Degradation of Highly Unsaturated Fatty Acids in Artemia sp.
Michael Oliewo Aluma Student number: 01801137
Promotor
Prof Gilbert Van Stappen
Tutor
Tânia Margarida Lourenço
Master’s Dissertation submitted to Ghent University in partial fulfilment of the requirements for the degree of Master of Science in Aquaculture
ii COPYRIGHT
The author and the promoter give the permission to use this master dissertation for consultation and to copy parts of it for personal use. Every other use is subject to the copyright laws, more specifically the source must be extensively when using from this thesis.
Gent, 21 August 2020
The promoter,
Prof Gilbert Van Stappen
……….
The tutor,
Tânia Margarida Lourenço ………..
The author,
Michael Oliewo Aluma ………..
iii
CONFIDENTIALITY NOTICE
This document may contain confidential information proprietary to the Universiteit Gent. It is therefore strictly forbidden to publish, cite or make public this document in any way or any part thereof without the express written permission by the Universiteit Gent. Under no circumstances this document may be communicated to or put at the disposal of third parties; photocopying or duplicating it in any other way is strictly prohibited. Disregarding the confidential nature of this document may cause irremediable damage to the Universiteit Gent.
Gent, 21 August 2020
The promoter(s), The author,
‘Signature’ ‘Signature’
Prof Gilbert Van Stappen Michael Oliewo Aluma
The tutor,
‘Signature’
iv
ACKNOWLEDGEMENTS
Sincere gratitude goes to my promoter, Prof Gilbert Van Stappen and supervisor Tânia Margarida Lourenço of the Laboratory of Aquaculture & Artemia Reference Center, Department of Animal Sciences and Aquatic Ecology, Ghent University, for the guidance throughout my thesis work. Their direction and assistance was valuable in ensuring that the process of undertaking this thesis was a success. Many thanks to my supervisor Tania was always available to assist.
v PREAMBLE
Originally, it was planned that I would investigate HUFA degradation in Artemia franciscana strains to identify any genetic variation among them. This would entail working with two strains from different parts of the globe - San-Francisco Bay (USA) and Vinh Chau (Vietnam). In order to do so, the experimental design included hatching the Artemia nauplii; enrich them; empty their guts; and follow their metabolism for the next 24h while stored at different temperatures. During the experimental period 7 time points were agreed to collect samples for FAME analysis and DNA extraction. Obtained data would then be analyzed in line with the specific objectives of the study. However, when Covid-19 struck, I was unable to enter the lab and run any of the planed experiments. As a solution, I focused on expanding and deepening the literature review chapter which covered additional relevant sections to the thesis topic such as fatty acid degradation pathway and the effect of environmental factors on HUFA metabolism in crustaceans. I also analyzed statistically, the data from a similar experiment conducted previously under the same conditions by my supervisor, Tânia Lourenço.
vi ABSTRACT
The enrichment and storage of Artemia nauplii is a practice that is common in many larviculture hatcheries across the globe. It is understood that under storage, enriched Artemia nauplii degrade fatty acids, particularly HUFAs, to meet their own metabolic needs. In line with the efforts to try and address this problem of HUFA degradation in enriched Artemia nauplii, this study sought to investigate the degradation of HUFA in two different strains of Artemia franciscana – Vinh Chau and San Francisco Bay. Part of the specific goals of this study included determining and comparing the degradation rates of HUFA as well as the effect of temperature on the degradation or synthesis
rate. The FAME (fatty acid methyl ester) analysis data obtained from a previous
experiment done by the supervisor following the same methodology were analysed by performing a one-way ANOVA test using JMP Pro 14 software. The results showed that the metabolism process in Artemia nauplii involved a number of fatty acids; some of which were common to both Vinh Chau and San Francisco Bay, while others were specific to each strain. Further analysis of the results revealed that during fatty acid metabolism, the metabolism curves of SAFAs, MUFAs, PUFAs and HUFAs behaved differently with some increasing, others decreasing and still others remaining constant as storage time and starvation elapsed. The analysis also demonstrated that temperature had an effect on the rate of degradation or synthesis with higher rates found to be at higher temperatures. With regard to HUFAs, the analysis showed that the degradation rate of DHA was the highest followed by the synthesis of ARA and then EPA during storage at 27°C and at I6°C in both strains. The analysis of the FAME data also revealed a number of differences between SFB and VC in their metabolism of HUFAs at 27°C and at I6°C. The study concluded that based on the findings that maintaining high levels of DHA in SFB or VC Artemia nauplii at higher temperatures (≥ 27°C) was a challenging task and that hatchery managers needed to find better feeding plans for the fish larvae.
vii TABLE OF CONTENTS
COPYRIGHT ...ii
CONFIDENTIALITY NOTICE ... iii
ACKNOWLEDGEMENTS ...iv
PREAMBLE ... v
ABSTRACT ...vi
LIST OF FIGURES ... x
LIST OF TABLES ... xii
LIST OF ABBREVIATIONS AND UNITS ... xiii
CHAPTER 1: INTRODUCTION ... 1
1.0. Introduction ... 1
1.1 Objectives ... 3
CHAPTER 2: LITERATURE REVIEW ... 4
2.1. Background ... 4
2.1.1. Aquaculture feeding the world... 4
2.1.2. Increasing seafood production through marine aquaculture ... 5
2.1.3. Fingerling availability and larval rearing ... 5
2.2. Live feeds for marine fish larvae ... 6
2.2.2. Rotifers use as live feed and their limitations ... 6
2.2.3. Copepods use as live feed and their limitations ... 8
2.3. Artemia ... 9
2.3.1. History of Artemia exploitation and use ... 9
2.3.2. Scientific classification ... 10
2.3.3. Morphology and life cycle ... 10
2.3.4. Ecology, natural and global distribution ... 13
2.3.5. Characterization of Artemia strains ... 15
2.3.7. Advantages and limitations of using Artemia as food for marine fish larvae 18 2.3.8. Nutritional value of Artemia ... 20
2.4. EFA and their roles in marine fish larvae development ... 22
2.4.1. Structure and classification of fatty acids ... 22
2.4.2. Saturated fatty acids (include FA structures) ... 22
2.4.3. Monounsaturated fatty acids ... 23
2.4.4. Polyunsaturated fatty acids ... 23
viii
2.4.4.2. Omega-6 (ω-6 or n-6) family ... 24
2.4.4.3. Omega-3 (ω-3 or n-3) family ... 25
2.4.4.4. Conversion of dietary PUFA to HUFA ... 27
2.5. Essential FA and roles of HUFA in the diet of marine fish larvae ... 29
2.6. Enrichment ... 31
2.6.1. Enrichment overview ... 31
2.6.2. Enrichment products and technique for Artemia nauplii ... 31
2.6.3. Enrichment, HUFA assimilation and composition ... 32
2.6.3.1. HUFA assimilation and rate during enrichment ... 33
2.6.3.2. HUFA Assimilation and starvation ... 34
2.6.3.3. Evacuation of gut content ... 35
2.7. Problems of enrichment ... 36
2.8. Fatty acid degradation pathway in crustaceans ... 37
2.9. Possible enzymes of interest in fatty acid degradation pathway in crustaceans... 39
2.10. Effects of environmental factors on HUFA metabolism and composition in crustaceans... 41
2.10.1. Temperature ... 41
2.10.1.1. Temperature effect on HUFA metabolism in crustaceans other than Artemia 41 2.10.1.2. Temperature effect on HUFA metabolism in Artemia ... 43
2.11. Salinity ... 44
2.12. Dietary source of fatty acid ... 46
CHAPTER 3: MATERIALS AND METHODS ... 48
3.0. Experimental setup ... 48
3.1. DNA extraction from Artemia using the CTAB-method ... 48
3.2. Restriction fragment length polymorphism (RFLP) procedure... 49
3.3. Analytical methods ... 51
3.4. Statistics ... 51
CHAPTER 4: RESULTS ... 53
4.1. Starvation and storage of SFB and VC Artemia nauplii at different temperatures ... 53
4.2. SAFAs and MUFAs metabolism curves ... 56
4.3. Metabolism of PUFA and HUFA ... 61
4.4. Effect of temperature on PUFA/HUFA metabolism ... 67
ix
5.1. Storage and starvation of SFB and VC treatments ... 70
5.2. Metabolism curves of SAFAs and MUFAs ... 71
5.3. Metabolism of PUFA and HUFA ... 72
5.4. Metabolism curves of PUFA ... 74
5.5. Effect of temperature on metabolism rate of PUFA/HUFA ... 76
5.6. DNA extraction and RLFP ... 78
CHAPTER 6: CONCLUSION AND SUGGESTIONS FOR FUTURE RESEARCH ... 79
x LIST OF FIGURES
Chapter 2: Literature Review
Figure 1 - Structural morphology of an Adult female Artemia franciscana, adapted
from Piper, 2018. ... 11
Figure 2- Life cycle of Artemia franciscana adapted from Hollergschwandtner et al., 2017. Two distinctly separate paths of reproduction are possible which are ovoviviparity and oviparity. ... 13
Figure 3- Structure of palmitic acid (Source: ChEBI) ... 22
Figure 4- Structure of palmitoleic acid(Source: ChEBI) ... 23
Figure 5-Structure of linoleic acid (Source: ChEBI) ... 24
Figure 6- Structure of arachidonic acid (Source: ChEBI)... 25
Figure 7- Structure of alpha-linolenic acid (Source: ChEBI) ... 25
Eicosapentaenoic acid (EPA or 20:5(n-3); Figure 8): is regarded as one of the most crucial fatty acids of the omega-3 family. EPA occurs widely in algae and in fish oils and has been shown to be a vital constituent of the phospholipids in animal tissues (Burdge, 2018). ... 26
Figure 8- Structure of EPA (Source: NCBI PubMed) ... 26
Figure 9- Structure of DHA (Source: NCBI PubMed) ... 26
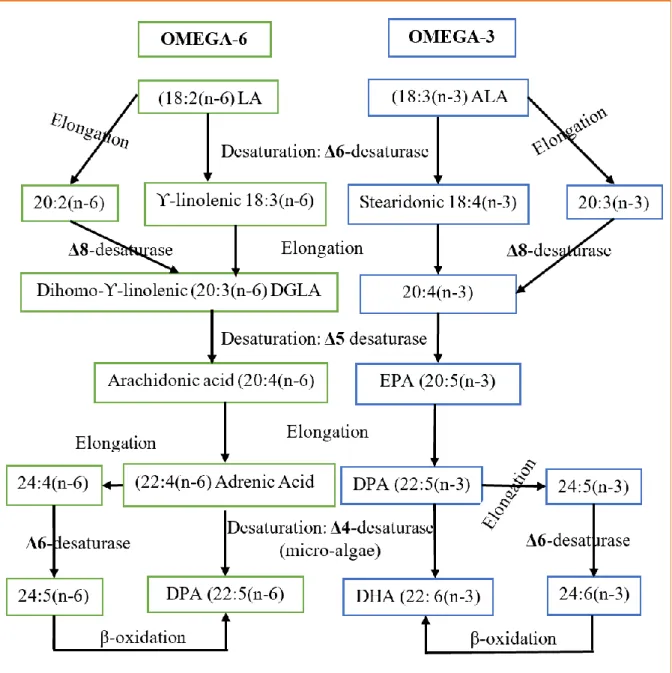
Figure 10 - Biosynthetic pathways showing elongation and desaturationconversions where both omega-6 and omega-3 PUFA form their equivalent HUFA (Garrido et al., 2019) ... 28
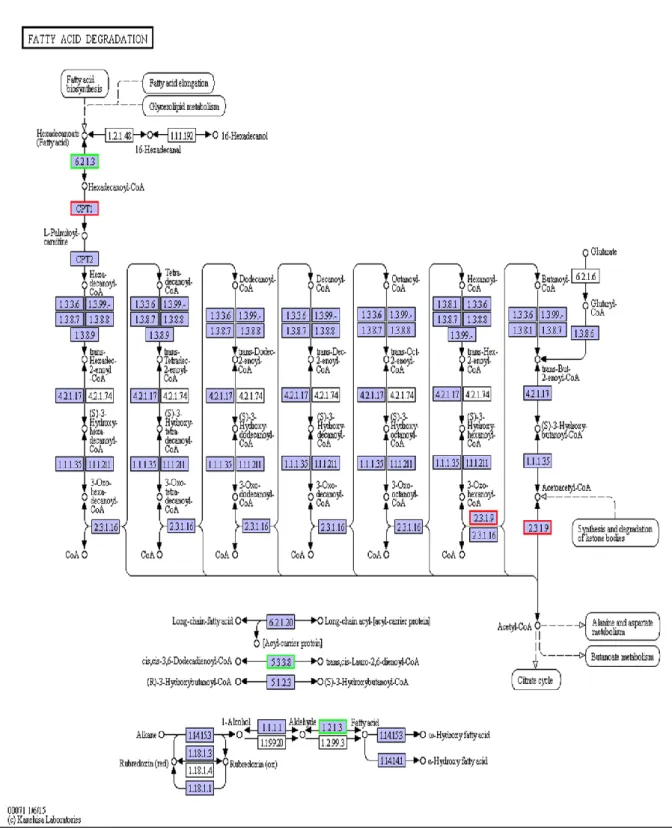
Figure 11- FA degradation in Pacific white shrimp (Source: https://www.genome.jp/kegg-bin/show_pathway?pvm00071) ... 38
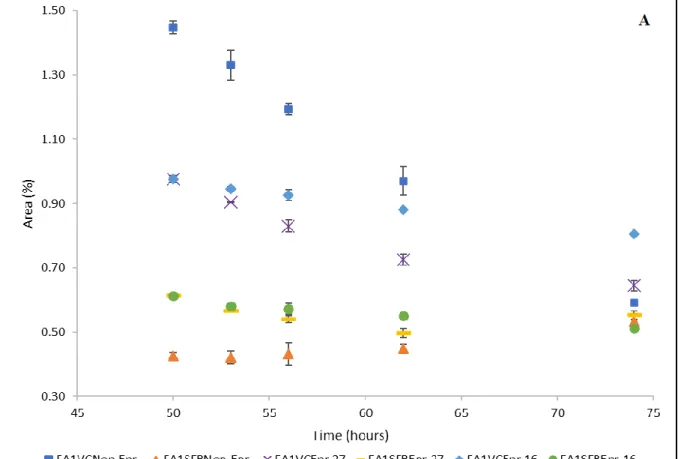
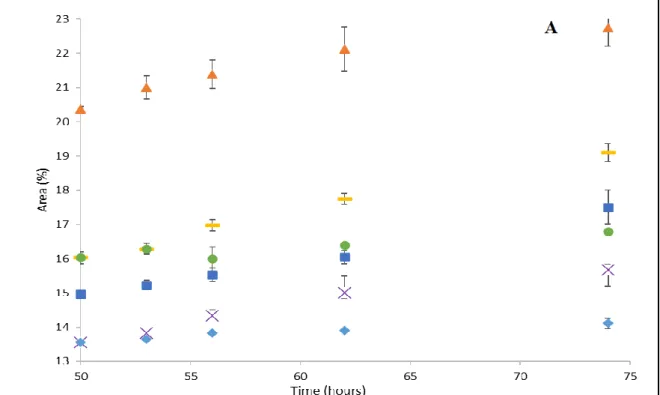
Chapter 4: Results Figure 1- Relative contents of myristic acid- FA1- (A) and myristoleic acid- FA2- (B) (% of total fatty acids) in VC and SFB Artemia nauplii during storage period (50-74 hours)………..57
Figure 2 Relative contents of SAFAs; stearic acid FA9 (A) and docosanoic acid -FA28- (B) (% of total fatty acids) in VC and SFB Artemia nauplii during storage period (50-74 hours) ………58
Figure 3- Relative contents of MUFAs; oleic acid -FA10- (A) and 11-Octadecenoic acid -FA11- (B) (% of total fatty acids) in VC and SFB Artemia nauplii during storage period (50-74 hours) ………..60
Figure 4 - Relative content of LA, ALA, ARA, EPA and DHA (% of total fatty acids) in VC (A) and SFB (B) at hatching, enrichment and after gut evacuation ……….62 Figure 5: Relative contents of linoleic acid (A) and arachidonic acid (B) (% of total fatty acids) in VC and SFB Artemia nauplii during storage period (50-74 hours).….64
xi
Figure 6: Relative contents of ALA (A) and EPA (B) (% of total fatty acids) in VC and SFB Artemia nauplii during storage period (50-74 hours) for enriched
treatments……….66 Figure 7: Relative contents of DHA (% of total fatty acids) in VC and SFB Artemia nauplii for enriched treatments during storage period (50-74 hours)……….67
xii LIST OF TABLES
Table 1- List of fatty acids displaying significant differences (P < 0.05) in their area percentages (%) mean, present in SFB nauplii from hatching (24h) to end of
starvation/storage period (74h). ... 53 Table 2- List of fatty acids present in VC nauplii with significant differences (P < 0.05) in their area percentages (%) mean across treatments from hatching (24h) to end of starvation period (74h). ... 54 Table 3 List of fatty acids in SFB and VC showing significant differences (P < 0.05) in their area percentage (%) mean during the storage period (50 to 74 hours). ... 55 Table 4- The degradation/synthesis rate (-K ± SD, day -1) of LA, ALA, ARA, EPA and DHA in enriched SFB Artemia nauplii stored at 27°C and 16°C for the period (50-74h). ... 68 Table 5- The degradation/synthesis rate constant (-K ± SD, day -1) of PUFA/HUFA in enriched VC Artemia nauplii stored at 27°C and 16°C for the period (50-74h). ... 68 Table 6- compares degradation/synthesis rate (-K ± SD, day -1) of selected
PUFA/HUFA in VC and SFB Artemia nauplii stored at 16°C. ... 69 Table 7- compares degradation/synthesis rate (-K ± SD, day -1) of LA, ALA, ARA, EPA and DHA in VC and SFB Artemia nauplii stored at 27°C. ... 69
xiii LIST OF ABBREVIATIONS AND UNITS °C Degree Celsius
% Percentage
/ Per μm microns mm millimetre g/l grams per litre ANOVA Analysis of variance
FAO Food and Agricultural Organization of the United Nations PCR Polymerase chain reaction
1
CHAPTER 1: INTRODUCTION
1.0. IntroductionA dramatic growth in human population over the last decade accompanied by increasing urbanization and affluence in emerging economies has and continues to trigger demand for more sustainable and quality food. In this context and given the better prospects for increased aquaculture production (FAO, 2018), marine aquaculture presents a good possible target to produce quality proteins for human consumption thereby addressing this demand. However, larviculture, particularly the start feeding of early larval stages still possess a huge challenge to fully exploiting marine finfish and shellfish production (Dhert et al., 2001; Megahed & Aly, 2009). This is mainly because successful larviculture of a majority of marine finfish as well as shellfish species still rely on live feeds like Artemia and rotifers, which are often of variable quality and expensive to produce. While the possibility of replacing the live feeds with formulated feeds has been tested as well, studies have demonstrated that the latter are nutritionally deficient in relation to all the requirements of larvae and are linked to poor larval growth and survival (Carneiro et al., 2003; Hamre et al., 2013). Therefore, this points to a proper selection of the right live feed and improving its nutritional quality as key to realizing success in marine finfish and shellfish larval rearing.
In the context of meeting all the nutritional requirements of marine fish larvae, research indicates that highly unsaturated fatty acids (HUFAs) are essential in the diet of these animals (Roo et al., 2019). This is because they are unable to synthesize HUFAs from their parent compounds, linoleic and Linolenic acid. Linolenic acid is easily converted to eicosapentaenoic acid (EPA) in fresh water fish but this conversion and subsequent synthesis of docosahexaenoic acid (DHA) is extremely small or absent in a majority of subtropical and tropical marine fish (Tocher, 2010; Williams et al., 2017). Therefore, as essential fatty acids (EFAs), HUFAs play a major role in the development of marine fish larvae. Good survival of marine fish larvae has been correlated with EPA and DHA linked to improved larval growth and quality (Reitan et al., 1994; Lavens et al.,1995). Moreover, studies show that high DHA/EPA ratios are important for stress tolerance, better growth and pigmentation of marine fish larvae (Kraul, 1993; Rezek et al., 2010).
2
In view of selecting the right live feed for marine finfish and shellfish larval rearing, Artemia is deemed as a good choice in comparison to the other live feeds. It has been used widely by most hatcheries and preferred because of a number of reasons. The production of storable dormant cysts, which can hatch swiftly into nauplii (Bengtson et al., 2018), allows for convenience during usage. The predictability of success in hatching of cysts, possibility of enriching nauplii (Abatzopoulos et al., 2013), and least labour intensive makes Artemia the most preferred live feed. The process of storing freshly hatched Artemia nauplii at around 4°C for 24 to 48 hours and providing moderate aeration (Léger et al., 1983; Almalul, 2000) economizes cyst hatching effort and offers an opportunity to supply food constantly to larvae.
On the contrary, the nutritional quality of Artemia, particularly their composition of EFAs, is a huge limitation in its usage despite its preference and merits. Studies have demonstrated that the content of EFAs, EPA: 20:5(n-3) and DHA: 22:6(n-3), is deficient and/or varies significantly from one species of Artemia to another and even within strains (Watanabe et al. 1978; Léger et al.,1986; Sargent et al., 1999; Yamamoto et al., 2008). Artemia has the capacity to convert linolenic acid to EPA for its metabolic needs. This is evident in their HUFA levels that resemble those of their recent foods (Webster et al., 1991). Therefore, it follows that this deficiency and/or variability in the content of their EFAs is in relation to the nutritional requirements of marine fish larvae (Ohs et al., 2012).
In regard to alleviating the limitation of Artemia nutritional quality, success has been realized in incorporating high levels of EFAs in live food via the enrichment procedure (Sorgeloos et al., 2001). This technique takes advantage of the primitive feeding characteristics of Artemia nauplii, non-selective particle filter feeders, to integrate lipid products containing EPA, DHA and in some cases, depending on the fish larvae requirements, arachidonic acid (ARA), into the Artemia prior to feeding them to the larvae (Lavens & Sorgeloos, 1996). While enrichment has been shown to boost the nutritional quality of Artemia (Abatzopoulos et al., 2013), it is believed that the problem of HUFA metabolism during storage or starvation of enriched Artemia nauplii once placed in the larval culture tanks has the potential to revert the enrichment gains. Evjemo et al. (2001) noted that the nutritional value of Artemia nauplii may be affected greatly if the nauplii reside for a longer period in the fish tanks before the larvae eats
3
them or when they are stored at ambient temperature awaiting transfer to the fish tanks.
1.1 Objectives
Knowing that under storage, enriched Artemia nauplii degrade fatty acids, particularly HUFAs, to meet their own metabolic needs, the aim of this study was to investigate the degradation of HUFA in two different strains of Artemia franciscana – Vinh Chau and San Francisco Bay. The specific objectives of this study are as outlined:
I. To assess the metabolism curves of different fatty acids in San Francisco Bay
(SFB) and Vin Chau (VC) Artemia strains
II. To determine and compare the degradation rates of HUFAs in SFB and VC
Artemia strains
III. To investigate the effect of temperature on the degradation rate of HUFAs in
SFB and VC Artemia strains
4
CHAPTER 2: LITERATURE REVIEW
2.1. Background2.1.1. Aquaculture feeding the world
The global demographic trends indicate that the world population is growing and will continue to grow. The United Nations prospects places the world population at 7.8 billion currently and projects that it will reach nine billion by 2050 (United Nations,
2019) – about 1.2 billion more mouths to feed. It is important that feed supply
accompanies this trend and thus the projected population increase will require that the food supply equally increase by 25% to 70% (Hunter et al., 2017). This will need to be attained despite the deteriorating natural resource base as well as the competing interests for agriculturally based input commodities. Alongside the population growth, the emerging economies like China, Brazil and India have led to increased affluence adding to the challenge of demand for food as people are earning more and are increasingly urbanized (Kharas, 2010). The rising incomes have led to a shift in dietary patterns as these new consumers option for more protein-rich meals with meat and fish (Kharas, 2010). These consumers are also increasingly aware of the quality of the products and thus the increase in demand will equally be accompanied by demand for quality products. While livestock food sectors are escalating production in an effort to meet the increasing demand, this comes with huge challenges that include water shortages, overgrazing and loss of natural biodiversity (Michalk et al., 2019). On the other hand, the activities of fisheries cannot be increased above the current levels as the stocks are already in danger. Additionally, there is the need to further decrease the catch from fisheries to be sustainable. It has therefore become more and more apparent that aquaculture will provide an increasingly significant component of the global animal-derived protein budget. Despite the decline in average annual growth rate from 11.3% and 10% (excluding aquatic plants) for the 1980s and 1990s respectively to 5.8% during the period 2000 to 2016, aquaculture remains the fastest growing food production sector (FAO, 2018). Out of the global fish production of 171 million tons in 2016, aquaculture accounted for 80.0 million tons of food fish and 30.1 million tons of aquatic plants in addition to 37900 tons of non-food products (FAO, 2018). This indicates that aquaculture now provides 53% of the fish for human consumption if the non-food uses that cover fishmeal and fish oil are excluded (FAO, 2018). Therefore, given the fisheries captures that have remained stagnant since the
5
late 1980s, it is evident from these findings that aquaculture has accounted for the continued remarkable growth in the supply of the fish consumed by humans.
2.1.2. Increasing seafood production through marine aquaculture The 80.0 million tonnes of aquaculture food fish produced globally in 2016 encompassed inland aquaculture at 51.4 million tonnes (64%) and marine aquaculture at 28.7 million tonnes (36%) (FAO, 2018); an indication for growth potential in marine aquaculture. According to Gentry et al. (2017), marine aquaculture presents an opportunity that can be exploited to increase the production of seafood especially in the wake of the growing demand for marine protein coupled with the limited scope for increasing the wild fishery harvests (Gentry et al., 2017). This points to the need to address a number of concerns such as fingerling availability and larval rearing that can aid in realizing increased production of sustainable marine protein. FAO (2018) indicated that if the marine-based proteins are responsibly developed as well as managed, they can make a significant contribution to attaining global food security. Additionally, Hua et al. (2019) showed in their study that these marine proteins are a vital source of the essential micronutrients such as minerals (zinc, selenium, iodine, and calcium), Vitamins (A, B and D), and omega-3 fatty acids required in the diet.
2.1.3. Fingerling availability and larval rearing
Fingerlings availability has been deemed as one of the critical factors for attaining commercial success in marine fish farms and consequently increasing marine-based proteins production (Bostock et al., 2010). For some species, domestication relies exclusively on wild captures as the source of fingerling. On the other hand, a majority of the species that are cultivated in Europe like sea bream and seabass rely on hatchery outputs owing to the availability of the requisite rearing techniques. Larval rearing can sometimes be done in outdoor ponds where the larvae are given natural food. In other instances, larvae can be reared under intensive conditions where they are fed using cultured live food. Studies from different authors, have indicated that regardless of the larval rearing technique employed, the start feeding of early larval stages remains a major impediment to realizing increased fish and shellfish production in marine fish farms (Dhert et al., 2001; Megahed & Aly, 2009). The small eggs and similarly small mouth gape of the larvae at first hatching has been shown to make feeding over the first few days critical (Yufera & Darias, 2007). The fry rely on the
6
nutrients that are stored in the yolk sac for the first few days. When the yolk is completely consumed, the larvae requires appropriate nutrients along with the right environment (Huntingford et al., 2012). Evolutionarily, the larvae of most fish and crustaceans are fixed on the system of motile prey organisms and thus confront problems in accepting inert or dry diets (Dhert et al., 2001). Even if they accept the diets, research has shown that their non-functional stomach along with poor enzymatic activity will not allow them to digest the present formulated diets (Dhert et al., 2001). Furthermore, the formulated feeds do not always meet all the requirements of larvae and studies have linked their use to poor growth and survival of the larvae (Carneiro et al., 2003; Hamre et al., 2013). While considerable effort has been directed towards improving the acceptance of the dry diets for fish larvae along with formulating more digestible and less polluting diets, live feed remains the most important food source for larvae. It appears to meet most criteria and thus deemed the most appropriate as first exogenous feed of early larval stages (Hamre et al., 2013).
2.2. Live feeds for marine fish larvae
2.2.1. Selecting the appropriate live feed for early larval stages
The selection and utilization of the right live feed during the first feeding phase of the larval cycle has been shown to determine the success of commercially producing grown marine species as well as the candidate species (Conceição et al., 2010). This occurs due to the importance of the first feeding period on early larval stages, which is critical for the optimal development of marine fish larvae. Therefore, a live feed that meets the larvae proper nutritional requirements , has a suitable size range and stimulates the feeding response is needed to increase not only the success of marine fish species produced but also to diversify the species produced. Studies indicate that among the most important starter feeds that have been used to successfully culture many species of marine fish through the larval phase are rotifers and newly hatched nauplii of Artemia (Agh & Sorgeloos, 2005; Bengtson et al., 2018). Equally reported as important feed for the larviculture of marine fish are copepods (Hansen, 2017).
2.2.2. Rotifers use as live feed and their limitations
Rotifers are microscopic aquatic animals classified under the phylum Rotifera and mostly inhabit the freshwater lakes and ponds (Fontaneto et al., 2006). There are two species of rotifers, Brachionus plicatilis and– Brachionus rotundiformis, that have been
7
extensively used as a first food for marine fish larvae and crustacean larvae (Dhert et al., 2001). The success in the use of Brachionus spp. as first feed in marine larviculture is attributed to their planktonic nature, small size (between 50 μm–2 mm in length), slow swimming, tolerable to a wide range of salinities (0–50 g/l), and relatively easy to culture (Lavens & Sorgeloos, 1996). Rotifers produce dormant cysts, following sexual reproduction, which can be collected or bought to start a new population. Alternatively, a part of the existing live population can be used to begin a new population - upscaling. Among the advantages that have been reported in the use of rotifers regards their quick propagation under suitable conditions (Dhert et al., 2001). In asexual reproduction, populations can double over a few days with cultures becoming quite dense exceeding 1000 rotifers/ml, a clear advantage for the hatcheries that require huge quantities of live feeds during the larval phase. In addition, rotifers are available commercially and thus possible to culture in sufficient numbers to meet the needs of the marine fish hatchery. They can be cultured with algal paste or with live algae further simplifying the process and reducing the costs involved.
Despite the success and advantages reported in using rotifers as live feed for marine larvae, several limitations have equally been highlighted. Rotifers are small with a body width that ranges between 90 to 350 μm (Ohs et al., 2012). When the body sizes are compared, literature shows that the lorica length and lorica width of rotifers vary from one strain to the other (Huang et al., 2017) and the width is equally larger than the mouth gape size range of a majority of the first feeding marine fish species. This points to the need to examine the possibility of culturing a much smaller marine rotifer such as Proales similis with a body width of about 40 μm for feeding the small mouth marine larvae (Wullur et al., 2009). The quantity of rotifers within the larval system may equally present another limitation if not monitored constantly to ensure that rotifers do not reproduce beyond the fish larvae grazing pressure. In the event that this happens, the fish larvae will consume rotifers that are nutritionally deficient with the possible rapid deterioration of water quality. High population of rotifers beyond that of the fish larvae will lead to reduction in the levels of dissolved oxygen in the fish tank. Furthermore, dense rotifer cultures are usually prone to crashing and when used may introduce bacteria and dead organisms in the tank, which will settle at the bottom and decompose thereby producing nitrogenous wastes that affect water quality. This will in turn decrease the survival of the fish larvae. The other limitation of using rotifers
8
relates to their nutritional profile, which lacks docosahexaenoic acid, eicosapentaenoic acid and arachidonic acid, fatty acids required by marine fish larvae for optimal development. Mæhre et al. (2013) indicated in their study that rotifers are deficient or low in a number of essential nutrients like highly unsaturated fatty acids (HUFA), some vitamins, amino acids, and minerals. Rotifers do not have the ability to elongate the shorter chain fatty acids and thus need to be enriched in order to meet the HUFA requirement of the marine fish larvae (Sargent et al., 1999). It therefore follows that despite rotifers having been used successfully to culture numerous marine fish species up to commercial levels, enrichment to boost their nutritional composition remains core to their success. However, these limitations demonstrate that they still do not meet the needs of all the marine fish species.
2.2.3. Copepods use as live feed and their limitations
Copepods are a tiny aquatic crustaceans and form one of the most numerous metazoan groups in aquatic communities. They are found in almost every freshwater as well as saltwater habitat. Copepods have been shown to form a major part of the marine zooplankton community (Selander et al., 2019). Hansen (2017) pointed out that in the wild, copepods form a key link in the nutrient pathway from primary producers (phytoplankton) to marine fish larvae. Regarding, nutrient pathway, copepod nauplii are eaten by marine fish larvae while the juvenile fish eat the adult copepods. Therefore, it follows that in the marine trophic system, copepods are crucial for the survival of the marine fish species. They have the correct size range, biochemical profile as well as triggering the right hunting behavior in fish larvae because of their zig-zag swimming patterns that larvae find attractive. However, with regard to using copepods to culture fish species and advance marine aquaculture, Cutts (2003) research has demonstrated potential but albeit with several challenges and/or limitations. Nonetheless, research works focusing on feeding marine fish larvae with copepods have highlighted a number of advantages in their usage. Their use was shown to improve survival, growth as well as development of fish larvae (Cutts, 2003; Hansen, 2017). Furthermore, copepods have the capacity to synthesize essential HUFAs as well as maintain the right DHA:EPA plus EPA:ARA ratios needed by marine fish larvae (Ohs et al., 2012). Equally, a larger percentage (90%) of the fatty acids present in copepods has been indicated to be in the form of phospholipids easily used by marine fish larvae (Evjemo et al., 2003).
9
Whereas studies have demonstrated that copepods remain as the preferred prey of wild marine fish larvae as well as a useful food for marine fish cultivation (Cutts, 2003), their use in commercial aquaculture has been slow. This has been attributed to the unreliable production of considerable numbers of nauplii and species-specific methods of culture known to vary greatly (Støttrup, 2000; Drillet et al., 2011). Therefore, the major limitation to the use of copepods as live feed for marine fish larvae regards the need to provide new technical solutions as well as improvements to specific copepod strains for production cultures, which points to Artemia as a better option. Furthermore, developing an indoor system with capacity to address the limitations that high density production generates holds the key to realizing largescale live feed copepod rearing (Hansen, 2017).
2.3. Artemia
2.3.1. History of Artemia exploitation and use
Artemia is a genus of anostracan crustaceans commonly known as brine shrimps and inhabitants of hypersaline habitats. The earliest scientific reference to brine shrimp was in 1756 when their existence was reported in the saltpans of Lymington, England by Schlosser. These saltpans are no longer there and presently, brine shrimp are not found in Britain (Abatzopoulos et al., 2013). While Artemia has been known to man for centuries, its use as food for culture of larval organisms seemingly started in the 1930s following a finding that it made a good food for newly hatched fish larvae. The most commercially available cysts of Artemia in the 1940s were collections from natural saline lakes and coastal saltworks. The growing interest for tropical hobby fish in the late 1940s made brine shrimp to be considered of commercial value leading to the establishing of a new industry. Early exploits of this industry (Artemia cyst production) in 1951 came from the Great Salt Lake (GSL) in Utah, USA with the first harvest yielding 16 tons of finished product. The mid 1950s saw commercial attention for brine shrimp shift to controlled sources for production in the San Francisco Bay region. This led to the realization that brine shrimp along with their cysts could be produced as a by-product of the solar saltworks. The commercial provisions in the 1960s originated from the few sources in North America. In the 1970s, a shortage was experienced following an expansion of the aquaculture production thereby triggering research to prove the possibility of producing Artemia in developing countries (Abatzopoulos et al., 2013). Presently, the global production of Artemia cysts is around 4, 000 tonnes
10
annually with about one third each produced from Great Salt Lake, Central Asia and China (MFL lecture by Van Stappen, 2019).
2.3.2. Scientific classification
The recent taxonomic classification of the genus Artemia according to the Integrated Taxonomic Information System is as outlined (ITIS Standard Report Page: Artemia, n.d.): Kingdom: Animalia Subkingdom: Bilateria Infrakingdom: Protostomia Superphylum: Ecdysozoa Phylum: Arthropoda
Subphylum: Crustacea Brünnich, 1772 Class: Branchiopoda Latreille, 1817 Order: Anostraca G. O. Sars, 1867
Suborder: Artemiina
Family: Artemiidae Grochowski, 1896 Genus: Artemia Leach, 1819
The genus Artemia has been outlined to encompass a number of species later listed in this review.
2.3.3. Morphology and life cycle
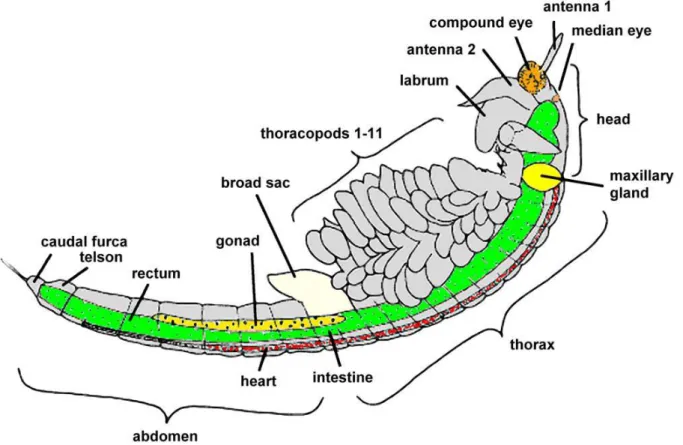
Artemia has a segmented body and has been shown to grow to an adult size of about 8-10 mm in length for adult males and about 10-12mm for adult females and about 4mm wide for both sexes (Abatzopoulos et al., 2013). The body is covered with a thin chitin exoskeleton layer and divided into head, thorax and abdomen (Abatzopoulos et al., 2013). The elongated body of an adult Artemia has a pair of stalked complex eyes, a linear digestive tract, long antennae, and eleven pairs of functional thoracopods each holding a pair of swimming legs (Figure 1). The adult males have a paired penis in the posterior part of the trunk and large claspers for clasping during mating (Criel & Macrae, 2002). On the other hand, the females have a brood pouch or ovisac located just behind the eleventh pair of thoracopods and used for storing eggs or nauplii during the cycles of reproduction (Sugumar, 2010).
11
Figure 1 - Structural morphology of an Adult female Artemia franciscana, adapted from Piper, 2018.
Free-swimming nauplii can be hatched from cysts placed in seawater after around 20 hours or develop from fertilized eggs depending on the type of reproduction. Cysts are usually produced by Artemia at certain times in their natural habitat and are metabolically inactive provided they are kept dry (Clegg et al., 1996). The first larval stage described as instar I, is usually 400 to 500 µm in length, has a brownish orange colour, a red nauplius eye in the head region, three pairs of appendages and the mandibles (Browne, 2018). It has a non-functional digestive system and thus relies completely on its yolk reserves. Instar I molts into instar II (second larval stage) after about 8 hours. Instar II has a functional digestive system and thus can filter small food particles ranging in size from 1 to about 16 µm as the optimum based on the study by Fernández, (2001) even though his study defined the particle size limit of metanauplii as ranging between 6.8 µm and 27.5 µm. In order to reach the adult stage, the larva grows and differentiates through about 15 molts undergoing various morphological along with functional changes that start from the tenth instar stage onwards (Parker, 2011).
12
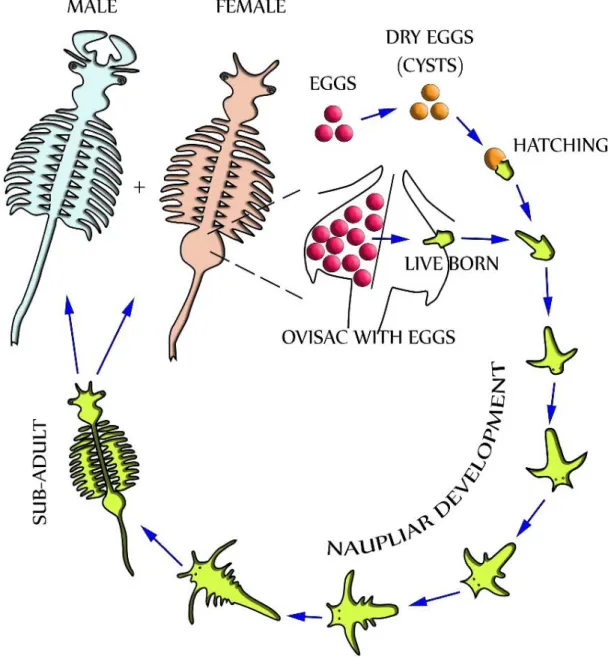
During favourable conditions, Artemia usually undergoes ovoviviparous reproduction where fertilized eggs develop into free-swimming nauplii measuring about 0.45mm. The adult females then release the free-swimming nauplii from their brood sac into the favourable environment (Baitchorov & Nagorskaja, 1999). On the other hand, during stressful conditions such as high salinity and low oxygen levels, Artemia undergoes an oviparous reproduction cycle (Figure 2). This involves the embryo developing up to the gastrula stage, and at this point surrounded by a thick shell, enters into a state of dormancy (diapause), and then released into the water by the adult female as cysts (Clegg et al., 1996). In principle, both ovoviviparity and oviparity are found in all the Artemia strains and females have the ability to switch in-between the two reproduction cycles from one to the other. The metabolically inactive cysts usually float in high salinity waters and move about on the surface at the mercy of the water currents as the parental animals die following the harsh prevailing conditions. The state of dormancy (diapause) of the cysts can be inactivated by dehydration process thereby making them quiescent (MacRae, 2016). Upon return of the favourable conditions, the quiescent cyst become hydrated and the embryo begins internal metabolic activity that ultimately breaks the cysts allowing them to hatch into free swimming nauplius (MacRae, 2016). It has been shown that under optimal conditions, Artemia can live for a number of months, grow from nauplius to adult in just 8 days, and reproduce at a rate of up to 300 nauplii or cysts every 4 days (Støttrup & McEvoy, 2008).
13
Figure 2- Life cycle of Artemia franciscana adapted from Hollergschwandtner et al., 2017. Two distinctly separate paths of reproduction are possible which are
ovoviviparity and oviparity.
2.3.4. Ecology, natural and global distribution
Artemia thrive in natural seawater owing to their physiological adaptation to high salinity. It has an incredibly efficient osmoregulatory system and produces effective respiratory pigments that assists it to cope with the low levels of oxygen at high salinities and warm environments (Clegg & Trotman, 2002). Equally critical to its survival is the capacity to produce dormant cysts during extreme environmental conditions and directly developing embryos during favorable conditions (Browne et al., 1990).
14
The growth of Artemia populations in their natural as well as introduced habitats has also been attributed to their selective filter feeding ability. Artemia is a non-selective filter feeder of microscopic algae, organic detritus and bacteria. Artemia biotopes show an extremely simple trophic structure as well as low diversity (Gajardo & Beardmore, 2012). At high salinities of above 100 g/l competitors and predators are usually eliminated and with the sufficient food coupled with favourable abiotic factors, Artemia develop into thick monocultures. Given that high salinity is deemed the prime abiotic factor that determines the presence of Artemia, the effect of the other factors such as temperature may at most affect the population abundance and ultimately cause a temporary absence of species (Van Stappen, 2002; Litvinenko et al., 2007). Furthermore, different geographical strains of Artemia have been reported to adapt to widely fluctuating local conditions in relation to salinity, temperature (6 – 35°C), and ionic composition of the environment (biotope). It is vital to indicate that while Artemia is only found at salinities where its predators are unable to survive, at extreme salinity levels, of above 250 g/l, Artemia tends to spend more energy on regulation of its physiological processes and the extreme stress and water toxicity leads to their death (Gajardo & Beardmore, 2012; MacRae, 2016). Studies have shown that the nauplius reproduction of Artemia is dominant at low salinity levels whereas the cyst production occurs at high salinities of above 150 g/l (Wurtsbaugh & Gliwicz, 2001; Dhont et al., 2013). Given that Artemia is incapable of active dispersion, the main natural dispersion vectors have been indicated to be wind and waterfowl particularly flamingos (Vanhaecke, 1987). The deliberate inoculation of Artemia in solar salt works by man, which has been a common practice in the past, forms the other means by which cysts are dispersed.
Artemia have been reported to have a wide global representation given their ability to inhabit a wide range of hypersaline ecosystems. Literature indicate that Artemia populations have been recorded in over 600 natural salt lakes and manmade salterns spread across the inland as well as coastlines of the tropical, subtropical and temperate climatic zones (Triantaphyllidis et al., 1998; Van Stappen, 2002). Furthermore, survey efforts are still ongoing with the intention of identifying more Artemia biotopes across the globe. While Artemia franciscana (Kellogg, 1906) is native to North, Central and South America, a number of species have equally been identified from different regions, which include Artemia salina (Triantaphyllidis et al., 1997) in the
15
Mediterranean basin, Artemia sinica (Yaneng, 1989) in China, Artemia persimilis described in the work of Peccinelli and Prosdocimi and cited by Støttrup and McEvoy (2008) found in the extreme south of South America, Artemia tibetiana (Abatzopoulos et al., 1998) from Tibet, Artemia urmiana (Günther, 1899) from Lake Urmia in Iran, and another Artemia sp. in Kazakhstan (Pilla & Beardmore, 1994). The parthenogenetic populations (A. parthenogenetica - Barigozzi, 1974) have been reported in different regions including Europe, Asia, Africa, and Australia. Since the discovery of Artemia species, a number of strains have equally emerged (Abatzopoulos et al., 2013). While these strains have been shown to be of inferior quality in reference to those of the Great Salt Lake and San Francisco Bay (Perumal et al., 2015), their supply is on the increase. Literature shows that in the 1980s, Artemia was inoculated in several countries including Vietnam, Indonesia, Philippines, Thailand, Sri Lanka, Iran, China, Egypt, India, and Panama (Lavens & Sorgeloos, 1996). Nevertheless, Artemia has equally spread unintentionally past the initial inoculation areas and invaded new territories in Spain, France, India, Portugal, Egypt, China and the United Arab Emirates (Van Stappen, 2002). Vietnam in South East Asia stands out having gained a considerable market share in the supply of Artemia cysts both locally and international (Le et al., 2018).
2.3.5. Characterization of Artemia strains
Strains are regarded as genetic variants or subtypes within a given biological species. In their study on determination of biological as well as physicochemical parameters of Artemia franciscana strains, Camargo et al., (2005) pointed out that to characterize Artemia strains, the aquaculture industry usually employ a range of evaluation tools. Some of the tools that have led to the identification of Artemia strain specific characteristics have been outlined to include cyst and nauplii biometry, cyst hatching characteristics (Sorgeloos et al., 1986), salinity and temperature tolerance (Clegg et al., 2001), and Essential fatty acid (EFA) profiles (Lavens & Sorgeloos, 1996). Vanhaecke and Sorgeloos (1980) indicated in their study that cyst diameter along with resistance to high temperature are deemed strain-specific and thus remain relatively constant. These characteristics have thus become genotypical subsequent adaptations of the strain to the local conditions. Therefore, with regard to biometry, cyst diameter of the same strain has been indicated to generally remain constant
16
despite the minute variations between batches that may arise as a result of processing and/or environmental factors. Literature has also shown that the other biometrical characteristics like cyst dry weight and volume, individual naupliar volume and weight, instar 1 naupliar length and energy content, among others have a high correlation with the cyst diameter (Vanhaecke & Sorgeloos, 1980). Subsequently, cyst diameter remains a good tool for characterizing Artemia strains as well as defining the origin of uneven or unknown mixed cyst samples. Correlations made between species siblings and size have demonstrated that A. persimilis and Artemia franciscana produce small or intermediate cysts having a thin chorion, Artemia salina produces large cysts with a thick chorion while the parthenogenetic Artemia produces large cysts.
Whereas cyst hatching characteristics have been used as an evaluation tool for characterizing Artemia strains, comparative studies indicate that the hatching behavior of cysts from different origins display considerable variation with regard to hatching rate, percentage, and efficiency (Vanhaecke & Sorgeloos, 1983). Therefore, given that the variations are as a result of the effects of environmental factors, genotypical conditions and/or improper storage/processing, none of these parameters is deemed strain specific.
Regarding salinity and temperature tolerance, these parameters have been shown to affect the growth and survival of Artemia with the effect of the latter being more pronounced. Furthermore, there appears to be some genetic adaptation of certain strains to high temperatures (Clegg et al., 2001). A broad range of salinities along with temperatures meets the requirements for over 90% survival. The strains from thalassohaline biotopes have a common temperature preference range of 20-25°C where mortalities are usually less than ten percent. However, considerable differences have been recorded at low salinities (5g/l) and high temperatures (30-34°C) with the GSL strain displaying significantly higher survival than the other strains at these elevated temperatures (Støttrup & McEvoy, 2008).
The Essential fatty acid profiles of Artemia as an identification tool for strains relates to the role of EFA in the diet of marine fish and crustacean larvae (Léger et al., 1986). The levels of EFA have been shown to tremendously vary from one strain to another and even from batch to batch from the same geographical source (Agh & Sorgeloos, 2005); something that has made the marine larviculture production facilities go in
17
search for EFA rich Artemia strains. However, it is important to point out that the commercial provision of Artemia cysts that have high EPA levels are limited and as a result they are extremely expensive. The use of these high-EPA cysts needs to be restricted to the feeding period when feeding of freshly hatched nauplii of a small size is needed (McVey, 1993).
2.3.6. Artemia franciscana strains
San Francisco Bay strain:-The San Francisco Bay area in the U.S.A along with several other hypersaline biotopes are home to a brine shrimp species, or subspecies frequently denoted as the "San Francisco strain" or the SFB strain of the more commonly found Artemia franciscana (Abatzopoulos et al., 2013). Other than being endemic to the New World, temporal and permanent population of A. franciscana exist across the globe because of deliberate inoculations of different strains (Amat et al., 2005). On a general note, at a wide range of salinities, A. franciscana appears to perform better in terms of reproduction and survival compared to the other bisexuals and parthenogenetic strains (Triantaphyllidis et al., 1995). A. franciscana cysts characteristics like hatchability along with easy diapause breaking makes them of high demand in larviculture. The San Francisco Bay strain cysts are small sized (cyst diameter 224.7- 228.7 µm) (Vanhaecke & Sorgeloos, 1980) and thus the newly hatched nauplii from these cysts are equally smaller in size and generally of higher HUFA value. They can be used as live food for the youngest as well as most sensitive larval stages of fish, thereby adding to their market value. The SFB strain has equally been shown to display better tolerance to low temperatures as well as high salinity ranges (Triantaphyllidis et al., 1995). With regard to temperature, a majority of the strains do not survive less than 6 ° C or greater than 35°C and tolerance to diverse temperatures has been indicated to be strain specific (Browne et al., 1988). Temperatures in the mid-twenties are expected to be optimal for survival with the SFB strains capable of tolerating both low and high temperatures (Triantaphyllidis et al., 1994).
The Vinh Chau (VC) strain:- VC-Artemia franciscana, originated from SFB and was inoculated in Vietnam over 20 years ago (Hoa, 2003). They are produced commercially in salt ponds via artificial inoculation and culture of the San Francisco Bay type Artemia franciscana (Kellogg, 1906) strain in Vietnam (Vanhaecke &
18
Sorgeloos, 1980). The Vinh Chau strains just like SFB are bisexual populations and thus reported to display higher survival and better performance (Sui et al., 2012). In comparison, Clegg et al., (1996) reported no marked differences between SFB and VC strain with regard to the metabolic status of diapause embryos of these Artemia. However, the VC strain cysts have been shown to be of larger size (cyst diameter 235.2 ± 1.3 µm (Van et al., 2014; Van Hoa & Sorgeloos, 2015) compared to those of the SFB strain. Furthermore, since its inoculation, the nature of genetic divergence between VC and the original SFB strain has been examined from a multidisciplinary approach employing reproductive traits, allozyme, and mitochondrial DNA analysis (Clegg et al., 2000; Kappas et al., 2004). For instance, having analyzed five reproductive traits at temperatures of 26°C and 30°C, the findings of (Kappas et al., 2004) revealed that the VC strain significantly outperformed the SFB strain at high temperatures compared (at 30°C). Allozyme analysis of 20 loci of the same strain showed considerable differences between the samples studied at the temperatures of 26°C and 30°C. The mitochondrial DNA Restriction fragment length polymorphism (RFLP) markers equally showed the same patterns of genetic differentiation with strong evidence suggesting a reduction in haplotype diversity from 40.6% in SFB to 10.5% in the VC strain (Kappas et al., 2004).
It is equally vital to indicate that while the VC strain cysts are readily available in the market, the San Francisco Bay strain cysts represent just a minute share of all the cysts harvested across the globe and thus their limited numbers, makes them more expensive to obtain.
2.3.7. Advantages and limitations of using Artemia as food for marine fish larvae
Brine shrimp remains the most widely used form of live feed for culturing larvae of fish and shellfish across the globe. This is attributed to the ability of Artemia to produce storable dormant embryos or cysts that can hatch swiftly into nauplii. The cysts of Artemia are usually harvested from natural hypersaline lakes in the wild or manmade salterns as dormant cysts. The fact that these dormant cysts can be stored in cans for long periods and then be used as an off the shelf food that only needs 24 hours of incubation at 28°C in saline water makes them the most convenient and attractive for culturing marine fish larvae (Bengtson et al., 2018). Other than convenience, they are
19
also preferred for their predictability of hatching success and least labor intensive among other benefits. Furthermore, freshly hatched nauplii can also be stored at temperatures near 4°C in densities of up to 8000 nauplii per milliliter for up to 24 to 48 hours when kept in moderately aerated cylindro-conical containers (Léger et al., 1983). The research by Anbaya Almalul (2000) showed that Artemia nauplii can be stored at 20 million per liter at 4°C for at least 48h with less than 20% mortality observed provided disinfection of the hatching and nauplii suspension is applied. The 24-h cold storage is deemed an advantage since the process economizes the Artemia cyst hatching effort, allows for a constant supply of product, as well as the possibility of frequent food distributions. Storing freshly hatched nauplii 4° C for 24 hours is equally helpful to the fish larvae for the reason that food retention time in larviculture tanks can be reduced thereby minimizing the growth of Artemia in the culture tank (Sorgeloos et al., 1986; Lavens & Sorgeloos, 1996). The other advantage of using Artemia as food for marine fish larvae regards the capacity to be enriched (Abatzopoulos et al., 2013). This will allow for boosting of the nutritional quality of Artemia and delivery of essential nutrients as required by the marine fish larvae.
Despite these advantages, a number of limitations have equally been reported when using Artemia in larviculture. For instance, the hatching along with the growth characteristics of Artemia have been shown to have the capacity to hamper the success of larval culture (Sorgeloos et al., 2001). The hatching of cysts may produce shells which, if not removed from the culture tank or system, could be ingested by the fish larvae (Sorgeloos et al., 1986, 2001; Bengtson et al., 2018). Furthermore, fish larvae may choke while trying to ingest these cysts shells and/or unhatched cysts and if ingested the larvae may not be able to digest them. The major limitation that has been reported when using Artemia in larviculture pertains to their nutritional value, especially in relation to their composition and/or deficiency of the essential fatty acids (Léger et al., 1985). While the other nutritional components of Artemia such as the total amount of free amino acids, vitamins, pigments, minerals and trace elements have been determined and shown to vary between strains, their effects on the success of larviculture has been deemed as less significant (Léger et al., 1987). This points to the composition of the essential fatty acids as the most significant and drawing much attention, focus as well as research (Watanabe et al., 1978; Sargent et al., 1999; Gonçalves et al., 2012).
20 2.3.8. Nutritional value of Artemia
The nutritional quality of a food organism is usually determined by a number of factors such as its ingestibility (and consequently its size and form) and its composition of dietary constituents comprising fatty acids, amino acids, minerals, and various vitamins (Yamamoto et al., 2008). However, in this review, the nutritional value of Artemia will focus on its fatty acid composition and deficiency because of the vital role it plays in larval rearing as will later be discussed. In the late 1960s and 1970s, a number of authors reported problems with success in rearing marine fish larvae and those of crustacean species when using Artemia sources other than SFB Artemia (Sorgeloos et al., 2001). While high doses of toxic compounds were initially suspected to be the cause of the poor nutritional value of Artemia from GSL and China, studies later confirmed that this was never the case. A comparative study examining eight Artemia spp. confirmed a variation in the nutritional quality among Artemia sources and further documented the nutritional variability in 11 batches of SFB Artemia nauplii for the mysid shrimp (Klein-Macphee et al., 1982; Léger et al., 1985). Findings from Léger et al., (1985) led to the conclusion that the major factor appearing to determine the nutritional value of Artemia nauplii to marine organisms was the content of the essential fatty acid, EPA: 20:5(n-3) (Léger et al., 1986). The findings of Watanabe et al. (1978) showed significant differences in the content of n-3 polyunsaturated fatty acids within several strains of Artemia. Several studies have further supported the findings of Watanabe et al. (1978) and Léger et al., (1985) and shown that Artemia are a nutritionally deficient live feed for a majority of the developing marine fish larvae (Sargent et al., 1999; Yamamoto et al., 2008). This is because despite the variability in the content of the essential fatty acids, that is DHA: 22:6(n-3), EPA: 20:5(n-3), and ARA: 20:4(n-6), Artemia also have insufficient levels compared to the requirements of marine fish larvae (Ohs et al., 2012).
In their summary of the feeding tests using a number of strains of Artemia for several species, Léger et al. (1985) suggested that Artemia nauplii containing at least 4% EPA of total fatty acid methyl esters was sufficient for better outcome with levels below 3% yielding poor results. Watanabe et al. (1982) concluded from their findings that Artemia containing at least 0.3% n-3 HUFA may be an adequate single feed for marine fish. However, with several other studies (Izquierdo et al., 1992; Ako et al., 1994; Zheng et al., 1996) indicating that the n-3 HUFA requirements may differ considerably among
21
the marine species, literature suggests that it is prudent to provide both EPA and DHA and possibly some ARA and linolenic acid in foods for marine fish (Jr, 2012). The suggested level for the dietary n-3 HUFA ranges between 2-4% of DW, including at least 1% EPA and 1% DHA deemed as sufficient to satisfy or exceed the requirements of most marine larvae (Jr, 2012). In contrast, studies have shown that Artemia are usually deficient in at least one of these fatty acids and perhaps other nutrients (Watanabe et al., 1982). The proximate levels of the essential fatty acid in Artemia were determined and shown to be low (Léger et al., 1986). Smith et al., (2002) reported findings consistent with those of Léger et al. (1986) values indicating that Artemia (non-enriched) contained only small amount of EFA ranging from 0.6 to 3.1% of the total fatty acid profile.
In the context of content variability between and within Artemia strains from different geographical areas, an assessment of the levels of EPA and linolenic acid showed much variability within strains in as much as there was already variability between the strains (Léger et al., 1986). In accounting for this variation and subsequently the levels of composition (deficiency in relation to the dietary needs of marine larvae) of fatty acid in Artemia, studies have demonstrated that the Artemia HUFA levels strongly resemble those of their recent foods (Millamena et al., 1988; Watanabe et al., 1983; Webster et al., 1991). This then shows that Artemia is definitely able to convert linolenic acid to EPA to meet its metabolic requirements, although the percentage of EPA required to meet those needs may be much less than the levels present in certain algae (Artemia food). In addition, for the newly hatched Artemia, the fat content and composition depend on the composition of the maternal diet (Millamena et al., 1988; Webster et al., 1991). Artemia cysts produced in large salt lakes such as the Great Salt Lake, Utah with one predominant algal species for instance is shown to have a more constant fatty acid profile over time as opposed to the cysts from ponds in the solar salt evaporation systems where the algal species can change noticeably spatially and temporally (Bengtson et al., 2018). Literature shows that in the recent years, the cysts from Great Salt Lake for example have contained high levels of linolenic acid 18:3(n-3) and low levels of EPA: 20:5(n-3), making them adequate for only culturing freshwater organisms (Bengtson et al., 2018).
With the realization that the nutritional quality of the commercially available Artemia strains is comparatively poor in EPA and particularly DHA, great effort has been
22
devoted towards incorporating high levels of these essential fatty acids in live food in a process described as enrichment (Sorgeloos et al., 2001).
2.4. EFA and their roles in marine fish larvae development 2.4.1. Structure and classification of fatty acids
Fatty acids (FA) accounts for a major part of phospholipids, triglycerides, and cholesterol esters (not found in a free state in nature but as organic acids). They are primarily composed of long chains of hydrocarbons that end with a carboxyl group (-COOH) (Ohs et al., 2009). The majority of naturally occurring fatty acids have been shown to contain 16- carbon atoms (palmitic acids) and 18-carbon atoms (stearic acid) (Gurr et al., 2016). FA are further classified into saturated (no double bond between carbon atoms), monounsaturated (one double bond) and polyunsaturated (two or more double bonds). The process of increasing the number of carbons in a fatty acid is denoted as elongation while that of increasing the number of double bonds is described as desaturation (Catala, 2017).
2.4.2. Saturated fatty acids (include FA structures)
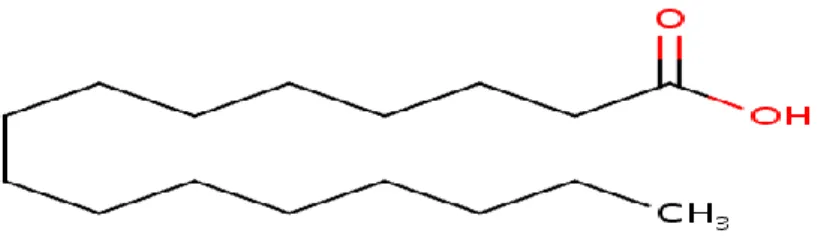
The saturated fatty acids have been described as straight-chain compounds having 14, 16, and 18 carbon atoms. The most abundant saturated fatty acids present in plant and animal tissues are esterified with odd-and even-numbered homologues with 2 to 36 carbon atoms. Some of the examples of saturated fatty acids include palmitic, stearic and eicosanoic acid (Catala, 2017).
23 2.4.3. Monounsaturated fatty acids
Monoenoic fatty acids, for example, are classified as monounsaturated fatty acids. They comprise of straight-chain fatty acids having 10 to 30 carbon atoms with a single cis-double bond. The double bond is located in different positions which is usually specified in the systematic nomenclature with regard to the carboxyl group. Some of the examples of these fatty acids are Tetradecenoic (14:1(n-5)), cis-9-hexadecenoic (16:1(n-7)) also known as palmitoleic acid (Figure 4), and trans-3-Hexadecenoic (Catala, 2017).
Figure 4- Structure of palmitoleic acid(Source: ChEBI) 2.4.4. Polyunsaturated fatty acids
2.4.4.1. Structure and classification of PUFAs
While the number of double bonds in fatty acids found in higher plants rarely exceeds three, those in algae and animals can be as much as six. And thus, the polyunsaturated fatty acids (PUFAs) are fatty acids containing multiple double bonds. They are subdivided into families according to their derivation from specific biosynthetic precursors. In each case, the families contain between two and six double bonds of the cis-configuration separated by a single methylene group and have the same terminal structure (Burdge, 2018). Two primary families of PUFAs occur in nature that are biosynthetically derived from linoleic (9-cis, 12-cis-octadecadienoic or 18:2(n-6)) and alpha-linolenic (9-cis, 12-cis, 15-cis-octadecatrienoic or 18:3(n-3)) acids. These comprise the omega-6 family of fatty acids whose parent compound (primary precursor molecule) is linoleic acid (LA) and omega-3 family whose parent
24
compound is Alpha-linolenic acid (ALA) (Burdge, 2018). While PUFAs can be found in most lipids, they have been shown to form a vital part of the phospholipids where they seem to confer unique properties to membranes, especially by decreasing their rigidity (Stillwell & Wassall, 2003).
2.4.4.2. Omega-6 (ω-6 or n-6) family
The omega-6 fatty acids have the first of their double bonds starting with the sixth carbon atom and largely consist of linoleic acid (LA) along with its derivative arachidonic acid (ARA).
Linoleic acid (LA or 18:2(n-6); Figure 5): forms a ubiquitous part of plant lipids along with those of all the seed oils of commercial importance; an example being soybean oils that have more than 50% of linoleate (Gunstone et al., 1994). While all the linoleate in animal tissues have to be acquired from the diet, it usually forms the most abundant di- or polyenoic fatty acid in most lipid classes. It equally forms a substantial part of fish oils even though the n-3 family tend to predominate in this case (Das, 2011).
Figure 5-Structure of linoleic acid (Source: ChEBI)
γ-Linolenic acid (GLA or 18:3(n-6)): is normally a small part of animal tissues estimated to be less than 1% given that it is converted rapidly to higher metabolites (Das, 2011). GLA is found in a few seed oils.
Arachidonic acid (ARA or 20:4(n-6); Figure 6): is deemed the most critical metabolite of linoleic acid in the tissues of animals. ARA is usually the most abundant polyunsaturated component of the phospholipids (Das, 2011).
25
Figure 6- Structure of arachidonic acid (Source: ChEBI) 2.4.4.3. Omega-3 (ω-3 or n-3) family
The first double bond of the omega-3 fatty acids starts with the third carbon atom. The n-3 fatty acids mainly consist of alpha-linolenic acid (ALA) along with its derivatives EPA and DHA.
Alpha-linolenic acid (ALA or 18:3(n-3); Figure 7): this is a major part of the leaves and particularly the photosynthetic apparatus of algae along with the higher plants, where most of it is synthesized. ALA can consist of up to 65% of the total fatty acids of linseed oil but varies in other plant oils such as rapeseed and soybean oils that have up to 7% of linolenate. In the animal tissue lipids, ALA tends to be a minute component (approximately <1%) with the exception being the grazing non-ruminants like goose whose amounts can be up to 10% of the adipose tissue lipids (Burdge, 2018). The other members of the n-3 family, EPA and DHA are synthesized from alpha-linolenate in plant and tissues of some animals via elongation and desaturation reactions as later described.
26
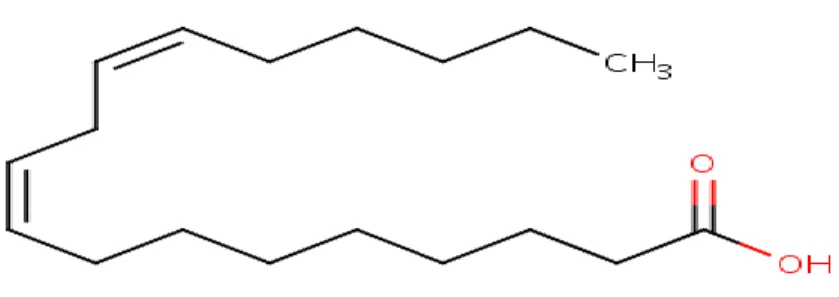
Eicosapentaenoic acid (EPA or 20:5(n-3); Figure 8): is regarded as one of the most crucial fatty acids of the omega-3 family. EPA occurs widely in algae and in fish oils and has been shown to be a vital constituent of the phospholipids in animal tissues (Burdge, 2018).
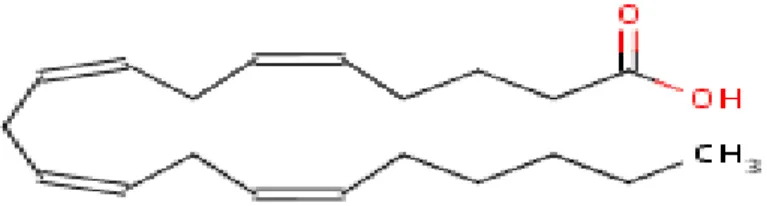
Figure 9- Structure of EPA (Source: NCBI PubMed)
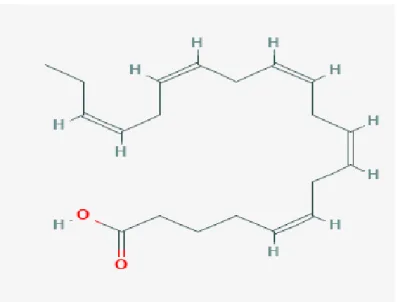
Docosahexaenoic acid (DHA or 22:6(n-3)): is normally the end point of alpha-linolenic acid metabolism in animal tissues. DHA forms a major component of fish oils, particularly the eyeballs of tuna and of phospholipids in animals. Literature also shows that DHA is found in high concentrations in various algae species, particularly the ones of the marine origin but absent in higher plants (Kim, 2015). As a constituent of phospholipids, DHA has great effects on the properties of membranes modulating their structure along with function. Structure of DHA is shown in Figure 9.