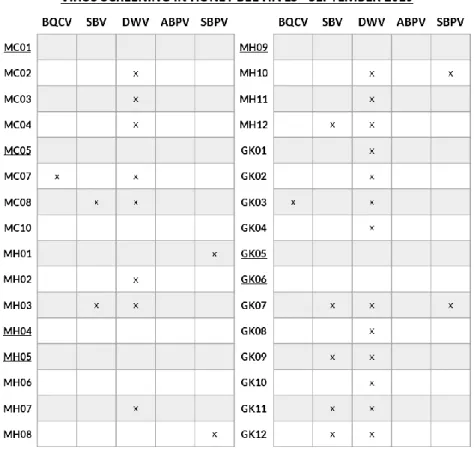
4. Detection of common honey bee viruses via MLPA
Samples obtained in September 2019 and March 2020 were screened for five of the most common honey bee viruses via MLPA (Appendix B). Out of the 32 samples taken in September 2019, 9 hives were found to be virus free. All other hives were infected with minimum one to maximum three viruses, DWV being the most occurring virus (figure 4.6). From the 22 samples collected in March 2020, only 3 hives were free from infection with any of the five viruses. Again, all other hives were infected with one to three viruses, the most common virus being DWV (figure 4.7).
Figure 4.6: Screening for five of the most common honey bee viruses in hives sampled in September 2019. Cells
marked with an ‘x’ indicate the presence of the virus, whilst empty cells indicate the absence. Underlined hives were completely virus-free.
Figure 4.7: Screening for five of the most common honey bee viruses in hives sampled in March 2020. Cells marked
with an ‘x’ indicate the presence of the virus, whilst empty cells indicate the absence. Underlined hives were completely virus-free.
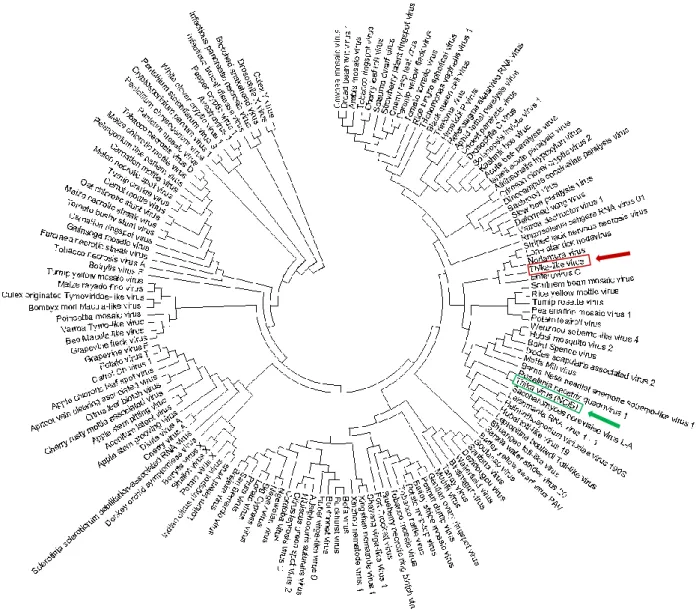
5. Phylogenetic tree
Based upon the obtained protein sequence of RdRp for Thika-like virus and the protein sequence of Thika virus found on NCBI, a phylogenetic tree was constructed (figure 4.8). Thika-like virus (indicated by a red square and arrow in figure 4.8) appears to be related to Enterovirus C, Nodamura virus, Lone Star Tick Nodavirus and Striped Jack Nervous Necrosis virus. The NCBI Thika virus (indicated by a green square and arrow in figure 4.8) appears to be related to Rosellinia Necatrix Quadrivirus 1.
Figure 4.8: Maximum Parsimony analysis of taxa. The evolutionary history was inferred using the Maximum Parsimony
method. The most parsimonious tree with length = 3133 is shown. The consistency index is 0,298755 (0,298755), the retention index is 0,590723 (0,590723), and the composite index is 0,176482 (0,176482) for all sites and parsimony-informative sites (in parentheses). The MP tree was obtained using the Subtree-Pruning-Regrafting (SPR) algorithm (pg. 126 in ref. Nei and Kumar 2000) with search level 0 in which the initial trees were obtained by the random addition of sequences (10 replicates). The analysis involved 144 amino acid sequences. All positions containing gaps and missing data were eliminated. There were a total of 63 positions in the final dataset. Evolutionary analyses were conducted in MEGA7 (Kumar et al., 2016).
5. DISCUSSION
1. Metagenomics studyRecently, the virome of honey bees in Flanders was investigated in a metagenomics study by Deboutte et al. (2020). Viral particles were purified from honey bee samples using the NetoVIR protocol (Conceição-Neto et al., 2016) and sequenced using NGS sequencing technology. Honey bee samples collected from across Flanders were homogenised, centrifuged and filtrated. Then, RNA or DNA was randomly amplified and prepared for deep sequencing using Ilumina. Finally, the raw sequence data was processed using specific bioinformatic tools. The study revealed a large number of known honey bee viruses, but also several unknown viruses. From the unknown viruses, 25 were selected based upon prevalence and probability of being honey bee viruses. Using approaches such as homology searches against viral databases or number of NGS reads, the exact host of the novel viruses was determined. Lastly, a limited number of these novel viruses was selected for future experiments, as they appeared to possibly be correlated with honey bee winter loss.
One of these selected novel viruses was the Thika-like virus, which was chosen based upon several observations. Firstly, Thika-like virus belonged to one of the viruses which were most closely related to families of known honey bee viruses, such as the Iflaviridae or the Dicistroviridae. Secondly, the virus also has a high probability of playing a significant role in honey bee mortality, as both the prevalence and viral load (number of NGS reads) in the samples appeared to be very high. Thus, the virus was deemed important enough for further identification and characterisation to determine its role in honey bee health.
2. Phylogenetic tree
A phylogenetic tree was constructed based on the protein sequences of RNA-dependent RNA polymerase from Thika-like virus, the Drosophila melanogaster Thika virus, and a list of important viruses from different orders and super families. The phylogenetic tree indicated that Thika-like virus is a different virus than the Thika virus which was isolated from Drosophilla species, as the viruses were found on different branches.
The D. melanogaster Thika virus has not yet been fully characterised. According to the study of Webster et al. (2015), the virus can be classified within the Picornavirales and was not only found in D. melanogaster, but also in D. virilis, D. ficusphila and D. simulans. The closest relative appeared to be the Rosy Apple Aphid virus, which is an unclassified positive single stranded RNA virus belonging within the realm of the Riboviria. The virus affects the production of winged morphs in the rosy apple aphid Dysaphis plantaginea (Ryabov et al., 2009). According to the phylogenetic tree constructed in this study, the Drosophilla Thika virus’ closest relative is the Rosellinia Nectrix Quadrivirus 1 (RnQV1), a double stranded RNA quadrivirus belonging to the family of the Quadriviridae within the order of the Toti-like viruses. It was first isolated from the fungus Rosellinia necatrix (Lin et al., 2012).
Contrastingly, Thika-like virus appeared to be related to Enterovirus C, Nodamura virus, Lone Star Tick Nodavirus and Striped Jack Nervous Necrosis virus. The latter three viruses all belong to the family of the Nodaviridae within the super family of the Sobemo-like viruses, whilst Enterovirus C belongs to the family of the Picornaviridae within the order of the Picornavirales. Enterovirus C is a virus that causes disease in humans and consists of 23 serotypes, including poliovirus and coxsackievirus A1. These serotypes cause a number of different illnesses such as encephalitis, myelitis, meningitis, upper and lower respiratory diseases (Tapparel et al., 2013). Nodamura virus was originally discovered in a species of mosquitoes (Culex tritaeniorhynchus) in Japan (Scherer & Hurlbut, 1967). Later studies determined that the virus also infects other insect species, as well as pigs and mice. The virus appeared to be highly lethal for honey bees and wax moths (Galleria mellonella), but could also kill mice (Bailey & Scott, 1973; Lauffer et al., 1976). The Lone Star Tick Nodavirus was isolated from the lone star tick Amblyomma americanum . This tick species is a
well-known vector of a large number of diseases such as Rocky Mountain spotted fever caused by Rickettsia rickettsia or tularemia caused by Franscisella tularensis. The virus was only discovered quite recently, and therefore not much is known about it (Tokarz et al., 2018). Lastly, Striped Jack Nervous Necrosis virus is a positive-stranded RNA virus which is known to infect fish and cause viral nervous necrosis (Iwamoto et al., 2005; Nishioka et al., 2016). All four of these viruses which are related to Thika-like virus seem to have a broad range in hosts and in pathogenicity. The only virus that belongs to the Picornavirales, and thus is closest related to the Picorna-like viruses, is Enterovirus C, which is placed directly next to Thika-like virus in the phylogenetic tree.
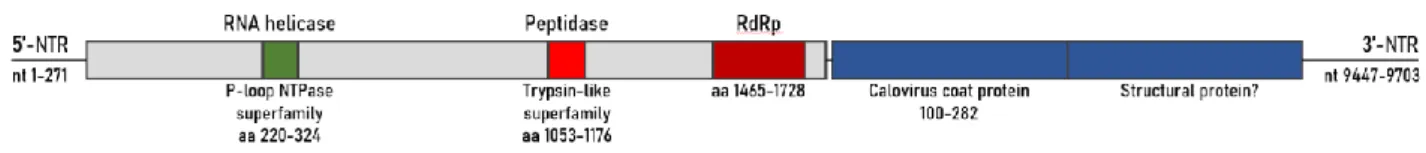
3. Thika-like viral genome
Figure 5.1: Schematic diagram of the genome of Thika-like virus. Based on Deboutte et al. (2020).
In the metagenomics study performed by Deboutte et al. (2020), the genome of Thika-like virus was described and schematically represented (figure 5.1). As expected, the Thika-like viral genome structure strongly resembles the genomic structure of viruses within the Picorna-like super family. More specifically, the genome structure resembles that of ABPV, BQCV and KBV, which have a monopartite bicistronic genome structure. The genome contains two ORFs: non-structural genes such as helicase and RdRp can be found at the 5’ end, whilst structural genes are present at the 3’ end (Chen et al., 2006). The ORF for the structural genes includes a region encoding for a coat protein, which is a feature that remained conserved within the Picorna-like viruses (Liljas et al., 2002).
4. Virus detection via PCR
To determine the prevalence of Thika-like virus in honey bees, adult honey bees were sampled from hives in four different time frames: spring (March/April ’19), summer (July/August ’19), early fall (September ’19) and winter (March ’20). The different time periods were chosen specifically to determine if there was seasonal variation of Thika-like virus prevalence in the apiary or, in other words, to determine the seasonal susceptibility of honey bees to the virus. It was found that during spring and summer, respectively 90% (36/40) and 69,7% (23/33) of the sampled hives were infected with Thika-like virus. During the other two time frames, early fall and winter, 100% (32/32 and 22/22 respectively) of the hives were infected.
The prevalence of Thika-like virus appears to be reasonably consistent, but shows a slightly higher infection rate for honey bee hives during fall and winter. If we compare with existing literature, several studies have conducted research towards the seasonal variation of honey bee viruses. DWV prevalence in French honey bees increased from spring on and reached its peak in fall. In contrast, SBV and BQCV showed highest viral infection rates in summer, followed by spring, and the lowest infection rates were present in fall (Tentcheva et al., 2004). In honey bees from Uruguay however, DWV appeared to be most prevalent in March and decreased towards spring and summer. For SBV and BQCV, inconsistent results were found: virus prevalence differed heavily between apiaries and between sampling years (Antúnez et al., 2015).
It makes sense that the prevalence of SBV is highest in spring and summer and low in fall. SBV mainly infects the early larval stage of honey bees, and thus sacbrood disease manifests itself in the infected brood (J. Li et al., 2019). The peak in brood population in a honey bee colony is reached around mid-June and declines
rapidly from there on (McLellan, 1978). Logically, the peak of SBV infection will be reached around the same time. The same logic can be followed for BQCV, as it infects and causes disease in queen prepupae and pupae (Leat et al., 2000). A high prevalence of DWV around fall can be explained by the fact that DWV infection is closely associated and driven by V. destructor mites, who reach peak infestation around early fall (van Engelsdorp et al., 2013; Wilfert et al., 2016).
Compared to these viruses, the seasonal variation of Thika-like virus appears to be most similar to the seasonal variation of DWV: high prevalence in fall and winter and a slightly lower prevalence in spring and summer. The results regarding other honey bee viruses seem to be too inconsistent to draw conclusions about their seasonal prevalence compared to like virus. Nevertheless, our data suggests that Thika-like virus is consistently present during the whole year and shows little seasonal variation. This is (partly) supported by unpublished data of Deboutte et al. (2020). Here, prevalence of Thika-like virus was examined in 2012 and 2013 and was found to be very high in the first year of screening. However, the following year it was almost non-existent. This suggests that future screenings should opt for a longer screening period, with more consistent screenings about every 3 months. Compared to the study by Antúnez et al. (2015), this experiment about Thika-like virus’ seasonal variance was carried out on a rather small scale and on a limited amount of colonies. Extending the sampling range to the entirety of Flanders might result in more representative view on the seasonal variability of Thika-like virus. The number of screened hives also varied for each season, which might have been disadvantageous as obtained results were not as consistent as they could have been.
Next, the parasitic V. destructor mite was screened for Thika-like virus, as it is known to be one of the most important vectors of many honey bee viruses, including DWV, Kashmir Bee virus (KBV) and Israeli Acute Paralysis virus (IAPV) (Bowen-Walker et al., 1999; Chen et al., 2004; Di Prisco et al., 2011; Rosenkranz et al., 2010). To determine whether V. destructor is a vector for the Thika-like virus, mites sampled from different honey bee hives in October ’19 were screened for the virus. It was found that nearly 93,5% (43 out of 46) of the hives were infested with Thika-like virus positive mites. This indicated that V. destructor most likely is a host or a vector for Thika-like virus and aides the transmission of the virus between honey bees.
In the same study mentioned before (Tentcheva et al., 2004), V. destructor mites from different hives were screened for the same number of common honey bee viruses. Only four viruses were detected in the mites, namely DWV, SBV, ABPV and KBV. The latter three viruses were present in 45%, 36% and 5% of the apiaries respectively. In contrast, DWV was present in 100% of the apiaries. Again, Thika-like virus shows a similar prevalence in V. destructor as DWV does. Here too, it could be suggested that for future research more screenings for Thika-like virus could be performed throughout an extended time period. This way, possible seasonal variation of the virus within the mites could be determined.
5. Negative strand detection via MLPA
After confirming the presence of Thika-like virus in the honey bee and V. destructor samples, the next step was to verify active virus replication. For positive stranded RNA viruses, the key indicator for active replication is the presence of a negative-strand RNA intermediate (K. A. White et al., 2011). Detection of the virus through PCR alone does not indicate whether the virus is actively or passively present within its host. If the virus is passively present, it is non-infectious. Thus, detection of a negative-strand intermediate proves active infection of the samples with Thika-like virus.
To detect this negative intermediate in the samples, MLPA-analysis was used. Negative strand detection through MLPA was preferred over strand-specific RT-PCR, as the latter method is highly sensitive to false-positive results caused by mistakes during reverse transcription. MLPA synthesis is favoured as it amplifies a probe rather than the original target. This probe is produced in a strand-specific manner, by ligation of two
oligonucleotide half-probes which hybridize to a complementary cDNA target. The ligase-65 used for this ligation step is not active on RNA-DNA hybrids, warding off false-positive results (De Smet et al., 2012).
Out of the 46 V. destructor samples, three samples were selected to be screened for the negative strand intermediate. These three samples were chosen based upon having the (subjectively) highest Thika-like virus quantity on gel after PCR. The negative strand intermediate was found to be present in all three mite samples, proving that active virus replication occurs within V. destructor mites and the virus is actively infecting mites. To verify active replication in honey bees, one of the hives sampled in September ’19 was chosen and screened. Again, selection was based upon having the subjectively highest quantity of Thika-like virus on gel after PCR. Negative strand detection was performed in different body parts of the honey bees, namely the head, thorax and abdomen. Results showed that active replication of the Thika-like virus takes place in honey bees, more specifically in the thorax and the abdomen, not in the head.
Despite proving the presence of the negative strand of Thika-like virus within the honey bees, we cannot completely rule out the possibility that the virus is a plant virus. The negative strand of plant viruses can be detected in honey bee samples if they are present in tissues of the intestines or the salivary glands. As these tissues reside in the thorax and abdomen, Thika-like virus could possibly not be a honey bee virus. Furthermore, a recent study stated that the plant-pathogenic Tobacco Ringspot virus was able to replicate within honey bees, therefore having a host range which spans both the plant and animal kingdom (J. L. Li et al., 2014). After publishing, the study received quite some criticism as no conclusive support could be found for the adaptation to honey bee hosts (Allen Miller et al., 2014; Cornman, 2017). Therefore, the subject of replication of plant viruses in honey bees is still very controversial and it is assumed that the negative strand of plant viruses can only be present in honey bees when taken up through feeding.
Thus, in order to gain more knowledge about the exact location of the negative strand, different tissues needed to be examined. By localising Thika-like virus infection in different honey bee tissues, more precise information can be obtained about the virus: it could not only reveal the virus’ origin, but could also tell more about its possible pathological effects in certain tissues or its route of transmission. Active infection of Thika-like virus was found in the thorax, which could indicate that the virus resides in and/or affects structures such as the flight muscles or in the salivary glands. Active infection in the abdomen could predict pathological effects in the gut, stomach, wax glands, or Malpighian tubules. If the virus is present within the gut, this could either indicate that the virus is transmitted via food or that the virus was solely taken up by food (e.g. the negative strand of a plant virus). The next step of the experiment would have been to dissect the different tissues of the thorax and the abdomen and to screen the tissues for the negative strand. This way, the distribution of the virus in the honey bee could have been uncovered which would reveal much more about Thika-like virus. Unfortunately, due to the COVID-19 measures, this part of the experiment could not take place and will have to be performed in a future study.
6. Quantification of Thika-like virus via RT-PCR
In nature, honey bees are often infected with a range of viruses without any apparent symptoms of disease. In such cases, honey bee health remains unaffected because the viruses are only present at very low levels. Under certain circumstances though, for example by increased stress, the immunity of the honey bees will lower to which the viral load can increase and reach a certain threshold. At this threshold, viral infection can lead to overt symptoms and impair honey bee survival (Chen & Siede, 2007). Thus, quantification of the viral load in a honey bee colony is of great importance as it can reveal much about the colony’s health status.
The viral load of many common honey bee viruses has been studied thoroughly in the past. Gauthier et al. (2007) determined the viral load of a number of honey bee viruses, including DWV, in asymptotic honey bees and V. destructor mites. Honey bee colonies sampled in fall had a viral load of circa 1.0e+06 DWV copies per adult asymptotic honey bee. In a study by Y. P. Chen, Higgins, and Feldlaufer (2005), relative