Biology Department
Research Group Evolution and Optics of Nanostructures
Angular dependency of heat load in
iridescent feathers
Debruyn Gerben
Student number: 0181149Supervisor(s): Prof. Dr. Matthew Shawkey Dr. Liliana D’Alba
Scientific tutor: Svana Rogalla
Master’s dissertation submitted to obtain the degree of Master of Science in Biology Academic year: 2019 – 2020
© Faculty of Sciences – research group Evolution and Optics of Nanostructures
All rights reserved. This thesis contains confidential information and confidential research results that are property to the UGent. The contents of this master thesis may under no circumstances be made public, nor complete or partial, without the explicit and preceding permission of the UGent representative, i.e. the supervisor. The thesis may under no circumstances be copied or duplicated in any form, unless permission granted in written form. Any violation of the confidential nature of this thesis may impose irreparable damage to the UGent. In case of a dispute that may arise within the context of this declaration, the Judicial Court of Gent only is competent to be notified.
Table of contents
1. Introduction ... 5
2. Objectives ... 11
3. Materials and methods ... 13
3.1. Sample collection and preparation ... 13
3.2. Measurements ... 14
3.2.1. Characterization of feather reflectance ... 14
3.2.2. Quantifying the heat load of feathers ... 16
3.2.3. Importance of feather structure on reflectance and heating load ... 17
3.3. Statistical analysis ... 18
3.3.1. Characterizing feather reflectance ... 18
3.3.2. Quantifying the heating load of feathers ... 19
3.3.3. Importance of feather structure to reflectance and heating load ... 19
4. Results ... 20
4.1.1. Characterizing feather reflectance ... 20
4.1.2. Quantifying the heating load of feathers ... 24
4.1.3. Importance of feather structure on reflectance and heating load ... 25
5. Discussion ... 29 6. Conclusion ... 33 7. Summary ... 34 8. Samenvatting ... 37 9. Acknowledgements ... 40 10. References ... 41 11. Appendices ... 46
5
1. Introduction
Animal coloration is extremely diverse. It is variable among different groups, families, species, individuals and sexes. The extreme variety in coloration in nature can be explained by their underlying function. Many functions often match the diversity in color, for example, colors can serve as camouflage as seen in nightjars, whose rufous coloration matches their background while roosting or breeding (Stevens et al., 2017) (Fig. 1F). Coloration as sexual signaling can help in mate choice as seen in guppies (Poecilia reticulata) (Houde, 1997) or as a status-signal (Tarsiger cyanurus) used in male-male interaction as in the plumage coloration of the red-flanked bluetail (Morimoto et al., 2006). More functions of coloration are warning, luring and schooling (Eaton & Cott, 1940). Different phenotypes can be maintained through some form of balancing selection (Roulin, 2004; Wright, 1930) alternative behavioral strategies (B. Sinervo, 2001; B. Sinervo & Lively, 1996) or the exploitation of different ecological niches (Roulin, 2004). Because they are so striking, researchers often focus on the visual functions of color. Especially the role in sexual signaling (Dreher & Pröhl, 2014; Morimoto et al., 2006; Pryke & Griffith, 2006) and camouflage (Chiao et al., 2011; Stevens et al., 2017) has been well studied. However, over the past decades there has been a growing interest in the non-signaling functions of coloration, for example as mechanical support of feathers (Bonser, 1995), protection against bacterial degradation (Burtt et al., 2011) or thermoregulation (Rogalla et al., 2019; Smith et al., 2016) Still, knowledge on the non-signaling functions of animal coloration and their underlying color creating mechanisms is limited. For example, how differently colored tissues absorb and radiate thermal energy is still largely unexplored. Dark materials can have either higher or lower heat loads compared to light materials (Burtt, 1981; Walsberg et al., 1978). In addition, the research that has been conducted so far, considers almost exclusively the electromagnetic wavelengths that are visible to animals (300-700nm). Yet, we know that there is a great variation in the capabilities to perceive these wavelengths across different species (Bowmaker, 2008). Also, the visible spectrum only represents a small portion of the sun’s radiation that reaches the Earth’s surface (Grace & Gates, 1982). Over half of this radiation is in the near infrared (NIR: 701-2000nm) and it is these wavelengths that have great potential to have significant thermal effects (Christian et al., 1996; Cowles & Bogert, 1944).
Most of the studies investigating the thermal effects of coloration have been performed on ectotherms, which derive their body heat from the environment (Bale & Davenport, 1992). Maintaining optimal body temperature is primarily achieved by behavioral adjustments, such as shifts to favorable thermal habitats (Barry Sinervo et al., 2010; Tattersall & Cadena, 2010). Color change is very often observed in these organisms (e.g. reptiles, amphibians, fish and invertebrates).
6 Dynamic integumentary coloration can happen for many reasons like camouflage (Chiao et al., 2011), communication (Dubuc et al., 2009; Yoshida et al., 2008). It is especially well documented in anuran amphibians (Fingerman, 1963; Parker, 1943). Where it allows the animal to adapt its color to that if the substrate to escape detection by visually hunting predators (Norris & Lowe, 1964). The use of cryptic coloration to escape predation is seen in many different animals (Cook et al., 2012; Kettlewell, 1955; Reimchen, 1979). However, many ectotherms are also able to rapidly change their coloration to modify their body temperature (Cowles & Bogert, 1944; Huey & Slatkin, 1976). Recent study shows that bearded dragons (Pogona vitticeps) will change coloration to improve both thermoregulation and camouflage (Smith et al., 2016). In general, darker individuals absorb more solar radiation when compared to lighter individuals (Clusella Trullas et al., 2007; Grace & Gates, 1982).
Endotherms (e.g. bird and mammals) differ in many aspects compared to ectotherms. Their metabolic processes are primarily responsible for the production and conservation of heat and physiological responses regulate the heat fluxes to the environment (Smit & Mckechnie, 2015; Tattersall & Cadena, 2010). For birds, it has been hypothesized that solar radiation is absorbed at the feather surface. Feathers would operate as insulation that prevents heat transmission to the skin (Dawson & Maloney, 2004). However, the degree of penetration of solar radiation through bird plumage has only been quantified in a couple of studies and only in one of those studies was the effect of feather coloration considered (Burtt et al., 2011). Light transmittance through feathers is also likely to vary extensively in relation to feather structure and environmental conditions. It has also been observed that birds shift position during periods of high environmental temperatures and prefer shaded areas over areas in direct sunlight (Pattinson & Smit, 2017; Xie et al., 2017).
Plumage coloration could possibly have significant thermal effects in birds. Colored tissues differentially reflect or absorb solar radiation that can directly affect feather surface temperature and thus determine the amount of radiative heat that can reach the bird’s skin (Wolf & Walsberg, 1996). A recent study shows that Carotenoid-based colors show the greatest reflectivity from 300-2100 nm, followed by non-iridescent and iridescent melanin-based colors (Shawkey & D’Alba, 2017). Even though iridescent (i.e. change color with angle of observation or measurement) feathers seemed, to the naked eye, more reflective in the visible range (300-700nm) than non-iridescent melanin based colors. This strongly suggests that not only the dominant hue but also the underlying color producing mechanisms as well as the orientation of feathers in relation to the light source and angle of observation strongly affects the reflectivity.
7
A) B) C)
D) E) F)
G) H) I)
Figure 1: Images showing the diversity of color and color creating mechanisms in birds (class Aves). Iridescent coloration in (A) indian peafowl (Pavo cristatus), (B) annas hummingbird (Calypte anna), (D) wild turkey (Meleagris gallopavo), (G) lawes’s parotia (Parotia lawesii), (I) satin bowerbird (Ptilonorhunchus violaceus). Non-iridescent structural coloration in (E) blue jay (Cyanocitta cristata). Pigmentary coloration: carotenoids in (H) northern cardinal (Cardinalis cardinalis), Psittacofulvin in (B) pesquet parrot (Psittrichas fulgidus) and melanosomes in (F) european nightjar (Caprimulgus europaeus). Photocredits (A )jvphoto, (B) TheCornellLab, (C) Greg Hume, (D) Riki7 (Wikipedia) , (E) Jongsun Lee, (F) Anonymous , (G) Worldlifeexpectancy, (H) Mark Nale , (I) Jurgen Otto.
8 Coloration in birds is created by two main mechanisms (Fig. 1, Table 1). One color producing mechanism, pigmentary coloration, is produced by the deposition of pigments in the integument that interacts with light on a molecular level. The pigments selectively absorb, reflect and transmit wavelengths. Thus, the hue is determined by the pigment’s molecular structure and its concentration within the integument. Pigments can either be metabolized (endogenous) or have to be acquired through dietary pathways (exogenous). Carotenoids are an example of exogenous bio-pigments. After acquisition through dietary pathways they are stored in the feather follicle (Brush, 1990). Carotenoids are strongly wavelength dependent and absorb light in the violet-blue region between 400-500nm (Shawkey & Hill, 2005). This absorption range results in red, orange and yellow coloration in birds plumages as we see in cardinals (family Cardinalidae, Fig. 1H) (Linville & Breitwisch, 1997). Psittacofulvin and Turacin pigments are similar to carotenoids. They have narrow-band absorbance spectra and are responsible for the red, orange and yellow colors in respectively parrots (order Psittaciformes, Fig. 1C) (McGraw & Nogare, 2004) and turacos (family Musophagidae) (Toral et al., 2008).
Another important pigment for color production is melanin (Figure 1, Table 1). Melanins are the most ubiquitous pigments found in nature (Solano, 2014), they are products of the melanogenesis pathway and thus not dependent on diet. Melanin is produced within melanosomes by specialized cells called melanocytes (Riley, 1980). Two distinct type of melanins are found within the integument of birds: eumelanin which results in black, grey, and brownish colors and pheomelanin which gives rise to more yellow and reddish colorations. Melanins found in feathers are often a mixture of both, however the relative amount of each pigment can differ between species (McGraw & Nogare, 2004). In contrast to other pigments, melanins are not wavelength dependent, and their absorption varies relatively little across the entire spectrum. They absorb 2-3 times as much light compared to carotenoids (Sarna & Swartz, 2007). More importantly, melanosomes are able to create a great diversity of coloration because of their high refractive index and the fact that, as independent particles, they can form arrays of organized structures within the surrounding feather keratin (Durrer, 1986).
This arrangement of structures within the integument constitutes a second category of color producing mechanism, structural coloration and is responsible for some of the most striking colorations in nature (Raman & Jayaraman, 1953; Snow et al., 2004; Wilts et al., 2014). In contrast to pigmentary coloration, structural coloration does not rely on the absorption of wavelengths, rather on the interaction of light with the structure of the material that it collides with (Prum, 2006). The most common structural color mechanisms are film interference, diffraction gratings, scattering and photonic crystals (Kinoshita et al., 2008; A. R. Parker, 2000; Seago et al., 2009; Srinivasarao, 1999).
9 First, film interference is most common in nature, it includes both thin-film interference as seen in satin bowerbirds (Ptilonorhunchus violaceus, Fig. 1I) (Doucet et al., 2006) and multilayer interference which is found in the breast-plate plumage of male Lawes’ parotia birds (Parotia lawesii, Fig. 1G) (Stavenga et al., 2011) . Second, diffraction gratings mechanisms are widespread in invertebrates (A. R. Parker, 2000), for example the visually striking butterfly genus Morpho (Tamáska et al., 2013) and present since at least 100 million years ago in hexapods (D’Alba et al., 2019). Third, scattering can be split in incoherent scattering which includes the blue hue of the sky (Prum & Torres, 2003) and coherent scattering (Fig. A8), which is responsible for most structural coloration in nature and is seen in the tail feathers of a peacock (Pavo cristatus, Fig. 1A) (Bagnara, 1977), the black coloration of a jungle crow (Corvus macrorhynchos) (Lee et al., 2010) and the blue coloration of a blue jay (Cyanocitta cristata) (Parnell et al., 2015). Fourth and perhaps the most intriguing mechanism: photonic crystals. Photonic crystals consist of mediums with refractive indices that vary in space periodically (Vigneron & Simonis, 2012). The striking tail feathers of the wild turkey (Meleagris gallopavo, Fig. 1D) are due to photonic crystals formed by melanosomes ordered in hexagonal formation inside their barbules (Eliason et al., 2013).
Color producing
Mechanism Pigment Iridescent Location in feather
Example
Pigmentary
Carotenoids No Cortex or barbs
northern cardinal Other
pigments No Cortex or barbs pesquet parrot
Melanin No
Medulla (sometimes cortex)
european nightjar Structural
Melanin No Barbs (Spongy layer) blue jay
Melanin Yes
Barbule (Organized melanosomes)
anna’s hummingbird
Table 1: Overview of the different color producing mechanisms. Underlined colorations are the colorations used in this research.
Figure 2: Structural differences between melanized feather coloration and iridescent feather coloration. (A) Achromatic color produced by melanins with (B) schematic of feather barb showing random distribution of melanosomes within barbs and barbules (C) Representative drawing of avian feather detailing the location of feather barbs and barbules. (D) Representative example of iridescent structural color with (E) schematic of feather barb showing organized melanosomes on the perimeter of barbules. On images indicating the location of barb (br), rachis (r), calamus (ca), barbules (bb), barb cortex (c), layer of melanosomes (m). Image credit : modified from (Shawkey & D’Alba, 2017).
10 Certain structural coloration mechanisms can result in a special type of coloration, namely iridescence, which is possibly best known from the visually striking displays of male hummingbirds, for example, the male Anna’s hummingbird (Calypte anna, Fig. 1B) (Giraldo et al., 2018). Structural coloration can thus also be divided in either being iridescent or non-iridescent (Table 1) (Shawkey et al., 2009). Iridescent coloration is characterized by a change in color appearance (hue) based on the angle of observation or illumination (Doucet & Meadows, 2009). In birds (Aves) iridescence can be produced by organized structures of melanin and air in feather barbules (Shawkey et al., 2009) (Figure 2). Functions of iridescence has been studied extensively in the last decades (Doucet & Meadows, 2009; Meadows et al., 2009; Sun et al., 2013). Studies have primarily focused on iridescence for communication because of its striking appearance. For example it has been hypothesized that iridescent coloration plays an important role in intraspecific communication in the courtship behavior of broad-tailed hummingbird (Selasphorus platycercus) (Hogan & Stoddard, 2018).
However, the evolution of iridescence precedes the evolution of eyes. Hypotheses that iridescence may help to avoid predation (Hanlon, 1982) or serve non-communicative functions such as water-repellant (Gower, 2003; Wang et al., 2012) or strengthening of the integument (Bonser, 1995; Butler & Johnson, 2004) are supported as well. Nevertheless, to our knowledge, the thermal properties of iridescent coloration have not yet been considered. While some suggest that iridescent coloration should decrease the absorbance of solar radiation because of its higher reflectance (Koon & Crawford, 2000) others show that iridescent coloration might do the opposite due to the air spaces within the iridescence-producing structures acting as heat collectors (Heilman & Miaoulis, 1994). In our research we investigated the effect of iridescence on heat load in feathers. Specifically, we hypothesized that the angle dependency of iridescent feathers extends into the longer wavelengths and thereby affects thermal properties. This would imply that it is possible for birds with iridescent coloration to modulate the amount of solar irradiance they receive though their plumage by changing their orientation relative to the sun.
11
2. Objectives
By combining different techniques including spectrophotometry, thermal imaging and microscopy we test whether iridescence affects thermal properties. For this we collected feather samples (iridescent and, as controls, melanized non-iridescent feathers) and tested the hypothesis that birds exhibiting iridescent plumage can modulate the amount of solar irradiance absorbed by their feathers and as a result can regulate heat gain, depending on how their feathers are angled. The project can be divided into three goals, each with their embedded specific objectives:
Goal 1: To characterize the angle dependency of feather reflectance
· To assess if reflectance in the near infra-red (NIR) is angular dependent.
· To determine whether changes in the visible spectrum (VIS) occur similarly in the near-infra red (NIR).
Goal 2: To quantify the heating load of feathers
· To determine whether iridescent feathers heat up more compared to control feathers.
· To evaluate if feathers heat up differently depending on angle of exposure.
· To assess whether maximal temperature correlates to differences in reflectance.
Goal 3: To determine the importance of feather microstructure on reflectance and heating load
· To compare feather barbule dimensions of iridescent and non-iridescent feathers.
13
3. Materials and methods
3.1. Sample collection and preparation
Feathers were acquired from different commercial sources or were part of the feather collection from the EON research unit (University of Ghent). When needed, feathers were washed in ethanol after which they were fully dried in an oven for 24h at 60°C. On some cases more than one feather of different colors were used from a given species.
We distinguished two types of coloration within our sample and categorized them either as
structural iridescent (hereafter referred to as iridescent feathers) or melanin-based, non-iridescent (hereafter referred to as control feathers) (Table 1) .We also measured one white feather which was included as a reflectance and heating reference showing the properties of achromatic feather keratin alone.
In total we collected iridescent feathers of 14 different species and control feathers of 10 different species. Number of samples used per experiment vary depending on limitations due to use of specific measuring equipment (Table A2).
14
3.2. Measurements
3.2.1. Characterization of feather reflectance
For the measurement of reflectance across visible light (UV-VIS; 300-700nm) and the near infra-red spectrum (NIR; 700-2000nm) we used a dual spectrophotometer – light source setup: AvaSpec-ULS2048L StarLine Versatile Fiber-optic Spectrometer + AvaSpec-NIR256/512-2.0/2.2TEC NIRLine Near-Infrared Fiber-optic Spectrometer + AvaLight-HAL-(S)-MINI Tungsten-Halogen Light Source + AvaLight-DH-S Deuterium-Halogen Light Source), which makes it possible to measure reflectance across 300-2000nm. This way, accounted for most of the solar radiation that reaches Earth’s surface. Reflectance spectra were measured relative to an Avantes 99% white reflectance standard and Avantes black standard. This makes it possible for specular reflectance of iridescent feather samples to exceed reflectance values of 100% in some cases.
Angle dependent reflectance
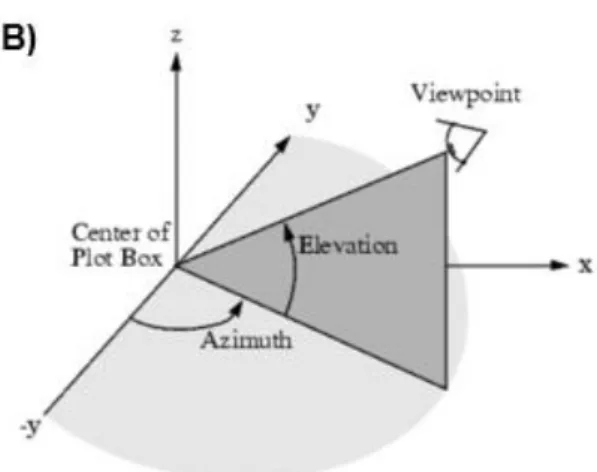
There are five different variables that can affect the reflectance spectrum of a fixed point on a surface viewed by a fixed observer. The light source can move in azimuth and elevation (Fig. 3B) and the object can rotate around 3 axes (Fig. 3A). In this experiment we take two variables into account, since incorporating all can lead to redundancy. We test the effect of feather orientation (based on the orientation of the barbs), which is the rotation of the feather around the y-axis and elevation of the light source (Fig. 3A, 3B).
Figure 3: Image showing the five different variables affecting reflectance spectrum. (A) three angles of rotation. (B) possible movements of the light source, elevation or azimuth. Image credit: (A) Javatpoint , (B) North western McCORMICK School of engineering
15 The feather orientated reflectance is measured at a 90 degree
elevation (light source being in the zenith) with the use of a RPH-1 reflection probe holder (Fig. ARPH-1). We covered the feather with a black piece of paper with a punched out hole that allowed the measurements for reflectivity and marked with the different feather orientations (0° – 45° – 90°) to create a more standardized set up according to the direction of the barbs. 0° indicating the barbs being in line with the line of sight (Fig. 4A), while 90° is perpendicular to the line of sight (Fig. 4C).
To characterize the effect of elevation on feather reflectance we connected the dual spectrophotometer and light source to two bifurcated fibre optic cables which were attached to the arms of a custom-made goniometer (Fig. A2). The feather was mounted on the sample holder in a fixed orientation and could be viewed from 5° up to almost 90° (the illumination- and viewing beam could not be coaxial). Specular reflection measurements were taken at different elevation angles (60°, 70°, 90°) (Fig. 5). This was performed in a dark room and the sample was shielded with cardboard to block any stray-light which could affect the measurements.
The feathers were put on a black paper to minimize background reflectance. Three measurements were collected per species per angle, if possible a different feather was used for a repeated measurement. Afterwards, the average was used for the characterization of reflectance of each species.
Figure 5: Schematic representation of specular reflection. Light coming from source (I) and reflection (R) at elevation of illumination (α). Image credit: Javatpoint
Figure 4: Feather orientation which is based on barb orientation. (A) 0° orientation with feather barbs in line of sight. (B) 45° orientation with feather barbs at 45 degrees. (C) 90° with feather barbs perpendicular to the line of sight. (D) example of how barbs are positioned within feather.
16
3.2.2. Quantifying the heat load of feathers
To determine the heat load of feathers when light arrives at different angles, we conducted a heating experiment. The experiment was performed inside a reflective test-box (Fig. A3), in which a feather was placed at a predetermined fixed spot on a white piece of paper. Directly next to the feather we attached a thermocouple attached to a data logger (TinyTag, Gemini data loggers, Chichester, UK ) on black tape (Fig. A4). Both are used to measure the temperature as a control to keep the temperature the same in between repeats of the experiment. The lightbulb (Philips BR125 IR250w Bulb) that was placed 45cm above the feather was turned on for 12 minutes. Simultaneously, we recorded the feathers with an infra-red camera (FLIR T530 Thermal Imager, FLIR Systems Inc. Oregon, USA) (Fig. A5). The experiment had two different set-ups. Firstly, the feathers were first placed horizontally (90 degree angle compared to the incoming light). Secondly, we placed the feathers at a 45 degree angle (Fig. A3). Each set-up was performed twice per species. So in total we had four measurements per species. More specifically, 2 measurements at the 45 degree angle and two horizontally to the incoming radiation. Afterwards we used the average per angle to quantify the heat load of feathers.
To quantify the heat load of feathers we obtained two parameters from each heating curve. On one hand we used the maximal temperature. The maximum temperature was calculated by taking the average values of the feather’s temperature from the last two minutes of the heating experiment where no changes in temperature were further observed, which is the asymptotic temperature of the heating curve. On the other hand we calculated the heat rate of feathers.Which is the slope of the formula: y = LN(TEt - TE∞) / (TE0 - TE∞), TEt = temperature at a given point in time, TE0 = initial temperature and TE∞
17
3.2.3. Importance of feather structure on reflectance and heating load
With a scanning electron microscope (FlexSem 1000II, Hitachi High-Tech Corporation, Japan) we imaged the feather’s microstructure to identify morphological correlates of reflectance and heating load. With a razor blade, we transversely cut small pieces of feather and placed individual barbs on a piece of double-sided adhesive carbon tape on the aluminum stub holder. Since we were interested in the cross sections of the barbules, we made sure some barbs were pointing up. Then we coated it with a mixture of gold/platinum and viewed them on the SEM.
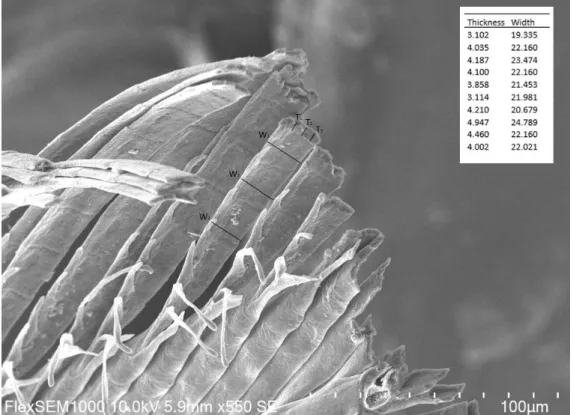
From the calibrated SEM micrographs we quantified the dimensions of feathers barbules using the ImageJ computer software (Fig 6). We measured the width (w) and thickness (t) of a feather barbule at minimum three different locations on the barbule and repeated it for three different barbules. Then we used the averages to calculate the ratio between barbule width and its thickness (𝑡
𝑤) and used it as a proxy for barbule flatness.
Figure 6: SEM image of Eurasian magpie feather barbules. Inset shows the measurements of barbule dimensions in μm. On image indicating the repeated measurements for 1 barbule. Width (W), Thickness (T).
18
3.3. Statistical analysis
Statistical analyses were carried out using R studio in the R statistical environment using core packages and packages ‘lme4’, ‘lmertest’, ‘car’, ‘rstatix’, ‘ggpubr’. We tested for outliers using the ‘identify_outliers()’ function. Normality was assessed using a Shapiro-Wilk normality test. The variance of the differences between groups were tested using Mauchly’s test of sphericity. The data was normal unless stated otherwise. The sample sizes experiments were small (Table A2), this can affect the statistical power of the analysis. P-values < 0.05 were taken as statistically significant.
3.3.1. Characterizing feather reflectance
We calculated the total and mean reflectance, and hue from the spectral curves of feathers using the R package PAVO (Maia et al. 2019). For control feathers, the calculation of Hue is not appropriate since these feathers show an achromatic color (reflecting similarly across wavelengths). Therefore, in the analyses of feather hue we only include iridescent feathers. The reflectance variables showed outliers. Normality assessment showed that the data was not normal. Outliers were not removed since the values did not seem wrong, since it is possible that the spectral reflectance of iridescent feathers exceed 100%. However this can affect the statistical power of the analysis.
Feather orientation
To test for differences in reflectance between the different angles of iridescent feathers a repeated measures ANOVA was performed. The analysis included mean reflectance as response variable and the type feather and degree of feather orientation as factors. The species id was included as repeated measure factor.
Elevation of incident illumination
To test for differences in reflectance for both feather type and the different elevations of light illumination a repeated measures mixed ANOVA was performed. With feather type (iridescent or control) and the elevation of light illumination (90o, 70 o, 60o) as between-groups variables and species
19
3.3.2. Quantifying the heating load of feathers
Maximum temperature and heating rate
To test for differences in either maximum temperature or heating rate for both feather type and the different degrees of heating a repeated measures mixed ANOVA was performed. With feather type (iridescent or control) and degree of heating (45 o or 90 o) as between-groups variables and species
id as within-subjects variables.
3.3.3. Importance of feather structure to reflectance and heating load
Barbule flatness
To test for differences in barbule flatness between feather type a t-test was performed. To compare the mean barbule flatness of the feather types. The data was not normal, probably caused by the small sample size. No outliers were removed. This will affect the statistical power of the analysis.
20
4. Results
4.1.1. Characterizing feather reflectance
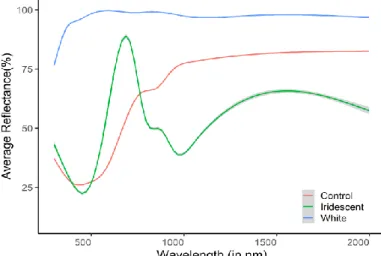
The reflectance spectra of iridescent feathers are characterized by a sinusoidal form, showing one or more peaks, within the UV-Visible and a relatively broad reflectance spectra in the near infra-red range. Control and white feathers produce overall broadly-tuned spectra across both UV-Vis and NIR range (Fig. 7).
Figure 7: Reflectance spectra (300-2000nm) example of the different feather types.
Feather reflectance (300-2000nm) was significantly different between feather types (FDF = 7.111,18 , p
= 0.018 ). Control feathers were on average more reflective compared to iridescent feathers (Fig. 8C). This was similar for reflectance in the NIR spectrum (700-2000nm; FDF = 55.2451,18 , p < 0.001 ).
Control feathers had 1.85 times greater NIR reflectance compared to iridescent feathers (Fig. 8B). However reflectance did not differ significantly for the UV-Visible (300-700nm) between feathers (FDF
= 0.1611,18 , p = 0.69 ) (Fig. 8A).
Figure 8: Boxplots showing the average reflectance (%) for different feather types with the dashed line representing a white control feather, showing overall higher feather reflectance in both Vis and NIR ranges. (A) UV-Vis (300-700nm). (B) NIR (700-2000nm). (C) Total spectrum (300-200nm).
21
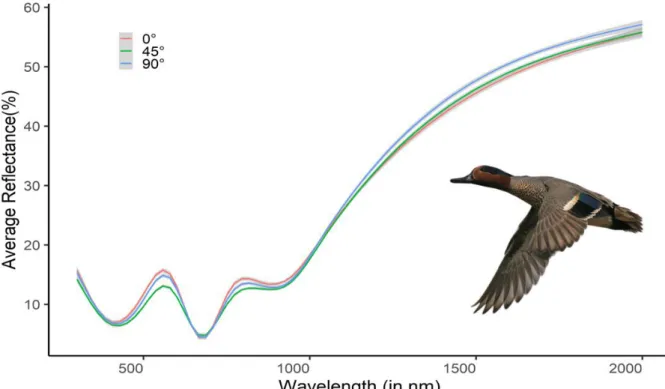
Feather orientation
Feather reflectance
Feather reflectance did no differ significantly with the angle at which feathers were orientated when rotated around y-axis in relation to the light source (Figs 3A, 4). This result was almost significant for reflectance in both the UV-Visible (FDF = 3.3381,11 , p = 0.056 ) and not significant in the NIR range
(FDF = 0.8071,11 , p = 0.46). In other words, iridescent feathers showed similar brightness regardless
of their orientation (Fig. 9, A6). However, we notice larger variation within the UV-Vis compared to the NIR.
Feather hue
Similarly, we found that hue did not differ between angles of feather orientation (Fig. A6D). There is no shift in the reflectance peaks (λmax) within the UV-Vis (FDF = 0.8211,11, p = 0.394 ) for iridescent
feathers (Fig 9.).
Figure 9: Representative reflectance spectra of an iridescent feather (of the eurasian teal (Anas crecca)) from the speculum for multiple feather orientations. (inset: Eurasian teal (Anas crecca) in flight showing iridescent speculum, Digital photograph (PNG), Pngwing, accessed 4 June 2020, <https://www.pngwing.com/en/free-png-yuwcl>.
22
Elevation of incident illumination
Feather reflectance
Feather reflectance did not differ significantly between feather types for the UV-Vis (FDF = 0.422,18 , p
= 0.53). Iridescent feathers do have significantly lower reflectance for the NIR wavelengths compared to control feathers (FDF = 672,36 , p < 0.001). This holds true for all angles of illumination (Fig. 10).
We found that overall feather reflectance increased with a decreasing angle of incident illumination for UV-Visible (FDF = 10.52,36 , p < 0.001) and NIR ranges (FDF= 17.42,36 , p < 0.001) (Fig. 10). This
happened in a similar manner for both feather types, since we found no interaction effect between angle and feather type in UV-Vis (FDF = 0.062,36 , p =0.941) or NIR spectrum (FDF = 0.2292,36 , p =0.79).
However there are slight differences.
Control feathers differed significantly between the 60° and 70° angle, this holds true for both UV-Vis and NIR. While iridescent feathers differed significantly between the 60° and 90° angle in the UV-Vis and in both the 60°- 70° and 60°- 90° angles for the NIR.
It should also be noted that the difference we perceive for both feathers between the 70°- 90° angle in the UV-Vis is different and opposite in the near infra-red. While feathers reflect more at 70° angle compared to 90° angle in UV-Vis, feathers seem to reflect slightly more at 90° angle compared to 70° angle in near infra-red (Fig. 10).
Figure 10: Reflectance curve ranging from 300-2000nm of the average of (A) Control feathers; (B) Iridescent feathers for different elevations of illumination.
23
Feather hue
Control feathers are achromatic, i.e. black or grey, and do not change in hue. In contrast, we found that for iridescent feathers their hue significantly varies with the angle of light incident (FDF = 0.5721,11 , p = 0.035).
Overall, iridescent feathers showed an increase towards longer wavelengths with increasing angle (Fig 11, 12). The average change in hue per 15° change is (19.662nm ± 6.387nm).
The iridescent Lahore pigeon demonstrate an example of the changes we observed in reflectance spectrum of iridescent feathers as a function of angle of elevation. The pigeon’s feather shows multiple sinusoidal peaks of roughly similar amplitude within the UV-Vis spectrum. Showing the shift of hue (Fig 12B, 12C) between angles of incident light. The hue, as mentioned above, shifts towards longer wavelengths with increasing angle (Figure 11). It should be noted that when the light elevation was 30°, feather reflectance flatten and was not reliably quantified (Fig. 12A).
Figure 12: Reflectance spectrum of lahore pigeon (Columbia livia) for different angle of incident light. (B,C) microscopic picture of the nape feather of the lahore pigeon.
Figure 11: Boxplot shows the differences in iridescent feather hue per 15 degree change of incident light for the UV-Vis (300-700nm).
24
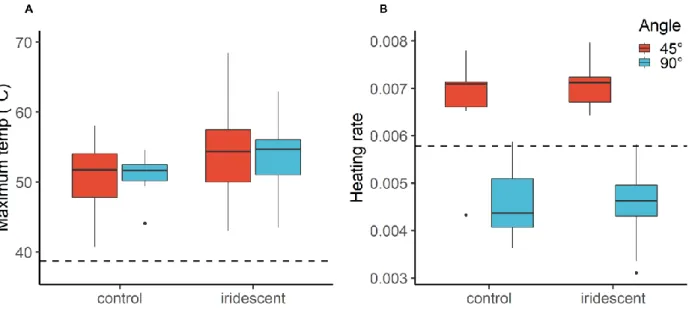
4.1.2. Quantifying the heating load of feathers
Maximum temperature
Iridescent feathers reached higher maximum temperatures compared to control feathers, however the differences were not significant (FDF = 3.772,42 , p = 0.067). We found that maximum temperature
was similar when feathers were heated at 45 degrees compared to 90 degrees (FDF = 0.3952,42 , p =
0.537). There was no significant difference between the changing angle and feather type (FDF =
2.472,42 , p = 0.132). In other words, the maximum temperature of iridescent- and control feathers
behaved similarly with a changing angle of heating (Fig. 13A).
Heating rate
Heating rate was significantly higher when feathers were heated at a 45 degree angle compared to when they are perpendicular (90 degree) to the light source (FDF = 133.232,42 , p < 0.001). We did not
found any significant differences between feather types (FDF = 0.2712,42 , p = 0.68) or the interaction
effect of changing angle and feather type(FDF = 0.662,42 , p = 0,43). Both control and iridescent feathers
showed similar heat rates depending on the angle they were heated at (Fig. 13B).
Figure 13: Boxplot shows feather heating load at different angles of heating for iridescent and control feathers.. (A) Maximum temperature, dashed line shows white control feather (37°). (B) Heating rate of feathers, dashed line shows white control feather (0.0058).
25
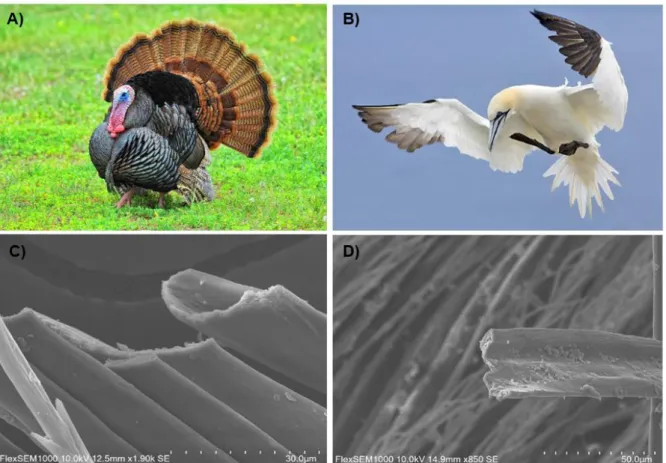
4.1.3. Importance of feather structure on reflectance and heating load
Barbule flatness
Barbule flatness differed significantly between iridescent feathers and control feathers (FDF = 7.111,14 , p = 0.0184).
Overall iridescent feathers are more flat (Fig. 14, 15C) compared to control feathers, which shape is more rounded (Fig. 14, 15D).
Figure 15: Images show representative species and corresponding SEM images of feather barbules cross sections in wild turkeys (A,C) and northern gannet (B,D). Photo credits: (A) Tes Randle Jolly, (B) Anonymous.
Figure 14: Boxplot of feather barbule flatness (ratio between the width and thickness) for control and iridescent feathers.
26 On average, feathers reach higher maximum temperatures when they absorb more light. We found a significant correlation between feather reflectance for the total spectrum and maximum feather temperature (r = 0.55, df = 16, p = 0.02). This result was true for both the UV-Vis (r = 0.52, df = 16, p = 0.02) and NIR (r = 0.55, df = 16, p = 0.02) range for both iridescent and control feathers (Fig 16A, 16B, 16C).
Figure 16: Scatterplots showing relationships between feather reflectance (%) for different ranges of the electromagnetic spectrum and maximal temperature (°C). (A) Total spectrum, (B) UV-Vis, (C) NIR.
27 We found no significant correlation between the reflectivity of feathers (300-2000nm) and their heating rate (r = 0.23, df = 16, p = 0.38) (Fig. 17A). The heating rate of feathers was similar for feathers of different brightness.
Figure 17: Scatterplot showing heating rate of feathers compared to feather reflectance (300-2000nm) and (B) barbule flatness.
28 Flatter barbules seem more reflective compared to more round barbules. There was a significant correlation (r = 0.67, df = 10, p = 0.01) between the reflectivity (300-2000nm) of iridescent feathers and the flatness of their barbules (Fig. 18B). This was similar for reflectance in both the UV-Visible (r = 0.64, df = 10, p = 0.02) and the NIR ranges (r = 0.64, df = 10, p = 0.02). Barbule flatness was not significantly correlated to heating rate (r = 0.20, df = 14, p = 0.43) (Fig 18C). Remarkably, there seem to be no clear correlation with the maximum temperature of a feather and barbule flatness (r = 0.03, df = 14, p = 0.90 Fig. 18A).
Figure 18: scatterplot showing the correlations between barbule flatness and (A) the maximum temperature of feathers. B) the feather reflectance (300-2000nm). (C) heating rate of feathers.
29
5. Discussion
In this study we first investigated whether iridescent feathers, which change color with the angle of view, would heat up faster or reach higher temperatures compared to non-iridescent, melanin-colored feathers. First, in contrast with the study by Shawkey et al (2018), we found that reflectance of iridescent feathers was not significantly less in the UV-Visible range than control feathers, we found a similar reflectance for both iridescent and non-iridescent control feathers. However, we found that the NIR reflectance of iridescent feathers was much lower than melanin-colored control feathers. The study by Shawkey was performed on sunbirds (Family Nectariniidae), where one similar color-producing mechanism is responsible for the iridescence of their feathers. The samples used in our research were from species in more distantly related clades, which display different color-producing mechanisms (Table A3). This larger variation in organization of nanostructures (melanosomes) could be responsible for increasing the variation in reflectance in the UV-Visible range, therefore decreasing the differences relative to non-iridescent control feathers.
Melanin-based colors reflected less incident light than white control feathers, similar to previous research (Shawkey et al., 2017; Ward et al., 2007). White feathers lack melanin and they contain more air cavities. Melanin is known for a very broad absorption that varies relatively little across the entire spectrum (Sarna & Swartz, 2007). The crystalline structure of a white feather keratin, combined with the lack of underlying melanin granules gives rise to a high reflection across the entire spectrum of the visible light. We speculate that the high variation we saw in reflectance, especially spanning the near-infra red might be due to micro- and nano structural differences between iridescent and control feathers. Moreover, we found a difference in microstructure between iridescent and non – iridescent control feathers. More specifically, iridescent feather barbules were flatter (Fig 14, 15C.), while those of the control feathers were rounder (Fig 14, 15D). In addition, there was a weak correlation between barbule flatness and reflectance, where flatter barbules show higher reflectance (Fig 16D.). It is not surprising that the shape of the reflecting surface can influences its reflectivity. As is the case for example in the sickle shape of hummingbird barbules, which positively correlates to more reflective colors (Gruson et al., 2019). There are many structural parameters that could possibly influence feather reflectivity. More closely packed barbules produce brighter colors. Barbules can be interlocked, leading to an overall greater spatial coherence and as result produce brighter colors.
30 As for the nano structural differences, we suspect that melanosome organization and concentration within the feather barbule might influence feather reflectivity. The ordered formation of melanosomes in iridescent feathers compared to the random – non organized distribution in non-iridescent feathers combined with melanosome concentration, which is higher in iridescent feathers (Lee at al. 2009, Maia et al., 2012) might increase the absorbance properties of the feathers, especially beyond the avian visible spectrum. Higher melanin concentration in iridescent feathers relative to control feathers and more evidently, unmelanized feathers could explain the heating patterns we observed in our study.
First we found that white feathers heat up less compared to melanin based colors and that iridescent feathers heat up more than non-iridescent feathers, even though it was clear, our analysis showed no significant differences in the maximum temperature between iridescent and non – iridescent melanin based colors. Future investigations of the effect of different melanosome organizations on thermal properties, particularly absorbance patterns will also help clarify the effect of iridescence on heat transfer in feathers.
Furthermore, there are other parameters that could affect the heat load of feathers. Within this study we only looked at the feather type (iridescent or non-iridescent). Parameters such as thickness, color, degree of iridescence al confer to the heat load. As is the case for the black winged stilt (Himantopus
himantopus), which is characterized by iridescent black feathers, but only showed relatively moderate
maximum temperatures. We suspect that this is caused by the thickness of the feather, being very thin with sparse barbs. Micro and macrostructural plumage characteristics need to be considered in future, comprehensive models of thermal effects of feather coloration.
Thus, our findings suggest that the content of melanin in feathers is responsible for both the high absorption of iridescent feathers, as well as their higher heating loads. We found a correlation in overall feather reflectance and maximum feather temperature (Fig 16A). Furthermore, this correlation was seen both similar in UV-Vis and NIR ranges. However, the NIR should always be included since UV-Vis reflectance is a poor predictor of the maximum temperature. The visible wavelengths only hold a small fraction of the thermally relevant radiation (Grace & Gates, 1982). Therefore if iridescent feathers absorb significantly more of the longer wavelengths compared to non-iridescent feathers, it is plausible that this is why they also heat up more compared to control feathers. Since wavelengths that range the near infra-red have great potential to have significant thermal effects. (Christian et al., 1996; Cowles & Bogert, 1944).
31 Despite the differences we observed in heat load between iridescent and control feathers, it is still unclear if these differences would influence the bird’s skin temperature and ultimately the internal thermal balance. Heat transfer through plumage is largely unexplored and the effect of coloration has only been considered in one previous study by Walsberg (1983). So far it is known that heat transfer is affected by the structure of the plumage, either by 1) radiation, 2) conduction through solid elements of the feather and 3) aerial conduction and convection through feathers (Wolf & Walsberg, 2000). However these factors are often under behavioral control, i.e. ptilo erection. Birds that show iridescent plumages may have an additional path to control this. The ordered nanostructures of iridescent feathers may enable a form of dynamic modulation of heating and cooling.
The most intriguing characteristic of iridescence is the change in color based on the angle of observation or illumination. We first quantified the change in total reflectance and hue depending on the angle of incident light in iridescent feathers, as it has been shown in other studies (Giraldo et al., 2018; Osorio & Ham, 2002). The hue shifted towards longer wavelengths, on average 20nm per 15 degrees change in elevation of illumination (Fig. 11, 12). The non-iridescent feathers are achromatic, i.e. black or grey and did not change in appearance. Feather reflectance was also correlated to the angle of incident light. We found that overall reflectance increased with a decreasing angle of elevation of illumination (Fig. 10). This was the case for both iridescent and non-iridescent feathers. Changing feather orientation had little effect on both feather reflectance and hue (Fig. A6). This variation in reflectance between different angles of illumination seemed to be less pronounced in the near infra-red compared to the UV-Vis, for both iridescent and non-iridescent feathers (Fig 10). This finding was mirrored in our heating experiment since we found no significant differences in the maximum temperatures of both iridescent and non-iridescent control feathers when they were heated under different angles (Fig. 13A). But the heating rate for both type of feathers was higher when heated at 45 degrees compared to when they were heated at 90 degrees (Fig 13B), in contrast to our reflectance measurements, where feathers absorbed less light under a lower elevation of illumination (Fig 10). Together these results suggest that variations in the angle of illumination and heating seem to have a similar effect on feathers regardless of whether they are iridescent or not. Thus, increase in feather reflectance and heating rate with decreasing angle seem more likely explained as being the result of slight changes in the setup as the angle was modified during the experiments. For example, in the reflectance measurements, shifts in the elevation of illumination will influence the area of the feather that is being illuminated by the beam of light and will affect the reflectance recorded by the spectrophotometer. As the angle decreases, a larger area of the feather seems to be illuminated by the light source (Fig. A7), decreasing the overall reflectance.
32 In the case of the heating experiment, inclination of the samples at 45 degree could have resulted in slight but impactful decreases in the distance between the feather and heating source, effectively increasing the amount of radiation impinged on feathers. Since we would expect the heating rate at 45 degree to be lower compared to 90 because of the angle of insolation. A lower angle would result in less intense heating since the light rays are spread over a larger area on the feather (Fig. A7). Overall, iridescent feathers seem to potentially incur a higher heat load than non-iridescent feathers. This could negatively influence the performance of a bird with iridescent plumage. Nevertheless animals make trade-offs. A certain trait can be beneficial in one life history trait, while being detrimental in other ways (Charnov & Krebs, 1974). The cost of iridescent feathers might overall be a good trade-off against reproduction, since the hypothesis that iridescent feathers function in sexual signaling as well supported (Doucet & Meadows, 2009). The thermal properties of animals will become increasingly important in warming climates and the trade-offs of a trait may shift as result. Although often barely known, more knowledge on the thermal properties is needed. Further elucidating the heating load of iridescent plumage is thus encouraged. Both micro- and macrostructural plumage variables should be considered in a more complete model of the thermal effects on feather coloration. Future research should be focused on the micro- and especially nanostructures of feathers. The effect of melanosome content and the different ordered formations within feathers seems to greatly influence the thermal properties of a feather. This will elucidate the effect of iridescence on heat transfer in feathers.
33
6. Conclusion
Our study showed that feather reflectance was significantly higher for non-iridescent feathers compared to iridescent feathers. Iridescent feathers absorb almost two times as much reflectance from the near infra-red (701-2000nm) relative to non-iridescent feathers. We hypothesize that this variation is mostly caused by larger amounts of melanin in iridescent feathers and also potentially to micro and nano structural differences between iridescent and non – iridescent feathers. We found a significant difference in microstructure, more specifically the barbules of iridescent feathers were more flat compared to the barbules of control feathers. The difference in reflectance was also evident in the heating experiments. Iridescent feathers tended to reach higher maximum temperatures compared to non-iridescent. Moreover, feather reflectance and maximum feather temperature were correlated. Feathers heated up more quickly at 45 degree, which could be explained by the feather being somewhat closer to the light bulb. Changing the inclination at which the feather was heated did not result in different maximum temperature for both iridescent and control feathers. Feather reflectance decreased with an increasing elevation of illumination, while the hue of iridescent feathers shifts toward longer wavelengths. Overall our findings suggest that iridescence has the potential to impose a thermal cost for birds, as a bird with iridescent plumage could incur in greater heat loads and physiological costs in order to maintain a thermal balance. These costs could also arise from producing larger amounts of melanin to deposit in iridescent feathers. Future studies should try to elucidate these two potential costs of producing and maintaining bright iridescent plumages.
To our knowledge, this is the first study testing the angular dependency of iridescent feathers beyond the visible light and contributes to the understanding of the thermal properties of iridescent coloration.
34
7. Summary
There are over 10.000 species of birds, while each year more species are being discovered. Birds are known for their great diversity in coloration, ranging from the snow-like appearance of the bali starling (leucopsar rothschildi) to the red, blue and yellows of the scarlet macaw (Ara macao). Matching this diversity in coloration are the underlying functions. Most studies only consider coloration as an agent for camouflage or as form of social and sexual signaling, both for attracting partners or to warn of possible opponents. However, non-signaling functions of coloration have rarely been considered. In this study, we’ll be focusing on a lesser known function of coloration, their thermal properties.
Studies on thermal effects of coloration have mainly been performed in ectotherms. They maintain an optimal temperature by shifting to more favorable thermal habitats. Color change has been observed in many species, mainly as camouflage. However many ectotherms are also able to rapidly change their coloration to modify their body temperature as form of thermoregulation.
In birds, plumage coloration could possibly have significant thermal effects. Different colored tissues will reflect or absorb solar radiation differently. This will directly affect feather surface temperature and thus determine the amount of radiative heat that can reach the bird’s skin. In general, white surfaces reflect more of the incoming solar radiation, therefore will heat up slower compared to a black surface. It should be noted that most research only consider the electromagnetic in the UV-Visible spectrum, but over half of the sun’s radiation is in the near infra-red and it are these wavelengths that will have a great potential to have significant thermal effects. However, bird coloration is often more variable than being white or black. The plumage can contain a combination of different colors and different color creating mechanisms.
Colors are often created by two different mechanisms. Pigmentary color, created by the selectively absorptance, reflectance and transmittance of wavelengths. While most pigments, like carotenoids, absorb a very narrow part of the spectrum. Melanosomes absorb over a broad spectrum and create less saturated when randomly ordered in the integument, they create colors like greys, browns and black. If ordered, these structures within the integument constitutes a second category of color producing mechanism, structural coloration. Structural colors can further be classified as being iridescent or not iridescent. There is some understanding about the reflectance of these different mechanisms. Carotenoid-based colors are most reflective (300 – 2100nm) followed by non-iridescent and iridescent melanin-based colors. Even though iridescent feathers seemed, to the naked eye, more reflective the visible range (300-700nm) compared to non-iridescent melanin based colors. This strongly suggests that not only hue but also the color producing mechanism and the orientation of feathers in relation to the light source and elevation of the light source strongly influences reflectance.
35 To examine the angle dependent properties of iridescent feathers beyond the visible light, we conducted the experiments on both non-iridescent, iridescent melanosome based colorations and added a white feather as comparison. First, we measured the feather reflectance (300 – 2000nm) for both changing feather orientation relative to the light source and changing elevation of illumination. We determined the changes in both overall reflectivity and changes in hue. Second, we conducted heating experiments that we recorded with the use of a infra-red camera. The feathers were heated at two different angles relative to the light source. With the use of thermal recordings, we calculated the maximum temperature and the heating rate. Third, with the use of an SEM we imaged the feather microstructure and measured barbule thickness and width, afterwards we quantified the barbule flatness as the ratio between thickness and width (𝑡ℎ𝑖𝑐𝑘𝑛𝑒𝑠𝑠
𝑤𝑖𝑑𝑡ℎ ) with the use of a ImageJ.
Feather reflectance was significantly higher for non-iridescent feathers compared to iridescent feathers. Even though their reflectance within the UV-Vis (300-700nm) was similar and to the naked eye iridescent feathers look brighter. Iridescent feathers absorb almost two times as much reflectance from the near infra-red (701-2000nm). The high variation we notice in reflectance, especially within the NIR, might be caused be micro and nano structural differences between iridescent and non-iridescent feathers. We found a difference in microstructure between non-iridescent and control feathers. Iridescent feather barbules were more flat, while the control feathers barbules are more round. This mirrored in our heating experiments. Iridescent feathers seemed to reach higher temperatures compared to non-iridescent feathers however the difference was not significant. But within this study we only looked at feather type, other parameters such as thickness, color, degree of iridescence al influence the feather’s heating load. Nevertheless, our finding suggest that the melanin content within feathers is responsible for the higher absorption of iridescent feathers, especially in the near-infra red. As well as their higher maximum temperatures. We found a possible correlation in overall reflectance and the maximum temperature.
For the heating experiment, feathers heated more quickly at the 45 degree angle, which is probably caused by the set-up of the experimental design. Changing the inclination might have decreased the distance to the light bulb. However, it did not result in differences for maximum temperature for both feather types. We see a similar result in our reflectance measurements. The reflectance of both the iridescent and non-iridescent feathers decreased with increasing angle of illumination, while the hue of iridescent feathers shifted towards longer wavelenghts. The reflectance and hue of iridescent feathers did not differ significantly when we changed the feather orientation relative to the light source.
36 Overall our data suggests that iridescent feathers seem to receive higher heat loads compared to non-iridescent feathers. This might negatively influence a bird with iridescent plumage. The cost of iridescence might overall impose a trade-off with its function for sexual signaling. Future research is needed to understand the thermoregulatory properties of iridescence.
37
8. Samenvatting
Wereldwijd zijn er meer dan 10 000 soorten vogels bekend en elk jaar worden er nog meer nieuwe soorten ontdekt. Vogels staan erg bekend om hun prachtige kleuren. Deze zijn erg divers en variëren van het sneeuwwitte verenkleed van de bali spreeuw (leucopsar rothschildi), tot de rood, blauw en geel tinten van de geelvleugelara (Ara macao). De functies van deze kleuren zijn net zo divers. Veel studies die uitgevoerd zijn omtrent de functies van kleur focussen zich vooral op kleur in functie van camouflage, zoals we in de europese nachtzwaluw terug zien, of als vorm van sociaal of seksuele communicatie. Vogels gebruiken hun kleuren vaak om indringers weg te jagen, of een partner te verleiden. Desondanks hebben kleuren ook niet communicatieve functies die minder onderzocht zijn. In ons onderzoek focussen we ons op een van de minder bekende functie van kleur, namelijk de thermische eigenschappen. De meeste studies op de thermische eigenschappen van kleur zijn uitgevoerd op ectothermen, zoals hagedissen. Deze behouden een optimale temperatuur door zich te verplaatsen naar een habitat met een optimale temperatuur. Het veranderen van kleur is een fenomeen dat in verschillende soorten terug gevonden word. Meestal wordt het gebruikt om aan een predator te ontsnappen. Niettegenstaande zijn er veel ectothermen die hun lichaamskleur veranderen voor thermoregulatie als doel om hun temperatuur aan te passen.
Het verenkleed van vogels kan ook significante thermische effecten hebben. Net zoals verschillend gekleurde oppervlakten op een verschillende manier zonlicht weerkaatsen en absorberen, doen veren dit ook. De oppervlakte temperatuur van de veer wordt hierdoor beïnvloed en dit zal bepalen hoeveel warmte de huid van de vogel kan bereiken. Over het algemeen zullen witte oppervlakte meer zonlicht weerkaatsen, t.o.v. een zwart oppervlakte. Hierdoor zal een zwart oppervlakte sneller opwarmen. Opmerkelijk is dat het meeste onderzoek uitgevoerd op thermische effecten van kleur enkel maar rekening houden met het zichtbaar licht, in tegenstelling tot het volledige elektromagnetische spectrum van zonnelicht. Het zichtbaar licht bevat minder dan de helft van de radiatie uitgezonden door de zon. Het grootste deel bevind zich in het infra rood gedeelte. Het zijn deze golflengtes die mogelijk een grote thermische invloed kunnen hebben. Toch is het verenkleed van vogels vaak niet volledig wit of zwart. Het bevat vaak een combinatie van verschillende kleuren en verschillende kleur creërende mechanismes.
De meeste kleuren kunnen ingedeeld worden onder twee verschillende mechanismes. Eerst, kleuren gecreëerd door pigmenten. Pigmenten zullen selectief bepaalde golflengtes absorberen, reflecteren en doorlaten. De meeste pigmenten, zoals carotenoïden, absorberen enkel een zeer smal deel van het totale spectrum. In tegenstelling tot melanosomen, een ander soort pigment. Deze absorberen over een zeer breed spectrum en creëren voornamelijk grijs en bruintinten.
38 Melanosomen kunnen ook zeer gestructureerd zijn. Dit zorgt voor een tweede categorie van color creërende mechanismes. Structurele kleuren, dit zijn kleuren die gevormd worden door de interactie van licht met de structuur waarmee ze botst. Verder vallen structurele kleuren nog in te delen als iriserende of niet iriserende. Iriserende kleuren zijn kleuren welke van uitzicht veranderen afhankelijk van de positie van waar er naar gekeken wordt. Recent onderzoek over de reflectiviteit van deze verschillende mechanismen toonde aan de kleuren gevormd door het carotenoïde pigment het meest reflectief zijn (300 -2000nm) gevolgd door niet iriserende gemelaniseerde kleuren en iriserende gemelaniseerde kleuren. Toch leken iriserende veren meer te reflecteren van het zichtbaar licht (300-700nm) t.o.v. de niet iriserende melanine kleuren. Dit laat blijken dat niet alleen de kleur maar ook het mechanisme achter de kleur een effect heeft op de reflectiviteit. Net zoals de oriëntatie van de veren t.o.v. het licht en de hoogte waarmee het licht invalt
Om de invalshoek-afhankelijke eigenschappen van iriserende veren op zowel het zichtbaar licht als infra rood te ontdekken, hebben we experimenten uitgevoerd op zowel niet iriserende als iriserende gemelaniseerde kleuren. Eerst hebben we met behulp van een spectrofotometer de reflectiviteit van veren (300-2000nm) bepaald. We veranderde de oriëntatie van de veren t.o.v. het licht en de hoogte van het licht (de hoek waarmee het licht op de veer invalt). We keken vervolgens naar de verandering in de totale reflectiviteit van de veren en de verandering in tint. Ten tweede hebben we een warmte experiment uitgevoerd, deze werd gefilmd met een infra rood camera. De veren werden op twee verschillende hoeken t.o.v. het licht verhit. Door de video beelden achteraf te analyseren kon de maximale temperatuur en de snelheid waarmee de veren opwarmden geanalyseerd worden. Ten derde, maakten we gebruik van een SEM om de microstructuur van veren in beeld te brengen. Hieruit werd de dikte en breedte van de veer barbule gemeten met behulp van het computer programma ImageJ, om vervolgens de barbule vorm te bepalen. Dit was de ratio tussen de dikte en de breedte. De reflectiviteit van niet iriserende veren was significant hoger dan iriserende veren. Hun reflectiviteit van het zichtbaar licht (300-700nm) is gelijkaardig, maar iriserende veren absorberen bijna tweemaal zoveel van het infra rood (700-2000nm). Deze variatie in reflectiviteit binnen het IR valt mogelijk te verklaren door verschillen in de micro en nano structuren van iriserende en niet iriserende veren. We vonden ook een verschil in de microstructuur. Iriserende veren hebben meer afgeplatte barbules t.o.v. niet iriserende veren, hun barbules zijn over het algemeen ronder. De warmte experimenten toonde aan dat iriserende veren over het algemeen hoge temperaturen haalde dan niet iriserende veren, maar was niet significant. Dit kan komen doordat we in deze studie enkel rekening houden met het type van veer als parameter (iriserend of niet). Andere parameters zoals de dikte, de kleur, hoe iriserend ze zijn kunnen ook veel invloed hebben op de temperatuur van een veer.
39 Niet tegenstaand toont onze studie aan dat de concentratie aan melanine in een veer een invloed kan hebben op de hitte absorptie. Het veroorzaakt dus mogelijk de hogere temperatuur die we waarnamen bij iriserende veren en de lagere reflectie van golflengten afkomstig uit het infra rood. We vonden ook een mogelijke correlatie van de totale reflectie en de maximum temperaturen, dit is mogelijk vooral veroorzaakt door reflectie van het IR. Het is dan ook niet verwonderlijk, aangezien het
zichtbaar licht slechts een fractie van het totale zonlicht is. Tijdens de warmte experimenten warmden veren sneller onder een hoek van 45 graden, dit is
waarschijnlijk veroorzaakt door de opstelling zelf. Bij het veranderen van de hoek kan het zijn dat de veren onder een hoek van 45 graden dichter bij de lamp zich bevinden, waardoor ze sneller opwarmen. We zagen geen verschil in maximale temperatuur bij de veren als we de hoek veranderde. De reflectiviteit van zowel iriserende als niet iriserende veren verminderde als we de invalshoek van de lamp verhoogde, terwijl de kleurtint van iriserende veren op schuift richting langere golflengtes. Als we de oriëntatie van iriserende veren veranderde, zagen we zowel de reflectiviteit als de kleurtint niet veranderen.
Onze data suggereert dat iriserende veren een hogere hitte belasting krijgen t.o.v. niet iriserende veren. Dit kan mogelijk een vogel met iriserende veren negatief beïnvloeden. Toch lijkt de kost van iriserende veren minder groot dan het voordeel dat het met zich mee brengt. Namelijk het gebruik van iriserende veren als vorm van seksuele communicatie. Toekomstig onderzoek is nodig om de thermische eigenschappen van iriserende veren te begrijpen.
40
9. Acknowledgements
First and foremost I would like to thank the University of Ghent and the research group Evolution and Optics of Nanostructures for giving me this opportunity.
I would like to express my gratefulness to my super visor Dr. Liliana D’Alba, who guided me through this entire project, while still allowing it to be my work. She learned me new techniques and helped me in all possible ways. Even under these strange circumstances, her virtual door was always open. I am really thankful for her support.
Next I would like to thank my co-super visor Prof. Dr. Matthew Shawkey, who’s knowledge helped me with the experiments and writing of the thesis.
I would like to thank my tutor Svana. Not only did she teach me to use new techniques, helped me with the analyzing of the data and provided useful insights for my thesis. She, together with the EON research group, has given me the opportunity to join her on a field season in South-Africa. She gave me hands-on experience for data collection and the opportunity of ringing my first bird, which I will never forget.
Further, my sincere thanks to the members of the research group Evolution and Optics of Nanostructures. For their openness, knowledge and insights.
Finally, I would like to thank my parents who made this all possible, their never ending support and trust throughout my years of studying and the thesis was unbelievable.