Technical evaluation of a potential
release of OX513A Aedes aegypti
mosquitoes on the island of Saba
RIVM Letter report 2017-0087Page 2 of 71
Colophon
© RIVM 2017
Parts of this publication may be reproduced, provided acknowledgement is given to: National Institute for Public Health and the Environment, along with the title and year of publication.
DOI 10.21945/RIVM-2017-0087
D.C.M. Glandorf (author), RIVM Contact:
Boet Glandorf GMO Office,
Centre for Safety of Substances and Products, RIVM boet.glandorf@rivm.nl
The technical evaluation has been performed by order and for the account of Council of Saba.
This is a publication of:
National Institute for Public Health and the Environment
P.O. Box 1 | 3720 BA Bilthoven The Netherlands
Synopsis
Technical evaluation of a potential release of OX513A Aedes aegypti mosquitoes on the island of Saba
The mosquito Aedes aegypti transmits viruses that cause diseases such as dengue, chikungunya and zika. Measures are taken to control the mosquito since these infectious diseases represent a significant health problem. This is the case on the island of Saba, a Dutch Caribbean island. In order to fight these diseases a British company has genetically modified the mosquito in such a way that it can suppress local mosquito populations. The modification causes the mosquitoes' offspring to die prematurely. The potential release of these mosquitoes on Saba is considered to result in negligible risks for human health and the environment.
This is the outcome of a technical evaluation of the potential release of these genetically modified mosquitoes. RIVM's GMO (Genetically
Modified Organisms) Office was commissioned by the Executive Council of Saba to perform this evaluation.
Among others, this evaluation looked into effects on the food chain to determine whether an important food source would disappear if the local mosquito population were to be eliminated. It was also considered whether it is unhealthy if people accidentally swallow a genetically modified mosquito. Another element of the evaluation was whether the genetic modification would increase the efficiency of the mosquito to spread diseases.
An evaluation of the efficacy of application of the genetically modified mosquitoes was not part of this technical evaluation. The same applies to socio-economic effects or the desirability of using these mosquitoes. Keywords: genetically modified mosquitoes, Saba, GMO, risk
Publiekssamenvatting
Technische evaluatie van een mogelijke inzet van genetisch gemodificeerde muggen op Saba
De mug Aedes aegypti brengt virussen over die meerdere ziekten kunnen veroorzaken, zoals dengue, chikungunya en zika. De mug wordt bestreden omdat deze infectieziekten een groot gezondheidsprobleem vormen. Dit is het geval op het eiland Saba, dat onderdeel is van Caribisch Nederland. Om de ziekten te bestrijden heeft een Brits bedrijf met behulp van genetische modificatie de mug zodanig aangepast dat lokale muggenpopulaties teruggedrongen kunnen worden. Door de modificatie sterft het nageslacht vroegtijdig. Deze toepassing blijkt op Saba verwaarloosbaar kleine risico’s voor mens en milieu met zich mee te brengen.
Dit blijkt uit een technische evaluatie van de mogelijke inzet van deze genetisch gemodificeerde muggen. Het Bureau GGO (Genetisch Gemodificeerde Organismen) van het RIVM heeft deze evaluatie in opdracht van het bestuur van Saba uitgevoerd.
Bij deze beoordeling is onder andere naar de voedselketen gekeken: verdwijnt er niet een belangrijke voedselbron wanneer de lokale muggenpopulatie wegvalt? Ook is onderzocht of het ongezond is als mensen per ongeluk een genetisch gemodificeerde mug inslikken. Een ander beoordelingspunt is of de mug door de genetische modificatie niet juist beter in staat wordt om ziekten over te brengen.
Evaluatie van de effectiviteit van de inzet van de genetisch gemodificeerde muggen was geen onderdeel van deze technische evaluatie. Hetzelfde geldt voor sociaaleconomische effecten of de wenselijkheid om deze muggen in te zetten.
Kernwoorden: genetisch gemodificeerde muggen, Saba, ggo, risicobeoordeling, milieu, infectieziekten, Aedes aegypti
Contents
Executive summary — 11 Introduction — 13
Technical evaluation of Oxitec’s documentation — 17 Part A Characterisation of OX513A — 19
Molecular characterization — 19
1 Recipient organism Aedes aegypti — 19
2 Donor organisms and general description of inserted genetic elements — 20
2.1 Trichoplusia ni (Cabbage looper moth) — 20
2.2 Drosophila melanogaster (Vinegar fly) — 20
2.3 Discosoma spp. — 20
2.4 Escherichia coli — 20
2.5 Herpes simplex virus type 1 — 20 2.6 Small synthetic linking sequences — 21
3 Plasmid used in the transformation of OX513A — 21 4 rDNA insert and characteristics of modification — 22
4.1 Detecting the absence of plasmid backbone in transgenic lines — 22 4.2 Number of copies inserted and insert stability — 22
4.3 Verifying the insertion site and sequencing the regions flanking the gene — 23
4.4 Nature of the inserted traits — 23 4.4.1 Fluorescent marker DsRed2 — 23 4.4.2 Self limiting trait tTAV — 24
4.5 Potential for toxicity and allergenicity of the introduced proteins — 25 4.6 Conclusions regarding the molecular characterization of the insert in
OX513A — 25
5 Further characterisation of OX513A — 25 5.1 Life table parameters — 25
5.1.1 Reproductive capacity — 26 5.1.2 Insemination capacity — 26 5.1.3 Mating competitiveness — 26
Mating competitiveness in the laboratory — 26 5.1.3.1
Mating competitiveness in semi field conditions — 26 5.1.3.2
Mating competitiveness in regulated environmental releases — 26 5.1.3.3
5.2 Response to abiotic factors — 27
5.2.1 Temperature response of OX513A — 27
5.2.2 Dose response to tetracycline and its analogues — 27 5.2.3 Susceptibility to chemical insecticides — 27
5.2.4 Behavioral responses of OX513A to insecticides — 27 5.2.5 Tetracycline loaded blood study — 27
5.2.6 Trait penetrance — 27
5.2.7 Non-penetrant OX513A progeny: longevity and fecundity — 28 5.2.8 Field penetrance — 28
5.3 Dispersal and longevity - regulated environmental releases of OX513A — 28
5.4 Potential toxicity - oral exposure studies — 28 5.4.1 Toxorhynchites spp (predatory mosquito) — 28
Page 8 of 71
5.4.2 Poecilia reticulate (guppy fish) — 29 5.5 OX513A morphology — 29
5.6 Analysis of expression of the introduced proteins in female mosquito saliva — 29
5.7 Vertical transmission of dengue and chikungunya viruses in OX513A — 29
5.8 Stability of the insert in OX513A — 29 5.8.1 OX513A quality control — 29
5.9 Conclusions regarding the phenotypic characterization of OX513A — 29 6 Detection and identification of OX513A — 30
6.1 Methods and sensitivity for detecting OX513A Aedes aegypti in the environment — 30
6.2 Monitoring the Aedes aegypti population in the environment — 30 7 Regulated environmental releases of OX513A — 31
7.1 Previous Aedes aegypti vector control projects using OX513A — 31 7.1.5.1 Environmental persistence — 31
7.1.6 Conclusions drawn from previous vector control projects using OX513A — 31
Part B Intended use of OX513A Aedes aegypti on Saba — 33
1 Details of the proposed release on Saba — 33 1.1 Anticipated outcomes for Saba Island — 33 1.2 Location of releases — 33
1.3 Determination of release rates - phased approach — 34 1.3.1 Preparation phase — 34
1.3.2 Intervention Phase — 35
Mosquito release and dispersal — 35 1.3.2.1
Adaptive management of release rate — 35 1.3.2.2
1.3.3 Maintenance Phase — 35
1.4 Containment measures prior to release — 36 1.4.1 Mobile Rearing Unit Overview — 36
Design and construction — 37 1.4.1.1
1.5 Rearing of OX513A from egg to adult mosquitoes — 37 1.5.1 Tetracycline use — 38
1.6 Transport and adult release — 38 1.7 OX513A program monitoring — 38 1.7.1 Ovitrap monitoring — 38
Ovitrap density, location and servicing — 38 1.7.1.1 Species identification — 38 1.7.1.2 Species Composition — 39 1.7.1.3 1.7.2 Ovitrap analysis — 39
1.7.3 Estimating mating fraction — 39 1.7.4 Adult Trapping — 39
2 Receiving environment — 39 2.1 Geography and Climate — 39 2.1.1 Geography — 39
2.1.2 Climate — 40
Weather conditions — 40 2.1.2.1
2.2 Aedes aegypti on Saba — 40
2.2.1 Habitat of Aedes aegypti on Saba — 40
2.2.2 Functions of Aedes aegypti in the ecosystem of Saba — 41 2.3 Flora and Fauna — 41
Part C OX513A Environmental risk assessment — 43
Introduction — 43
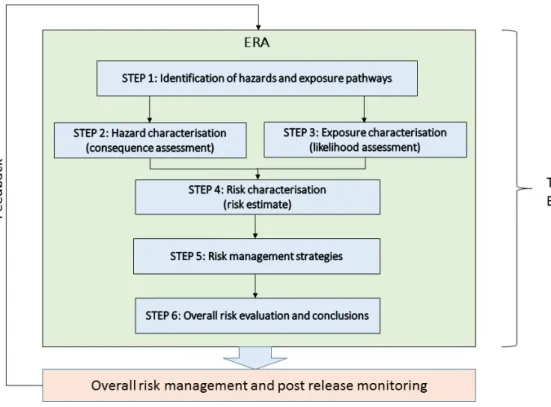
1 Approach of the environmental risk assessment — 43
1.2 Molecular characterization and phenotypic characterization — 45 2 Specific areas of concern — 45
2.1 Persistence and invasiveness, including vertical gene transfer — 45 2.2 Horizontal gene transfer — 49
2.3 Pathogens, Infections and diseases — 51 2.4 Interaction with target organisms — 56 2.5 Interactions with non-target organisms — 59
2.6 Environmental impacts of the specific techniques used for management — 63
2.7 Impact on human and animal health — 65 3 Overall risk evaluation and conclusions — 66
3.1 Uncertainty in the Environmental Risk Assessment — 66 3.2 Conclusions — 68
References — 69
Executive summary
Request for technical evaluation
The GMO Office of the National Institute of Public Health and the Environment (RIVM) is requested by the Council of Saba to provide a technical evaluation of the environmental risk assessment (ERA) for the release of Aedes aegypti on Saba submitted by Oxitec Ltd. The
evaluation focuses on the consequences of a potential release of
genetically modified (GM) Aedes aegypti (strain OX513A) mosquitoes on the island of Saba. The aim of the release is to suppress the local Aedes aegypti population, which is a known vector for human diseases such as dengue fever, chikungunya and zika. Aedes aegypti is considered an invasive species in several jurisdictions and is subject to vector control on Saba.
This technical evaluation considers potential adverse effects on human health and the environment of Saba as a consequence of the release of OX513A on Saba, in case this release may take place in the future. Potential adverse effects are compared to those of the non-modified Aedes aegypti and are considered in the context of local vector control measures. This evaluation does not consider the efficacy of the release of OX513A Aedes aegypti in Saba in suppressing local populations of wild type Aedes aegypti and the diseases they transmit. Socio-economic aspects are also not part of the scope of this evaluation.
Proposed release
The proposed activities involve the repeated release of male (non-biting) OX513A Aedes aegypti mosquitoes in the inhabited parts of the island of Saba. This strain has been used for releases in the Cayman Islands, Panama and Brazil. The OX513A mosquitoes are genetically modified so that their offspring die before adulthood, due to the introduction of a repressible lethal genetic system. When these male OX513A Aedes aegypti mosquitoes mate with wild females in the field, offspring is produced of which over 95% express the lethality trait. This trait causes mortality in larval stages in the absence of tetracycline that is not present in the environment. OX513A also contains the fluorescent marker gene DsRed2 in order to visualize and monitor this strain after its release. The proposed release is foreseen to take place in all inhabited parts of the island of Saba and aims to suppress the local population of Aedes aegypti to very low levels and potentially to
elimination with demonstration of continued elimination for a period of a year thereafter. Monitoring of the local Aedes aegypti populations is part of the proposed activities. Earlier releases of OX513A mosquitoes in the Cayman Islands, Panama and Brazil have demonstrated a clear
reduction in Aedes aegypti mosquito populations. Environmental risk assessment
The environmental risk assessment is performed according to Annex II of European Directive 2001/18/EC (EU, 2001)1. The Guidance document on the Environmental Risk Assessment (ERA) of GM insects of the European Food Safety Authority (EFSA, 2013)2 is used to evaluate potential risks for human health and the environment. Where relevant,
Page 12 of 71
also other, guidance documents with respect to GM insects are considered.
This EFSA Guidance describes seven ‘areas of concern’ that are to be taken into account in the ERA of GM insects. For each of these areas of concern it is considered whether the release of OX513A in Saba could lead to adverse effects on human health and the environment, in comparison to effects of the non-modified Aedes aegypti. Effects are considered in the context of current vector control program.
These areas of concern are the following:
1. Persistence and invasiveness of GM insects, including vertical gene transfer
2. Horizontal gene transfer
3. Pathogens, infections and diseases
4. Interactions of GM insects with target organisms 5. Interactions of GM insects with non-target organisms
6. Environmental impact of the specific techniques used for the management of GM insects
7. Impacts of GM insects on human and animal health
The molecular characterization of OX513A together with its phenotypic and behavioral characteristics are considered to establish whether there are any intended or any unintended changes in OX513A, as a result of the genetic modification, that could lead to a potential adverse effect on human health and the environment. The result of this comparative safety assessment demonstrates that the only differences of biological relevance are the expression of the two introduced traits; the self-limiting trait (tTAV) and the fluorescent marker trait (DsRed2). Therefore the ERA focuses on potential adverse effects of these new traits in OX513A, in comparison to effects of the non-modified Aedes aegypti.
For all areas of concern (EFSA, 2013)2 there is sufficient information to conclude that adverse effects on human health and the environment are unlikely. Adequate measures and controls are taken to prevent
unintended environmental release of OX513A during rearing of OX513A Aedes aegypti before their release. Moreover, in all environmental releases to date OX513A has performed consistently with respect to parameters identified in risk assessments and no unanticipated results or unintended effects have been observed.
The GMO Office concludes that potential adverse effects on human health and the environment as a consequence of the potential release of genetically modified OX513A on the island of Saba, under the conditions as described in the documentation of Oxitec and in the context of
standard vector control, are considered to be negligible as compared to effects of non-modified Aedes aegypti. This is in line with recent related environmental risk assessments such as fromBrazil 3,4 and the United States Food and Drug Administration 5.
The GMO Office recommends post-release monitoring by an independent party, as advised by the WHO6, on a monthly basis until populations of OX513A are below the level of detection.
Introduction
Request from Saba
The GMO Office of the National Institute of Public Health and the
Environment (RIVM) in the Netherlands was requested by the Council of Saba to evaluate documentation from Oxitec Ltd for a planned release on Saba with genetically modified (GM) male OX513A Aedes aegypti mosquitoes. Aedes aegypti is a known vector for human diseases such as dengue fever, chikungunya and zika.
This request of Saba for a technical evaluation was received by the GMO Office on February 29 (2016). On April 28 (2016) the GMO Office
requested from Oxitec all documentation with respect to the potential release on Saba, including raw data and an environmental risk
assessment of the planned release. These documents of Oxitec were received on September 28 (2016). Additional information was requested by the GMO Office on October 24 (2016) and January 19 (2017) and this information was received on November 30 (2016), January 11 (2017) and March 13 (2017), respectively. All documents and additional data were evaluated by experts from the RIVM, Wageningen University & Research and Saba. The resulting technical evaluation was thereafter reviewed by other experts of the RIVM, Wageningen University & Research and by international experts from Belgium and the United Kingdom.
Scope of the environmental risk assessment (ERA)
This technical evaluation concerns the assessment of potential risks for human health and the environment of Saba as a consequence of the release of OX513A Aedes aegypti, in case this release may take place in the future. The main question therefore focusses on the potential adverse effects on the flora and fauna of Saba and on the health of human inhabitants of Saba, in comparison to effects caused by non-modified wild type Aedes aegypti. These effects are considered in the context of the local vector control program of Aedes aegypti on Saba. Potential effects of the release on the wider environment of Saba are also taken into account in this environmental risk assessment.
This evaluation does not consider the efficacy of the release of OX513A on Saba in suppressing local populations of wild type Aedes aegypti and the diseases they transmit, nor does this evaluation takes into account socio-economic aspects of this potential release.
Documentation sent to Saba and evaluation by the GMO Office
The documentation of Oxitec follows Annex III of the Cartagena Protocol on the Biosafety to the Convention on Biological Diversity (CBD, 2000)7. The evaluation by the GMO Office (RIVM) is based on the data and information included in Oxitec’s documentation, but also takes into account other relevant data such as scientific literature and published risk assessments of similar releases with the OX513A Aedes aegypti in other countries. The evaluation of the GMO Office as reflected in this document follows the structure as given in Oxitec’s documentation and conclusions of the GMO Office are indicated in bold.
Page 14 of 71
The Guidance document on the environmental risk assessment) of GM insects of the European Food Safety Authority (EFSA, 2013)2 was used to evaluate the documents of Oxitec. Other available guidance on the ERA of GM insects such as the Guidance on risk assessment of living modified organisms under the Cartagena Protocol (Part II on living modified mosquitoes) (CBD, 2000)7, the WHO guidance framework for testing of genetically modified mosquitoes (WHO, 2014)8 were also taken into account when relevant.
The ‘areas of concern’ as described in the EFSA Guidance for the ERA of GM insects were used to structure the ERA. These ‘areas of concern’ cover elements that are generally taken into account in the ERA of GMOs in most countries to assess potential adverse effects on human health and the environment of GMOs as a consequence of the genetic modification on a case-by-case basis.
The areas of concern for GM insects are:
• Persistence and invasiveness of GM insects, including vertical gene transfer
• Horizontal gene transfer
• Pathogens, infections and diseases
• Interactions of GM insects with target organisms • Interactions of GM insects with non-target organisms
• Environmental impact of the specific techniques used for the management of GM insects
• Impacts of GM insects on human and animal health
Evaluation of Oxitec’s documentation and review of this evaluation was carried out by experts of the RIVM, Wageningen University and Research in the Netherlands, the Biosafety and Biotechnology Unit (SBB), WIV-ISP in Belgium, Department for Environment, Food and Rural Affairs (DEFRA) in United Kingdom and the Saba Conservation Foundation on Saba (Annex 1).
Planned release
The planned release involves the repeated release of male (non-biting) Aedes aegypti mosquitoes (strain OX513A). This strain has been studied for over 12 years. Male OX513A mosquitoes mate with the wild type females of their own species only, leading to a reduction of the local population of Aedes aegypti. The mosquitoes are genetically modified so that their offspring dies before adulthood (as fourth larval instar or pupae). This is caused by the introduction of a repressible lethal genetic system based on the widely used tetracycline transcriptional activator (tTAV) into Aedes aegypti, which results in lethality of the offspring of OX513A males and wild type females in the absence of tetracycline (such as in the environment). When GM male Aedes aegypti mosquitoes mate with wild females in the field, offspring is produced of which over 95% express the lethality trait in the absence of tetracycline. The GM Aedes aegypti also contains a fluorescent marker gene, DsRed2, so that they can be identified and monitored.
The proposed repeated release is foreseen to take place in all inhabited parts of the island of Saba and aims to suppress the local population of Aedes aegypti to very low levels and potentially to elimination with demonstration of continued elimination for a period of a year thereafter. To demonstrate continued elimination, monitoring of the local population
of Aedes aegypti will take place until a year after the release has taken place. Earlier releases of OX513A mosquitoes have resulted in a clear reduction of local Aedes aegypti mosquito populations during the program. No other monitoring is foreseen.
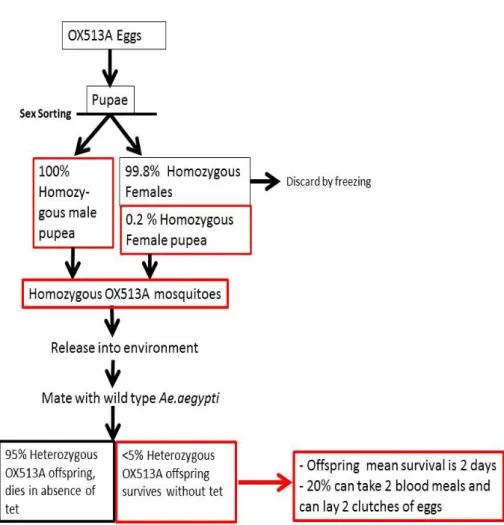
Specific aspects of the environmental risk assessment (see Figure 1) (1) Only male (non-biting) OX513A Aedes aegypti will be released in
the environment. Male pupae are selected by sorting. However, sorting of the pupae results in ≤0.2% of the pupae to be female. Therefore, the ERA takes into account that 0.2% of the released OX513A population is female.
(2) Trait penetrance (probability of a gene or genetic trait to be expressed) is 95%. After mating of OX513A with wild type Aedes aegypti the majority (95%) of the offspring will express the lethality trait and will die. About 5% of the offspring (male and female) will survive, also in the absence of tetracycline. This aspect is taken into account in the ERA.
Figure 1. Schematic representation of the process of sorting, environmental release and survival of male and female OX513A Aedes aegypti (drafted by the GMO Office).
Technical evaluation of Oxitec’s documentation
This technical evaluation summarizes relevant parts of Oxitec’s documentation and follows the same numbering is used in this documentation. Conclusions of the GMO Office are indicated in blue boxes. For further details, reports and references, the reader is referred to the full document and the additional information, as supplied by Oxitec, which can be accessed through the links below.
Submission to RIVM, September 28, 2016
http://www.rivm.nl/bibliotheek/rapporten/2017-0087_Submission Additional information November 30, 2016
http://www.rivm.nl/bibliotheek/rapporten/2017-0087_AI1 Additional information January 11, 2017
http://www.rivm.nl/bibliotheek/rapporten/2017-0087_AI2 Additional information January 11, 2017
http://www.rivm.nl/bibliotheek/rapporten/2017-0087_AI3 Additional information March 13, 2017
Part A Characterisation of OX513A
Molecular characterization
1 Recipient organism Aedes aegypti
The recipient organism is the Aedes (Stegomyia) aegypti (L.) mosquito that belongs to the genus Diptera: Family Culicidae, Genus Aedes. Aedes aegypti is a (sub)tropical species of mosquito found between 15oN and 15oS, typically in Africa and parts of South America but has not been reported to have a cosmopolitan habitat extending from 40oN and 40oS latitude. It is considered as an invasive species in several
jurisdictions.
Aedes aegypti originated from Africa and now has a worldwide distribution in all tropical and subtropical habitats.
Aedes aegypti is closely associated with human habitations. Breeding is tied to artificial water containers, such as potted plant holders, water tanks, tires, discarded plastic and metal containers such as soda cans, drains and roof guttering as well as ephemeral containers, such as puddles. Once eclosed, the adult Aedes aegypti mosquitoes live in and around houses where females have easy access to the blood meals necessary for egg development. The mosquito eggs are laid individually by females in the damp walls of both natural and artificial containers that can hold water. Eggs are the long-term survival structures of these mosquitoes, surviving, on average, up to 6 months. The larvae and pupae prefer relatively clean water typically found in containers such as water storage containers, flowerpots and waste materials such as tires, cans and bottles. In tropical countries waste material containers are year-round sources of mosquitoes. The duration of the larval stages is approximately 7-9 days and of pupae 2-3 days but these developmental rates are temperature dependent. The preferred sites for adults are domestic urban environments in sheltered dark spaces within houses/ apartments.
Aedes aegypti is a day biting mosquito with two peaks, one mid-morning and one mid-afternoon. Aedes aegypti bites humans and birds. The average adult lifespan for mosquitoes in nature (outside of the laboratory) is estimated to be 8-15 days.
Spontaneous flight of adults is limited to around 200m depending on availability of breeding sites, and hosts from which to take a blood meal. However the species can be dispersed by passive transport on boats, trains and modes of long distance transport. International Sanitary Regulations require ports and airports to be clear of Aedes aegypti up to 400m from the site.
Climate and the availability of breeding sites are the two main factors that regulate the populations of Aedes aegypti in urban environments. The effect of temperature on larval development of Aedes aegypti has been well studied, and has an ecological temperature range of 14-30oC, at which the larval development is a function of temperature.
Temperature also affects adult size, dry weight and ovariole number all of which fall as the temperature rises. Temperature at different altitudes
Page 20 of 71
is thought to affect distribution, with an elevation of 1800-2400 m likely to be limiting to the species and lower levels in temperate latitudes.
2 Donor organisms and general description of inserted genetic elements
The genetic elements in the recombinant DNA construct inserted in OX513A and their function, along with the origin of the DNA sequence are detailed in Table 1 of the documentation. Further information on each of the elements and the organisms where DNA sequences originated from is given below:
2.1 Trichoplusia ni (Cabbage looper moth)
The transposable element piggyBac, used as a carrier to introduce the intended genes into Aedes aegypti, was isolated from a cell culture of cabbage looper (Trichoplusia ni). Cabbage looper is a pest that feeds on the leaves of cruciferous plants but does not have any known toxic or pathogenic properties.
The piggyBac transposon is non-autonomous transposon that has been well studied and used to transform insects from a range of taxa:
Diptera, Lepidoptera, Coleoptera and Hymenoptera.
2.2 Drosophila melanogaster (Vinegar fly)
Several regulatory elements of the inserted DNA are derived from Drosophila melanogaster. D. melanogaster is not known to have toxic or allergenic properties. Due to their short generation time they make excellent model organisms for developmental biology and other
disciplines and have been well studied in laboratories for over a century.
2.3 Discosoma spp.
The DsRed marker gene is derived from Discosoma spp. Discosoma species are also known by their common name of mushroom corals and are found throughout many marine environments. Discosoma spp have particular fluorescence proteins that are similar to the green fluorescent protein (GFP) family of proteins. A mutation of DsRed enabled the generation of a close variant, DsRed2, which has improved expression and solubility, assisting its use as a marker. The fluorescent DsRed2 has been used extensively as a marker in a wide variety of organisms from viruses, to fungal species and mammals.
2.4 Escherichia coli*
The tTAV gene (coding for lethality) and the non-coding binding site for the tTAV protein are derived from Escherichia coli. This is an intensively studied bacterium which serves as a model organism across a range of disciplines. The E. coli strains used in the generation of the tetracycline-repressible system are all laboratory strains that are non-pathogenic.
2.5 Herpes simplex virus type 1*
VP16, used as a transcriptional activator of the tTAV gene, is derived from Herpes simplex virus type 1. Herpes simplex virus type 1 (HSV-1) is a human virus usually associated with infections of the lips, mouth, * In OX513A VP16 is used in a fusion protein with domains from E. coli and known as tTAV. Activating regions derived from the HSV-1 have been coupled to control elements derived from E. coli in order to develop the conditional lethal tetracycline-repressible transactivator element, tTA, widely used as the tet-repressible control system.
and face. It is the most common herpes simplex virus and many people develop it in childhood. VP16 is a virion phosphoprotein of HSV and a transcriptional activator of viral immediate-early (IE) genes and requires an acidic transcriptional domain. If absent, VP16 is impaired in its
capacity to support the infectious cycle.
2.6 Small synthetic linking sequences
Synthetic linking sequences are used to connect genetic elements within the construct. They do not have any function.
3 Plasmid used in the transformation of OX513A
The plasmid used is pOX513, containing the piggyBac transposable element. This transposable element is only capable of integrating into DNA flanked by an open reading frame (ORF) within the element when its inverted terminal repeats (ITRs) are intact. In the construct used for transformation the transposase gene of the piggyBac element was irreversibly destroyed by deletion of a section of that gene.
Transformation is done by using a helper plasmid that supplies the piggyBac transposase activity but that is in itself unable to transpose into other DNA. One of the ITR’s that flank the wild type piggyBac transposase has been removed in the helper plasmid so that the helper plasmid itself cannot integrate.
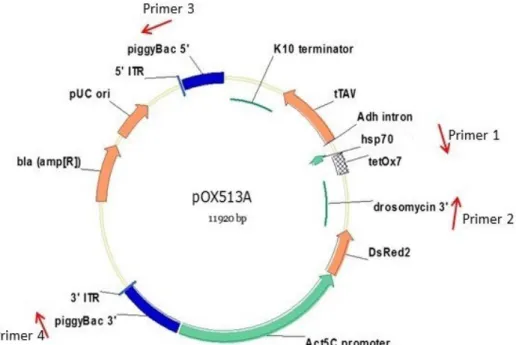
Transformation of Aedes aegypti to obtain OX513A was achieved through microinjection of individual eggs of the Rockefeller background strain. The microinjection consisted of the vector plasmid, pOX513 (Figure 2) co-injected with a piggyBac ‘helper plasmid’ as the source of piggyBac transposase. Once a stable transformed line of laboratory reared Aedes aegypti was identified it was made homozygous. The OX513A strain has been continuously maintained since 2002, representing over 115 generations.
After transformation the inserted DNA in strain OX513A was sequenced and proven to be identical to the sequence as present in plasmid
Page 22 of 71
Figure 2. Map of the plasmid used in the transformation of OX513A. Primer locations are a schematic representation intended to represent the general regions of the plasmid amplified as described in Section 4.1 of part A.
4 rDNA insert and characteristics of modification
4.1 Detecting the absence of plasmid backbone in transgenic lines
To check the absence of the vector backbone (including the bla gene and the pUC origin of replication) primers 3 and 4 were used to amplify a fragment of 4045 bp that contained the complete vector backbone (Figure 2). No signal was found in the genomic DNA of OX513A, whereas a positive signal was found in the pOX513A plasmid used to construct the strain. Proper controls were used to verify that the genomic DNA of strain OX513A was of sufficient quality for PCR. In addition, analysis of the flanking sequences of the insert indicated the absence of the vector backbone.
The absence of the bla gene in strain OX513A was demonstrated by Southern analysis, using the bla gene as a probe (additional info March 13, 2017). No signal could be detected in the OX513A strain, whereas the bla gene was detected in the pOX513A plasmid DNA and DNA obtained from an Aedes aegypti strain known to contain the vector backbone. The tTAV gene was detected in all samples except for the wild type sample, demonstrating the presence and integrity of the DNA used in the assay. These results demonstrate the absence of the vector backbone in OX513A.
4.2 Number of copies inserted and insert stability
Three restriction enzymes (AgeI, BglII, and SalI), each cutting once in the insert present in strain OX513A were used to digest genomic DNA of OX513A. Southern analysis using AC5/DsRed and tetR as a probe, demonstated the presence of one single insert. Moreover, analysis of the ratio of numbers of fluorescent progeny to non-fluorescent progeny, confirmed the expected Mendelian ratios for a single insertion.
OX513A has been maintained in continuous culture in the laboratory since 2002 (>115 generational equivalents) with no observation of genetic or phenotypic instability.
4.3 Verifying the insertion site and sequencing the regions flanking the gene
Inverse PCR was used to identify the genomic sequence adjacent to the insertion site of OX513A. DNA flanking sequences of 307bp and 315bp on either side of the insertion site were obtained. The Aedes aegypti genome has been fully sequenced, assembled, and annotated with respect to known genes, ESTs and transcripts. The combined flanking sequence of 622bp was compared with the Aedes aegypti genome sequence, transcript and EST databases using the BLAST tool on the Vectorbase website (www.vectorbase.org). Both Blastn and Blastx functions were used to compare the sequence in both orientations at the nucleotide level (Blastn) and translated sequence level in all 6 reading frames, to deposited amino-acid sequences (Blastx). The flanking sequence shows 94.6% identity across its entire length with a single genome sequence contig (1.859), showing an unambiguous match with this contig in Aedes aegypti genome sequence.
No homology to known open reading frames was identified, so no genes appear to be disrupted in Aedes aegypti by the insertion. In addition, results indicate the nearest gene/EST hit to be 30.5kb away and it is therefore unlikely to be affected by the insertion.
4.4 Nature of the inserted traits
Aedes aegypti OX513A is biologically similar with respect to its life-history characteristics to the non-modified populations of Aedes aegypti except for the introduction of two traits.
4.4.1 Fluorescent marker DsRed2
The fluorescent marker protein (DsRed2) enables the detection of OX513A in the field, and allows evaluation of the dissemination of OX513A Aedes aegypti and its genes resulting from the release of OX513A males. The DsRed2 is from Clontech Laboratories and was artificially developed from DsRed to enhance the fluorescence and improve the solubility, which in turn increases the sensitivity. In OX513A, there are three additional amino acids (MAR) at the N‐
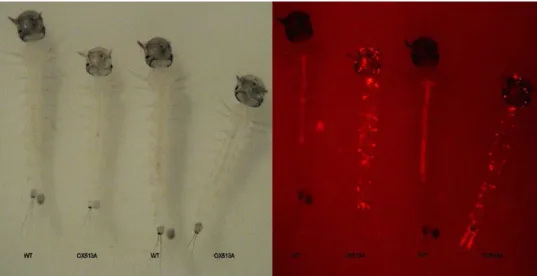
terminus, which are from a cloning linker sequence. The DsRed2 protein is expressed constitutively in the developmental stages (larva stage) of the OX513A mosquito and results in a fluorescent phenotype when viewed with diagnostic equipment (Figure 3).
Page 24 of 71
Figure 3. Expression of fluorescent marker (DsRed2) in OX513A Aedes aegypti under diagnostic fluorescence microscope. The fluorescent marker is strongly expressed in a characteristic punctate manner, allowing easy identification of OX513A individuals.
4.4.2 Self limiting trait tTAV
An insect-optimized tetracycline repressible transactivator protein (tTAV) is integrated into OX513A to produce a phenotype whereby the offspring after mating has increased mortality. tTAV acts as a
tetracycline-regulated switch (Figure 4) which confers conditional cell death and thus enables the mass rearing of the mosquito in the laboratory when
tetracycline is supplied.
Figure 4. Schematic representation of the tTAV system. In the absence of tetracycline (left panel), small amounts of tTAV protein generated by the effect of the hsp70 promoter (hsp70) can bind to the tetO binding sites (tetO7), creating a positive feedback loop that enhances expression of tTAV. When the tTAV protein accumulates in sufficient quantities it affects cellular function, resulting in cell death in the developing larvae. In the presence of tetracycline (right panel), tTAV is prevented from binding to the tetO sites and can therefore not enhance the expression from the hsp70 promoter. This prevents the
The self-limiting trait in OX513A works via a tTAV system (a variant of tTA) which elicits cell death. High-level expression of tTA is deleterious to cells as it can repress normal transcription. tTAV is a tTA variant sequence optimized for expression in D. melanogaster and other insects. tTA and its variants, such as tTAV, have been used in fungi, rodents, plants, and mammalian cultures.
4.5 Potential for toxicity and allergenicity of the introduced proteins
To assess whether the tTAV or DsRed2 protein produced by OX513A contain sequences that may be toxic or allergenic to humans or animals as a result of biting by genetically modified females or after (incidental) ingestion, bioinformatic analyses were conducted. Results do not
indicate any significant homology of the tTAV and DsRed2 protein sequences to known toxins and allergens. In addition, potential toxicity and allergenicity of the inserted proteins tTAV and DsRed2 was studied based on reviews of the scientific literature and other relevant studies with these proteins. Additionally, a literature search was performed on additional elements of the OX513A rDNA construct. None of these studies indicated any significant risk for human and animal health resulting from the newly expressed proteins in OX513A.
4.6 Conclusions regarding the molecular characterization of the insert in
OX513A
The GMO Office concludes that the molecular characterization of Aedes aegypti OX513A does not indicate any safety issues for human health, animal health and the environment.
This is based on the following observations:
• The sequence of the insert in OX513A is similar to the sequence in pOX513A, based on sequence similarity between the
introduced plasmid and the insert in OX513A
• OX513A does not contain vector backbone sequences from the plasmid used for transformation, based on PCR and Southern analysis
• The insertion does not disrupt endogenous gene functions in OX513A as demonstrated by sequencing of the flanking regions and subsequent sequence comparison with Aedes aegypti genome sequence
• The insert consists of an intact single copy, as demonstrated by Southern analysis and Mendelian inheritance
• The inserted sequences has been shown to be stable over many generations
• No sequences have been inserted that code for toxins or allergens as evidenced by bioinformatic analyses and literature searches
5 Further characterisation of OX513A
5.1 Life table parameters
Several life table parameters have been examined in the laboratory for the OX513A strain in comparison with its non-modified Aedes aegypti comparator strain: larval mortality, developmental rate (i.e., time to pupation), adult size, and longevity. Larvae were grown in the presence of tetracycline. Only two statistically significant differences were found:
Page 26 of 71
the OX513A larval survival was 5% lower than that of the non-modified strain and there was a reduced longevity of OX513A.
OX513A was also compared to laboratory reared Aedes aegypti of wild origin (wild caught) originating from two regions of India. They did not significantly differ with respect to the following parameters under laboratory conditions: blood meals per female; oviposition events per female; eggs laid per female; hatch rate; pupation rate and adult emergence. In these tests only developmental time from first instar to adult emergence was slightly longer for OX513A than for wild type. 5.1.1 Reproductive capacity
Several parameters regarding reproductive capacity have been measured for both the non-modified and the OX513A strain in two independent laboratory studies. These studies indicate that only minor life table differences of limited biological relevance exist between OX513A and wild type Aedes aegypti populations.
5.1.2 Insemination capacity
The insemination capacity of males (i.e., the number of females a male is capable of inseminating over the course of his lifetime) of a non-modified strain of Malaysian origin and OX513A was evaluated. Results show that OX513A males inseminated just over half as many females compared to the wild type males during their lifetime, indicating a slight fitness penalty in OX513A compared to the non-modified strain.
5.1.3 Mating competitiveness
Extensive testing of the OX513A strain mating competitiveness in a range of environments has been carried out. This includes studies in laboratory cages and in regulated environmental releases in the Cayman Islands and Brazil. A summary of the results is given below.
Mating competitiveness in the laboratory 5.1.3.1
Mating competitiveness studies for OX513A against wild‐type strains from around the world have been carried out in a wide variety of international laboratories. The OX513A strain performed successfully against all the Aedes aegypti wild type strains tested regardless of the genetic background.
Mating competitiveness in semi field conditions 5.1.3.2
Mating competitiveness in a purpose-built field house in Kuala Lumpur (Malaysia) showed that approximately 50% of the OX513A males found mates. This is equivalent to a fully competitive strain.
Mating competitiveness in regulated environmental releases 5.1.3.3
Regulated environmental releases resulted in mating competitiveness estimates of 0.56 (Cayman Islands) to 0.0004 to 0.059 (Brazil),
indicating that mating competitiveness depends on many factors such as environmental factors, timing of release and immigration of wild Aedes aegypti.
5.2 Response to abiotic factors
5.2.1 Temperature response of OX513A
The temperature response of heterozygous OX513A has been evaluated in the laboratory in order to determine:
• If the penetrance of the phenotype of OX513A heterozygotes varies when reared at temperatures different than that the laboratory standard (penetrance is the probability of a gene or genetic trait being expressed)
• If OX513A has altered survival at temperatures outside of Aedes aegypti’s natural range.
Experiments in the absence of tetracycline demonstrated that
penetrance of the phenotype is independent of the temperature and that survival rates of OX513A and the wild type strain are similar at different temperatures, also outside the natural temperature range of Aedes aegypti.
5.2.2 Dose response to tetracycline and its analogues
The response of OX513A heterozygous larvae to different doses of tetracycline, chlortetracycline, oxytetracycline, and doxycycline has been evaluated to identify the lowest concentrations which allow for greater survival of heterozygous OX513A as compared to larvae reared in the absence of tetracycline or its analogues.
The experiments show that concentrations at and below 3 ng/mL tetracycline, 1 ng/mL chlortetracycline, 10 ng/mL oxytetracycline and 0.1 ng/mL doxycycline do not increase survival of OX513A larvae, i.e. do not increase the proportion of functional adults. These levels are higher than the mean published concentrations of each analogue found in environmental bodies of water.
5.2.3 Susceptibility to chemical insecticides
OX513A and its non-modified wild type strain with similar background were found to be equally susceptible to four commonly used insecticides (temephos, permethrin, deltamethrin and malathion) and showed similar significant survival to bendiocarb. Two kdr mutations associated with pyrethroid and DDT resistance were absent in the OX513A strain. 5.2.4 Behavioral responses of OX513A to insecticides
Behavioral responses of OX513A male mosquitoes were overall similar to those displayed by the wild type strain, including significant contact irritancy to pyrethroids and significant spatial repellence to DDT. 5.2.5 Tetracycline loaded blood study
It was demonstrated that the penetrance of the OX513A phenotype in hemizygous offspring of female Aedes aegypti is not influenced by the presence of tetracycline in blood. This indicates that tetracycline present in human or animal blood, for example as a consequence of antibiotic treatment, would not affect the percentage of OX513A offspring that expresses the conditional lethality trait.
5.2.6 Trait penetrance
Penetrance is the probability of a gene or genetic trait being expressed. Under laboratory conditions the observed penetrance of the self-limiting trait in OX513A is always found to be over 95%. This means that less than 5% of the offspring of a cross between OX513A males and wild
Page 28 of 71
type Aedes aegypti females will survive if reared without tetracycline in the rearing water or the environment.
5.2.7 Non-penetrant OX513A progeny: longevity and fecundity
The survival and fecundity (fertility) of the small observed proportion (<5%) surviving heterozygous OX513A offspring has been investigated in the laboratory.
Median survival of both OX513A males and females in the absence of tetracycline was 2 days compared to the non-modified males and females that have a much higher median survival under laboratory conditions. In contrast to the wild type, substantial mortality was observed in the surviving OX513A progeny. A small fraction (~20%) does survive long enough to take two blood meals and some produced two clutches of eggs. The OX513A strain lays a statistically significant larger egg clutch (69,9) during the first gonotrophic cycle, compared to the wild type (54,8). Hatching rate is somewhat higher for OX513A eggs, but this is not statistically significant.
5.2.8 Field penetrance
Penetrance of the trait in the field was studied in the Cayman Islands (East End) and in two release sites in Brazil (Itaberaba and Mandacaru). Overall estimates of the percentage of incomplete penetrance ranged from 0-4.28%, falling below the ~ 5% reported in laboratory studies.
5.3 Dispersal and longevity - regulated environmental releases of OX513A
In a typical Aedes aegypti urban habitat in Brazil, the mean
dissemination of OX513A genes (as determined from eggs in ovitraps) was estimated to be 64 m and 79 m from the place where the
mosquitoes were released for the two periods evaluated, which corresponds with the dispersal of OX513A males and the wild type comparator strain observed at the same site.
In Malaysia, in an uninhabited forested area, maximum dispersal distances of OX531A and the wild type comparator strain were similar (220 m), but mean distance travelled by the OX513A strain was lower (52 vs. 100 m). Life expectancy of the OX513A males and the wild type comparator strain used in this study in Malaysia was similar (2.0 vs. 2.2 days).
In the Cayman Islands results demonstrated an average life expectancy of OX513A ranging between 0.1 to 1.6 days. No wild type comparator was released in this study.
This indicates that dispersal rates and longevity of OX513A are not greater than that the wild type.
5.4 Potential toxicity - oral exposure studies
5.4.1 Toxorhynchites spp (predatory mosquito)
Toxorhynchites larvae feed on small aquatic organisms. No significant impact was found on the development, fecundity and longevity of two predatory Toxorhynchites species (Tx. amboinensis and splendes) that were fed exclusively with OX513A larvae, compared to the same species that were fed with a diet consisting of wild type larvae. These results indicate no toxicity as a consequence of the genetic modification.
5.4.2 Poecilia reticulate (guppy fish)
To determine the effects of OX513A on the guppy fish Poecilia reticulate, a 14-day feeding study was performed. No significant differences were observed with regard to mortality, fish length, weight, appearance and behavior between fish fed with a diet of OX513A and fish fed with a diet of wild type Aedes aegypti. These results indicate no toxicity as a consequence of the genetic modification.
5.5 OX513A morphology
There are no genes introduced into the male mosquitoes that are intended to alter the morphology of the insect and no morphological differences between OX513A males and the wild type males have been observed.
5.6 Analysis of expression of the introduced proteins in female mosquito
saliva
An analysis was conducted to detect the presence of the tTAV and DsRed2 proteins in OX513A female mosquito saliva using Western blot analysis. Using the proper controls, the tTAV and DsRed2 proteins could not be detected in female OX513A saliva.
5.7 Vertical transmission of dengue and chikungunya viruses in OX513A
The passage of a disease causing agent or pathogen from an infected female to its offspring is known as vertical transmission. To assess whether OX513A females were more competent for vertical transmission of dengue and chikungunya viruses than females from a wild type strain, vertical transmission of dengue (serotypes 1-4) and chikungunya viruses in OX513A females and a wild type comparator strain have been
evaluated.
No differences were found between the homozygous OX513A strain and the non-modified comparator strain, nor were there significant
differences in fecundity observed.
5.8 Stability of the insert in OX513A
See section 4.2 of part A. 5.8.1 OX513A quality control
Regular strain integrity quality control assays are carried out on the OX513A colony with respect to:
• Colony genotyping;
• Penetrance, stability and tetracycline dose response; • Mating competitiveness.
5.9 Conclusions regarding the phenotypic characterization of OX513A
The GMO Office confirms conclusions of Oxitec that no phenotypic changes were detected in OX513A relative to the comparator(s) that suggest unintended effects as a consequence of the genetic
modification with respect to:
• Life table parameters, including mating competitiveness • Response to abiotic factors including temperature and
insecticides
• Adult dispersal and longevity
• Potential toxicity by oral exposure studies
Page 30 of 71 following:
• Mean reported concentrations of tetracycline and its analogues in water are well under the concentrations necessary to allow phenotype rescue in OX513A
• Trait penetrance in progeny produced by male OX513A and wild type females is observed to be around 95% or higher, both under laboratory conditions as under field conditions. This means that less than 5% of the offspring will survive in the absence of tetracycline and thus in the environment
• About 20 % of this surviving progeny is able to survive for about 2 days, long enough for the females to take two blood meals and produce two clutches of eggs
• Vertical transmission of DENV and CHIKV is not changed in OX513A
• Female OX513A do not contain tTAV and DsRed2 proteins in the saliva
6 Detection and identification of OX513A
6.1 Methods and sensitivity for detecting OX513A Aedes aegypti in the
environment
There are two primary detection methods available: fluorescence-based detection and DNA sequence-based detection.
Fluorescence-based detection is possible by microscopic detection of fluorescent larvae and pupae as a consequence of the production of the DsRed2 protein in OX513A. This marker has proven to be stable over 115 generation equivalents since 2002. This marker is not visible in eggs and adults of Aedes aegypti OX513A. Eggs of OX513A are
therefore required to be hatched under controlled laboratory conditions for visualization of the DsRed2 marker gene expression (additional information November 30, 2016).
DNA sequence-based detection visualizes a PCR-amplified unique
genetic fragment within OX513A, based on the genomic regions flanking the insertion site. Adults require molecular analysis by PCR to detect whether they are genetically modified or not.
6.2 Monitoring the Aedes aegypti population in the environment
The trapping methods used in an OX513A program to monitor Aedes mosquitoes are well established. There are two principle trapping methods used either in combination, or in isolation depending on the phase of the program and the intent of the monitoring.
Ovitrap (egg catch) surveys are the principal monitoring tool deployed, mimicking natural breeding sites in which females lay eggs and have been adopted as a standard monitoring tool for Aedes aegypti
populations. The World Health Organization (WHO) recommends use of ovitraps for Aedes aegypti surveillance.
The BG-Sentinel adult trap was developed specifically targeting Aedes (aegypti and albopictus) mosquitoes. These traps use a combination of visual and host mimic olfactory cues to attract adult (male and female) Aedes mosquitoes that are captured in a suction trap.
7 Regulated environmental releases of OX513A
7.1 Previous Aedes aegypti vector control projects using OX513A
Regulated environmental releases of OX513A males have been
conducted since late 2009 in collaboration with partners in both vector control programs and academia.
Releases in 2009 in Grand Cayman served principally to demonstrate that released OX513A males could successfully compete with wild males in their natural urban setting and mating is not compromised by
insertion of the OX513 recombinant DNA (rDNA) construct. Additionally, in Malaysia the 2010 releases also served to demonstrate that the insertion of the rDNA construct has not altered the dispersal range of in the OX513A strain. Subsequent releases of OX513A conducted to date in Cayman Islands, Brazil and Panama demonstrated the efficacy of the release of the OX513A strain in the context of a vector control program. In sections 7.1.1 to 7.1.5 of part A of the documentation of Oxitec descriptions are given of the releases in Cayman islands, Brazil and Panama including the number of OX513A introduced and the level of suppression of local Aedes aegypti populations. These are not reported here, since they are not considered to be relevant for the environmental risk assessment.
7.1.5.1 Environmental persistence
In Panama a post release environmental monitoring survey was performed to assess the persistence of the OX513 genetic construct in the environment. Final releases of OX513A took place on 31 October 2014. Environmental monitoring in control and release sites continued 138 days post-release through a network of 60 ovitraps and
fluorescence screening of larvae for the DsRed2 marker. Over 20,000 Aedes aegypti larvae where individually screened. Prior to the final release, OX513A fluorescent larvae comprised 100% of the trapped larvae in the treated area, 25 days post-release it was 5%, 84 days post-release it was 0%. Although the absence of OX513A genes could only be confirmed from 12 weeks post-release onward due to a disruption in data collection, the available data suggest that OX513A genes are unlikely to occur in the environment 6-8 weeks post-release. 7.1.1 Conclusions drawn from previous vector control projects using OX513A The GMO Office confirms the conclusions of Oxitec with respect to data supplied on environmental releases in Cayman Islands, Malaysia, Brazil and Panama performed since 2009 on:
• mating competitiveness • adult dispersal and longevity
• dissemination and persistence of OX513A genes into the environment
The GMO Office also remarks that no unintended effects on human animal health and the environment have been observed in any of the releases. However, there was no dedicated environmental monitoring in place during these releases.
Part B
Intended use of OX513A Aedes aegypti on Saba
1 Details of the proposed release on Saba General overview
Eggs are shipped in regular shipments throughout the course of the program to the facility on Saba near the release site where they are reared to obtain pupae, sex sorted to select male pupae and where the males are matured to adults for release. Sexually mature OX513A males are released from specialized release devices in a grid-like pattern from predefined release points to ensure even coverage of the area.
OX513A integrated into the Latin-American wildtype will be used for the release on Saba. The Latin-American strain used is the lead strain subject to regular quality testing as described in the Standard Operating Procedures (SOPs) and is used in field programs around the world. There is a high degree of confidence of the genetic integrity of the insert in this particular background strain and its mating competitiveness (additional information November 30, 2016).
The OX513A program can be divided into three sequential phases: (1) a preparation phase, (2) an intervention phase and (3) a maintenance phase. Release rates are informed by ongoing monitoring and evaluation of various metrics.
1.1 Anticipated outcomes for Saba Island
The proposed OX513A Aedes aegypti suppression program on Saba aims to suppress the local population of Aedes aegypti to very low levels and potentially to elimination with demonstration of continued elimination for a period of a year thereafter
Aedes aegypti is considered an invasive pest on Saba.
Sustained releases of OX513A on Saba aim to have several measurable effects on the wild Aedes aegypti population, including, in temporal order:
1. An increase of the overall male-to-female ratio for Aedes aegypti; 2. Wild type Aedes aegypti females mating with OX513A males; 3. Suppression of the target Aedes aegypti population leading to
potential elimination of Aedes aegypti on the island;
4. Continued elimination of Aedes aegypti for a period of one year. The OX513A program proceeds with an ongoing evaluation of the population dynamics of the wild Aedes aegypti population, and release rates are adapted based on monitoring throughout the release period as control/elimination is achieved. Once control is gained and the island is effectively free from wild Aedes aegypti, continued close monitoring and low level releases of OX513A at points at risk of potential re-introduction will be required to maintain the wild Aedes aegypti free status.
1.2 Location of releases
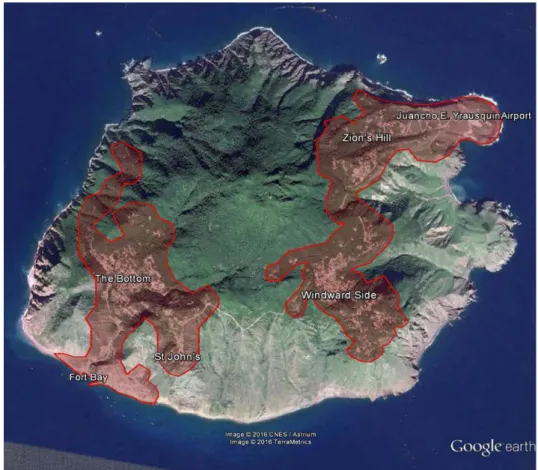
The proposed release on Saba is island wide throughout the four principle human populated areas of The Bottom, Windward side, Zion’s
Page 34 of 71
Hill and St. Johns, and any minor inhabited areas in between (Figure 5). Releases will also take place in the areas of the port at Fort Bay Harbor, and Juancho E. Yrausquin Airport. Releases of male OX513A are done typically up to three times a week at predetermined geo-referenced locations generally not more that 100m apart to ensure coverage of the release area.
The island may be subdivided into areas with different release rates depending on the heterogeneity of inhabited areas and corresponding Aedes aegypti infestation but the broad areas of release will be aligned with road patterns in the predicted habitat of Aedes aegypti on Saba (i.e. the areas inhabited by humans) as illustrated in Figure 5 below.
Figure 5. Principle habitat of Aedes aegypti on Saba represented as urban areas including an approximately 100m distance from human habitation (shaded in red). Total shaded area is equivalent to approximately 3.7 km2.
1.3 Determination of release rates - phased approach
As described earlier, OX513A releases are administered in a phased program and release rates are informed by ongoing monitoring and evaluation of various metrics.
1.3.1 Preparation phase
This phase will be used to evaluate the initial densities of Aedes aegypti mosquito populations in the treatment areas and optimize the OX513A rearing methodology to local conditions on Saba. Initial densities are monitored by ovitraps and adult trapping methods as well as using best available information including historical surveillance data, seasonality,
epidemiology records, existing mosquito abatement and qualitative factors such as housing type and proliferation of breeding sites. Based on the densities of wild Aedes aegypti determined, an initial release rate of the OX513A males is chosen. An initial release rate (IRR), is typically between 100 and 300 male OX513A per person per week in most projects to date.
1.3.2 Intervention Phase
Mosquito release and dispersal 1.3.2.1
During the intervention phase, OX513A is released in a systematic manner from a predetermined georeferenced grid of release points at regular time intervals, for even and consistent coverage of the
treatment area. Release points will be spaced no more than 100m apart, and releases will occur up to 3 times per week. In past projects,
substantial suppression was observed 4-6 months following initiation of releases, but depending on local conditions and mosquito densities, this could be up to 12 months.
The key factor governing the rate of Aedes aegypti population reduction is the fraction of local wild Aedes aegypti females mating with released OX513A: this mating should be in a greater proportion with OX513A than with local wild Aedes aegypti males.
The release rate necessary to achieve a given mating fraction is proportional to the local Aedes aegypti population. The greater the OX513A male: local Aedes aegypti male ratio achieved, the greater the mating fraction and likelihood of having an impact on local Aedes aegypti population.
Adaptive management of release rate 1.3.2.2
Release rates are dynamically adjusted in response to local Aedes aegypti populations which are expected to fall during the period of the intervention. OX513A male releases will continue as the local Aedes aegypti population is suppressed, but at a reduced level chosen to maintain the mating faction at >0.5. The release rates will be evaluated every 6-8 weeks during the suppression phase and adjusted based on the mating fraction.
Significant suppression of the local Aedes aegypti population is typically expected within 4-6 months of initiation of releases. However, to
achieve effective elimination of Aedes aegypti from Saba it is expected to take longer and may tend towards 12 months.
1.3.3 Maintenance Phase
When the local Aedes aegypti population drops sufficiently to
demonstrate convincing suppression or elimination, the program enters a maintenance Phase, where releases and monitoring will continue, but without estimating the mating fraction. Resurgence of the local Aedes aegypti population, as monitored by ovitraps, will trigger an increase in release rates.
Resurgence is categorized as 4 consecutive weeks with an ovitrap index >10 %. An ovitrap index defined as the traps in which one or more eggs confirmed as Aedes aegypti is found, divided by the total amount of traps. Once the wild Aedes aegypti population has been effectively suppressed (<10% ovitrap index) the program enters the maintenance phase designed to stop resurgence of Aedes aegypti population and to sustain the attained goals. This approach can be applied to contiguous
Page 36 of 71
subareas within the program as they become well controlled, even while other areas remain in the intervention phase. Resurgence could arise from small pockets of residual Aedes aegypti populations, egg bank and/or immigration.
The maintenance phase protocol is similar to that used in the intervention phase – with releases and/or use of other vector
management tools being planned and targeted based on monitoring data. The areas of high risk and the loci of re-infestation will be principally the transport hubs at the port at Fort Bay Harbor and Juancho E. Yrausquin Airport, which will be further characterized through the monitoring during the intervention phase. These areas are at a minimum proposed for sustained releases on an ongoing basis, with program objectives assessed annually in consultation with the Saba Public Health officials and appropriate government and other
stakeholders.
In the remaining areas, treatment is anticipated to stop as the Aedes aegypti population is eliminated, and be subject to ongoing monitoring, with targeted OX513A releases if reinfestation is detected.
Estimated numbers of OX513A Aedes aegypti to be released on Saba Estimates for release numbers in the context of the proposed Saba project can be made with a reasonably high degree of confidence based on experience in suppression projects to date (add. info. November 30, 2016).
Taken on average over the first 12 months, releases are anticipated to average 160 OX513A males/person/week. For a population of
approximately 1800 on Saba this would mean that up to approximately 15 million OX513A males would be released during the initial 12-month period. After the first 12 months, averaged over the whole island, the release rates are not expected to exceed on average 50 OX513A males/person/week. This would equate to up to approximately 4.7 million OX513A males during the subsequent 12 months (additional information November 30, 2016).
1.4 Containment measures prior to release
Eggs of OX513A will be produced in the UK under the Genetically Modified Organisms (Contained Use) Regulation 2014 and the
production is handled under Containment Level 1 (CL1) conditions. Upon export to Saba, OX513A eggs (amounts to be stated in a separate advanced notification) will be packaged in three layers of shatter-proof containment, and shipped by air by a commercial courier service transiting through St. Maarten.
1.4.1 Mobile Rearing Unit Overview
OX513A will be imported to a locally established Mobile Rearing Unit (MRU). MRUs are insect production laboratories fabricated within standard shipping containers which conform with relevant ISO
(International Organization for Standardization) standards for shipping containers, and are adapted as mobile insectaries that provide
compliance up to and including ACL2 (Arthropod Containment Level 22). Standard Operating Procedures (SOPs) are provided that detail all measures that are relevant to containment.