International Master of Science in Environmental Technology and Engineering
Master’s dissertation submitted in partial fulfilment of the requirements for the joint degree of EU Erasmus+ Master course organized by
University of Chemistry and Technology, Prague, the Czech Republic IHE Delft Institute for Water Education, Delft, the Netherlands
Ghent University, Ghent, Belgium Academic year 2018-2020
Assessment of sustainable
technologies for pig manure
valorization – a meta-analysis
Ghent University, Ghent, Belgium
Vaibhav Shrivastava
Thesis ID identifier: ES-IMETE.20-28 Promotors: Prof. Filip Tack, Prof. Erik Meers
International Master of Science in Environmental Technology and Engineering
Master’s dissertation submitted in partial fulfilment of the requirements for the joint degree of EU Erasmus+ Master course organized by
University of Chemistry and Technology, Prague, the Czech Republic IHE Delft Institute for Water Education, Delft, the Netherlands
Ghent University, Ghent, Belgium Academic year 2018-2020
Assessment of sustainable
technologies for pig manure
valorization – a meta-analysis
Ghent University, Ghent, Belgium
Vaibhav Shrivastava
Thesis ID identifier: ES-IMETE.20-28 Promotors: Prof. Filip Tack, Prof. Erik Meers
i Copyright Statement
This is an unpublished M.Sc. dissertation and is not prepared for further distribution. The author and the promoter give the permission to use this Master dissertation for consultation and to copy parts of it for personal use. Every other use is subject to the copyright laws, more specifically the source must be extensively specified when using results from this Master dissertation.
The Promoter 1: The Author:
Prof. Filip Tack Vaibhav Shrivastava
The Promoter 2:
ii Preamble
This preamble concerns the impact of Coronavirus COVID-19 (outbreak in 2020) on the master's dissertation work conducted on the initial topic of “Use of digestate and charred digestate (biochar) to increase stable organic carbon in soils”.
The intended focus of the study was to employ the use of various valorization mechanisms for the recovery of phosphorous from raw pig manure. Initially, for the month of February and March 2020, the experiments with pig manure were conducted at the Faculty of Bioscience Engineering (ECOCHEM lab), Ghent University. However, after the ban on 16th March 2020 on all lab activities at UGent, no further experiments could be carried out. Due to this inaccessibility, the application and analysis of prepared samples in pot experiments could not be continued, and not enough data was obtained from the previous experiments for proper interpretation.
To deal with the following crisis, a reorientation towards a detailed critical literature-based statistical study (meta-analysis) was proposed for the final thesis writing. This further led to the modification of the master’s dissertation title to “Assessment of sustainable technologies for pig manure valorization – a meta-analysis”. The current thesis focuses majorly on the trend of nutrients recovery (particularly focusing on N and P) via use of various valorization mechanisms over the span of last two decades.
This preamble was drawn up after consultation between the student and the supervisor and is approved by both the parties.
The Promoters: The Author:
Prof. Filip Tack Vaibhav Shrivastava
iii Abstract (English)
During the last decades, the increasing concern about the environmental sustainability, especially in terms of natural resources consumption and intensive agro-livestock practices, became widely recognized. In this context, the production of fertilizers from waste resources (manure) plays a crucial role. Moreover, the livestock sector produces an important source of nitrogen and untapped nutrients whose direct use is restricted due to potentially negative environmental consequences. Accordingly, there is an increasing interest in translating a quantitative waste problem into an important recovery and reuse opportunity. The goal of this study was to evaluate the valorization mechanisms related to different scenarios focused on pig manure treatment: (a) ammonia stripping (b) pyrolysis and hydrothermal carbonization (c) crystallization (d) solid-liquid separation and (e) biological processes. The following categories were considered for assessment in accordance with literature: yearly distribution, country wise distribution, recovered nutrients, technological feasibility, and economics. The reported factors such as nutrient concentration in initial and final product were analyzed by the software Review Manager 5.4 to identify the effectiveness of different valorization pathways in comparison to initial products. Pyrolysis and crystallization have come forward as the most efficient options for N and P recovery, respectively. Furthermore, an economic analysis was performed. The price trend for fertilizers over the period of last 20 years has shown an overall growth trend, attaining an all time high in the year 2009. Additionally, comparison between the cost-to-benefit ratio of different valorization mechanism evaluated crystallization and pyrolysis with the lowest payback periods and a good profit margin over initial products.
iv Abstract (Dutch)
In de afgelopen decennia werd de toenemende bezorgdheid over de ecologische duurzaamheid, vooral in termen van het gebruik van natuurlijke hulpbronnen en intensieve landbouwhuisdieren, algemeen erkend. Hierbij speelt de productie van meststoffen uit afvalbronnen (mest) een cruciale rol. Bovendien produceert de veehouderij een belangrijke bron van stikstof en onaangeboorde nutriënten waarvan het directe gebruik beperkt wordt vanwege mogelijk negatieve gevolgen voor het milieu. Dienovereenkomstig is er een toenemende interesse om een kwantitatief afvalprobleem te vertalen naar een belangrijke kans op terugwinning en hergebruik. Het doel van deze studie was om de valorisatiemechanismen te evalueren die verband houden met verschillende scenario's gericht op de behandeling van varkensmest: (a) strippen van ammoniak (b) pyrolyse en hydrothermische carbonisatie (c) kristallisatie (d) vaste stof-vloeistofscheiding en (e) biologische processen . De volgende categorieën kwamen in aanmerking voor beoordeling in overeenstemming met de literatuur: jaarlijkse distributie, verdeling per land, teruggewonnen nutriënten, technologische haalbaarheid en economie. De gerapporteerde factoren zoals nutriëntenconcentratie in begin- en eindproduct werden geanalyseerd door de software Review Manager 5.4 om de effectiviteit van verschillende valorisatiepaden in vergelijking met de oorspronkelijke producten te identificeren. Pyrolyse en kristallisatie zijn naar voren gekomen als de meest efficiënte opties voor respectievelijk N- en P-terugwinning. Verder is er een economische analyse uitgevoerd. De prijsontwikkeling voor meststoffen over de periode van de afgelopen 20 jaar heeft een algemene groeitrend laten zien, en bereikte een recordhoogte in het jaar 2009. Bovendien evalueerde een vergelijking tussen de kosten-batenverhouding van verschillende valorisatiemechanismen kristallisatie en pyrolyse met de laagste terugverdientijden en een goede winstmarge ten opzichte van initiële producten.
v Acknowledgement
I would like to express my appreciation and gratitude for the ones who have been supporting and involving for the completion of this master thesis.
First and foremost, my praise is to ALLAH, the Almighty, the most Merciful and Beneficent, for giving me opportunity, determination, and strength to do my research. His continuous grace and mercy were with me throughout my life and ever more during the tenure of my research. My sincere gratitude goes my promoter, Prof. Filip Tack and Prof. Erik Meers of the Laboratory of Applied Analytical and Physical Chemistry, for allowing me to embark on this project. His valuable guidance, advice and feedback during the course of this thesis helped make this work a success.
I would like to thank my tutor, Caleb Elijah Egene, for his continuous assistance ranging from setting up laboratory experiments, practical advice, editing of the thesis and full cooperation during the entire period of this study. Even off-field, Caleb helped me by going off his way many times, hence making the word “tutor” whole in every aspect. During my journey with him, I not only earned a “Guru” but also a true friendship, which will hopefully last for our lifetimes.
I must also thank European Union for providing me with opportunity to take part in Erasmus Mundus and made it possible for me to study at Ghent University. Furthermore, I would also like to thank all the staff members at UCT Prague, IHE Delft and Ghent University for organizing an excellent IMETE programme.
Finally, this document will go incomplete without thanking my parents for their prayers, support and encouragement that have helped me get to where I am today. And how can I forget to thank the most priceless person, my friend, Izba Ali, who has stick to me through all thick and thin and has been always been on my side. This is a debt that I can’t pay back in their entire lifetime (but perhaps small installments in the form of cakes, chocolates and sweets can appease a bit). I dedicate this work to all of them.
vi Acronyms and abbreviations
CI: Confidence Interval df: Degrees of Freedom
DOC: Dissolved Organic Carbon DOI: Digital Object Identifier
EEA: European Environmental Agency EPA: Environment Protection Agency EU: European Union
FAO: Food and Agriculture organization of United Nations
HM: Heavy metals
HTC: Hydrothermal Carbonization JSTOR: Journal storage
LPELC: Livestock and Poultry Environmental Learning Community MD: Mean Difference
MPCA: Minnesota Pollution Control Agency
MT: Metric Tons
NPK: Nitrogen-phosphorus-potassium NVZ: Nitrate Vulnerable Zones OC: Organic Carbon
OM: Organic matter p: Probability
RE: Recovery Efficiency RevMan: Review Manager SD: Standard Deviation SP: Struvite precipitation TKN: Total Kjeldahl nitrogen
UNDESA: United Nations Department of Economic and Social Affairs
USGS: US Geological Survey
vii Table of Contents:
Copyright Statement ... i
Preamble ... ii
Abstract (English) ... iii
Abstract (Dutch)... iv
Acknowledgement ... v
Acronyms and Abbreviations ... vi
List of figures ... ix
List of tables ... x
Table of Contents: ... vii
Introduction ... 1
Literature review ... 4
2.1 Manure as a fertilizer ... 4
2.2 Relevance of N and its availability in the ecosystem ... 4
2.3 Relevance of P and its availability in the ecosystem... 6
2.4 Concerned legislation... 7
2.5 Use of manure for agriculture ... 9
2.6 Heavy metals ... 10
2.7 Factors affecting composition of pig manure ... 11
2.8 Valorization technologies... 14
2.8.1 Hydrothermal carbonization ... 14
2.8.2 Ammonia stripping ... 14
2.8.3 Crystallization ... 15
2.8.4 Pyrolysis ... 15
2.8.5 Mechanical Solid-liquid separation ... 17
2.8.6 Biological Treatment ... 17
Methodology ... 21
3.1 Data and literature sources ... 21
3.2 Study selection ... 21
3.3 Data extraction ... 22
3.4 Data analysis ... 22
3.5 Strategy for data assessment ... 22
viii
3.5.2 Quantitative assessment ... 23
3.5.3 Heterogeneity assessment ... 23
3.6 Risk-of-Bias assessment ... 24
Results and discussion ... 25
4.1 Overview of the data collection ... 25
4.2 Data distribution ... 27
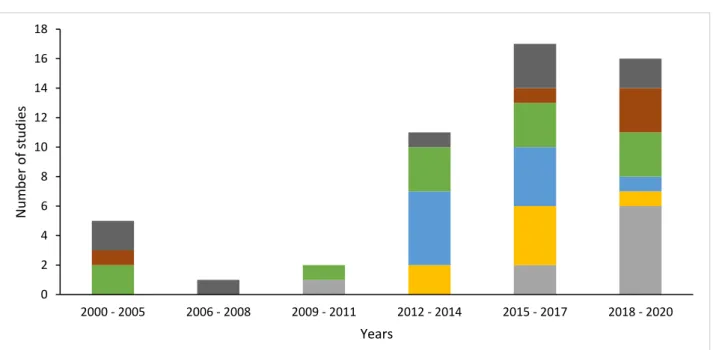
4.2.1 Year wise distribution of studies ... 27
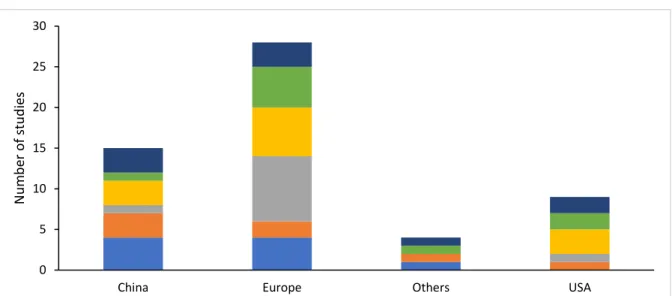
4.2.2 Country wise distribution of studies ... 28
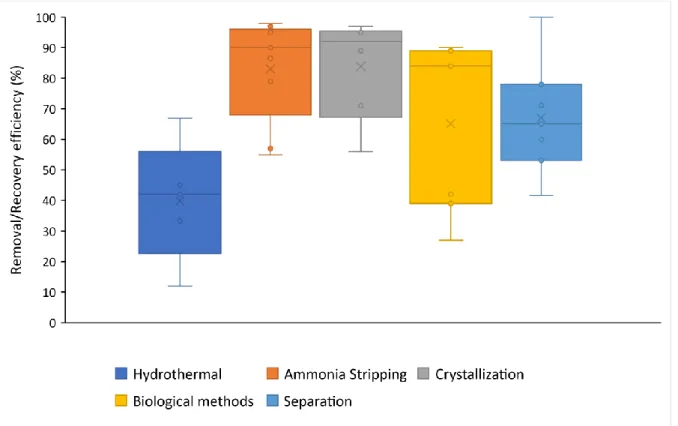
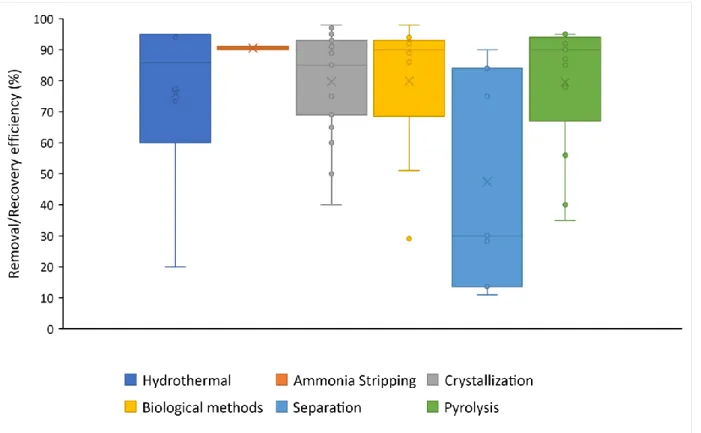
4.2.3 Nutrient wise distribution of studies ... 29
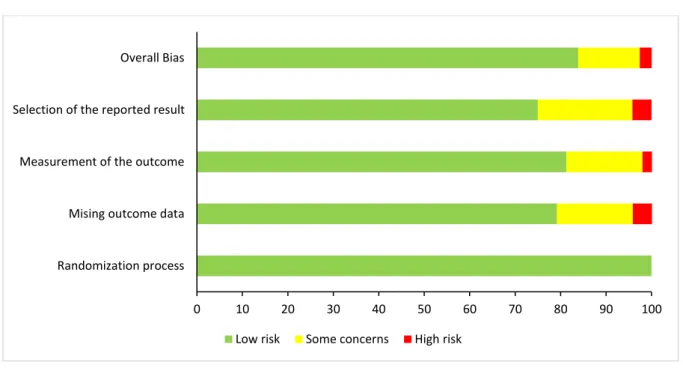
4.3 Risk-of-Bias ... 32
4.4 Quantitative analysis of studies ... 32
4.4.1 Ammonia Stripping ... 32 4.4.2 Pyrolysis ... 33 4.4.3 Crystallization ... 34 4.4.4 Hydrothermal Carbonization ... 35 4.4.5 Separation ... 36 4.4.6 Biological ... 37 4.5 Economics ... 39
4.5.1 Economic evaluation of fertilizers ... 39
4.5.2 Economic assessment of available technologies ... 41
4.6 Overall techno-economic comparison ... 43
Conclusions ... 44
References ... 45
Annex 1 ... 60
ix List of figures
Figure 1. Cycle for biogas generation and utilization (Shrivastava et al, 2019). ... 19
Figure 2. Data screening and selection steps for systematic review ... 26
Figure 3. Distribution of concerned dataset across last two decades ... 27
Figure 4. Country wise distribution of studies ... 29
Figure 5. Comparison of RE various valorization technologies for nitrogen (x = mean, --- = median and dot = datapoints) ... 30
Figure 6. Comparison of RE various valorization technologies for phosphorous (x = mean, --- = median and dot = datapoints) ... 31
Figure 7. Risk-of-bias assessment for the dataset ... 32
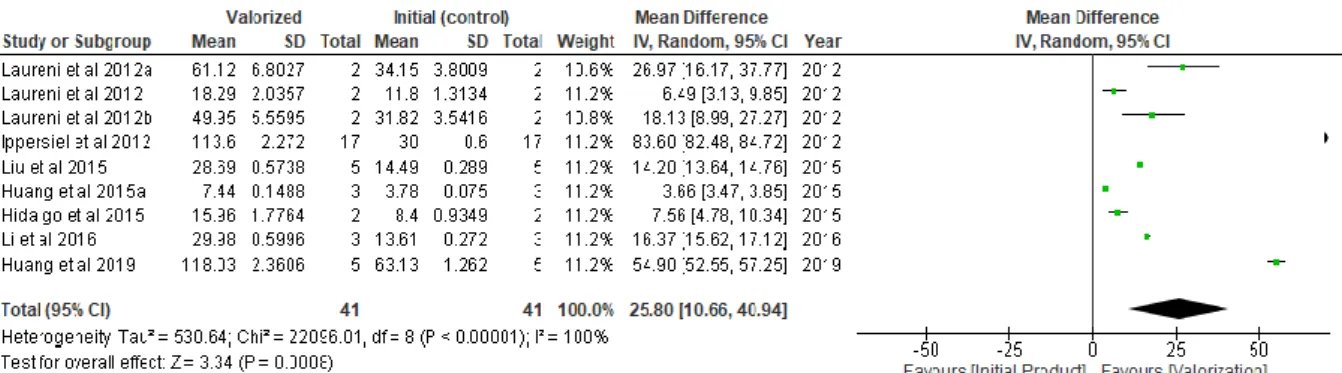
Figure 8. Trend of valorization against initial product for nitrogen recovery across studies (values in dg/L) ... 33
Figure 9. Trend of valorization against initial product for phosphorous recovery across pyrolysis (values in mg/g) ... 34
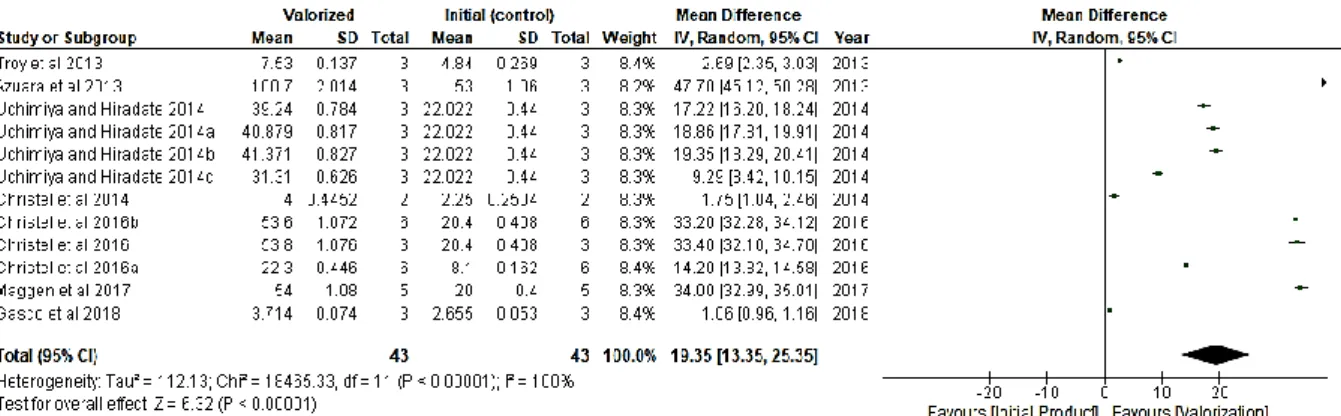
Figure 10. Trend of valorization against initial product for nitrogen recovery across crystallization (values in mg/kg) ... 34
Figure 11. Trend of valorization against initial product for phosphorous recovery across crystallization (values in mg/g). ... 35
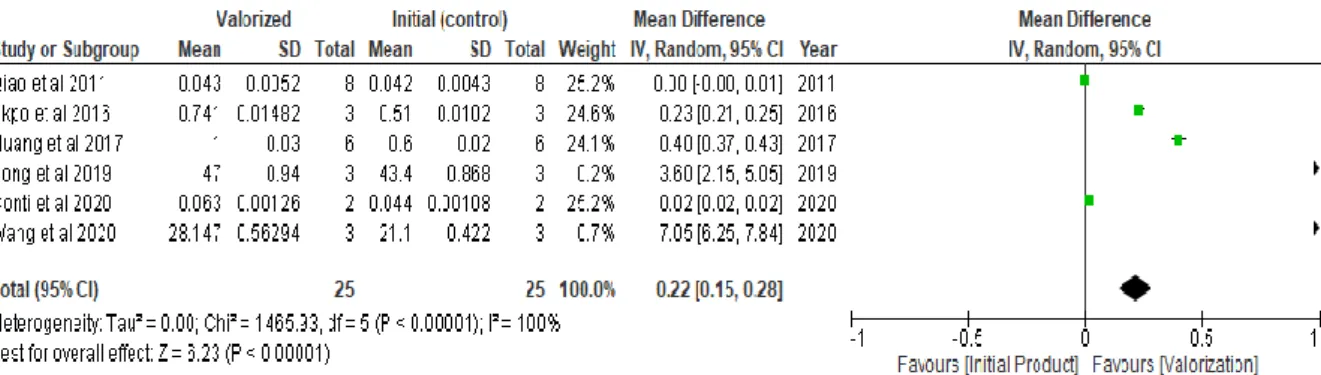
Figure 12. Trend of valorization against initial product for nitrogen recovery across hydrothermal carbonization (values in g/kg) ... 36
Figure 13. Trend of valorization against initial product for phosphorous recovery across hydrothermal carbonization (values in g/kg) ... 36
Figure 14. Trend of valorization against initial product for nitrogen recovery across separation processes (values in mg/dL) ... 37
Figure 15. Trend of valorization against initial product for phosphorous recovery across separation processes (values in mg/L) ... 37
Figure 16. Trend of valorization against initial product for nitrogen recovery across biological processes (values in mg/L) ... 38
Figure 17. Trend of valorization against initial product for phosphorous recovery across biological processes (values in mg/L)... 38
Figure 18. Trend of market price (global) for fertilizers over the last 20 years (The World Bank, 2020). ... 40
x List of tables
Table 1. Phosphorus (total) application standards in Flanders region (Amery and Schoumans, 2014) ... 8 Table 2. Nutrient application rate requirements for manure (MPCA, 2019) ... 9 Table 3. Manure production (liquid content) and nutrient content for major livestock species (Shrivastava et al, 2019). ... 10 Table 4. Quantity of nutrient elements excreted in manure of various animals during
reproduction cycles* ... 13 Table 5. Treatment processes diversification in the selected dataset... 27 Table 6. Economic comparison for various valorization technologies ... 42
1
Introduction
The European Union is the world's 2nd largest meat producer, behind China, with 63.85 million tons contributing to about 14% of the world's production (Ritchie and Roser, 2019). The United states comes 3rd in the list, with a meat production of 46.66 million tons. In January 2020, the swine herd population in the world was estimated at 677.6 million animals (Shahbandeh, 2020). This activity resulted in generation of 178 million m3 per year of swine manure in Europe alone, a volume that would fill up to 71,000 Olympic-sized swimming pools (European commission, 2018). This has shifted focus of environmental enthusiasts on manure related problems such as emissions of greenhouse gases from livestock production, runoff, over fertilization of soils and leaching. It is estimated that pig supply chains generate 0.7 gigatons of CO2 equivalent per
year, representing 9% of total emissions from the livestock sector of world (MacLeod et al, 2013). Furthermore, emissions from cattle production contributes to 41% of total emissions from livestock sector.
Pig manure is rich in organic matter and nutrients including nitrogen, phosphorus, and potassium. However, use of pig manure for agriculture is only possible when it is a balance with the number of livestock. The EU Nitrate directive recommends maximum dosage quantity as 170 kg of nitrogen per ha per annum (Council Directive 91/676/EEC). When animal husbandry is intensive, production of pigs with enhanced diets will release large quantities of nitrogen and phosphorus and lead to accumulation of copper and zinc, fed to pigs, in the soil. Such imbalances can lead to severe environmental problems affecting soil quality (drainage, plants toxicity, pathogenic activity), water (nitrate pollution of the groundwater or eutrophication of the surface water) and air (bad odours, greenhouse gas emissions) (Camargo-Valero et al, 2015). On the other hand, production of conventional synthetic fertilizers has tremendous environmental impacts. Alone, fertilizer industry accounts for 1.2% of the overall energy consumption of the planet. Ammonium is the basic component for nitrogen fertilizer production, but the process of its production is very energy intensive (36.6 GJ/t NH3) and
produces high CO2 emissions (1,966.8 kg CO2 eq/kg) and nitrogen oxides (European commission, 2018).
For dealing with the problems of manure management and fertilizer production sustainability, different valorization approaches of manure have been proposed. More rudimentary approaches involve designing storage such that the release of greenhouse gases is significantly reduced. However, more focus has recently been given to the application of different
2 technologies to valorize pig manure as organic soil amendments or mineral fertilizer substitutes.
Among the alternatives for pig manure treatment, aerobic composting is a relatively simple operation that yields a valorized product with high nutrient content (Chen et al, 2010). Another useful method to treat pig manure is biogas production through anaerobic digestion. The process of anaerobic digestion can also yield renewable energy (clean and cheap methane), soil conditioner and liquid fertilizer. Moreover, the biogas production could be enhanced by the anaerobic co-digestion technique compared to the single-substrate digestion (Wang et al, 2012).However, the anaerobic digestion is a complex process and microbial reactions can be inhibited by many factors such as sunlight-dark conditions. Recycling of pig-manure can also be achieved by applying it as a fertilizer for pond carp production (Zoccarato et al, 1995). Furthermore, it can be incorporated into algal biomass production, via aerobic fermentation, which can be used for animal feeding (Martin, De la Noüe and Picard, 1985). Swine manure derived biochar through slow pyrolysis has the potential to be used as an excellent soil amendment (rich in NPK) as well as a cheap adsorbent to immobilize contaminants (Tsai et al, 2012; Zhang et al, 2013).
The goal of this study is to quantitatively assess and compare the techno-economic sustainability of existing valorization technologies for pig manure by pooling the results of several previously conducted studies.
The specific objectives of this study are to:
• Summarize the state of the art of options for the down processing and valorization of pig manure.
• Evaluate and compare the various transformed products based on the recovery efficiency and mean difference obtained for various nutrients.
• Quantitatively compare the replacement value of products derived from pig manure for mineral N and P fertilizers based on nutrient availability and nutrient use efficiency.
This work is organized under 5 chapters. Introduction (chapter 1) provides a brief summary and statistical data about the background information on manure, problem statement and objectives of the thesis. Literature Review (chapter 2) consists with an overview of the environmental relevance and significance of nutrients (nitrogen and phosphorus) and importance of manure in agricultural application and nutrient valorization. It also describes various legislations and other concerns regarding agricultural application of manure
3 Methodology (chapter 3) describes the procedure of data extraction from various online databases as well as the inclusion/exclusion criteria. Furthermore, procedure of data interpretation through RevMan and other methods are also explained. Results and Discussion (chapter 4) provides a summary of the research trends on the basis of year wise and country wise studies and explains the significance of the results obtained from the statistical analysis (through RevMan and recovery efficiency) of the collected dataset. A brief discussion on economics of various technologies is also performed. Conclusion (chapter 5) conveys the conclusion of this study discussing best valorization technology for different nutrients.
4
Literature review
2.1 Manure as a fertilizer
Manure is in many cases the main fertilizer source for farmers, which has long been closing the nutrient cycling. However, the overall sum of nutrients in regions with intensive livestock farming systems often exceed the nutrient requirements of the crops. In these areas, the excess nutrients such as nitrogen (N) and phosphorus (P) results in their high concentrations in soil, surface waters, and atmosphere (Velthof et al, 2014). The demands for minerals, on the other hand, are high, and many minerals, such as phosphorus (P) and potassium (K), now mined, are becoming increasingly scarce. Therefore, nutrient removal and recovery from manure has recently received a lot of attention in regions with intensive livestock farming such as The Netherlands and Belgium (esp. Flanders) (Schoumans et al, 2015).
In order to boost water quality and to compel the member states to set up an action plan, the European Nitrates Directive was been implemented in 1991 (EEA, 2020). Manure production was developed in these vulnerable areas since the 1990's to extract excess nitrogen or export nutrients to less nutrient-dense areas (Velthof et al, 2014). Now, with the high costs of fossil-based fertilizer and lower stocks of phosphorus and potassium, the focus of the farmers and fertilizers producers is towards shifting from the manure processing to more advanced techniques for nutrient recovery.
In the recent times, the extraction of nutrients from pig slurry has gained a strong interest among researchers. However, handling and application of the valorized product always remain a question of interest. Nowadays, the most effective method of handling pig slurry is to biologically treat the thin fractions and dry them or composting the dried solid fraction and export them to nutrient deficient areas (Schoumans et al, 2015). The composting and pelleting processes are well known and relatively easy to execute. However, in contrast to raw manure, the costs of transport are not much lower and much compost in crop farms is denied because it has a low N/P ratio that does not meet the needs of the majority of arable crops. Therefore, it is more interesting to valorize the components of manure into valuable products (Schoumans et al, 2015).
2.2 Relevance of N and its availability in the ecosystem
Nitrogen is an abundant element on Earth; it makes up 78.1% of Earth's atmosphere and is an essential nutrient for all forms of life. Most of this Nitrogen is available in the form of unreactive nitrogen (N2) that most living organisms cannot use. However, part of it, which is
5 fixed by natural or anthropogenic processes [N, which includes nitrogen oxides (NOX), reduced nitrogen (NHX), nitrous oxide (N2O), nitric acid (HNO3), and other organic and inorganic
forms] is available for use by living organisms (Stevens, 2019). The quantity of N released into the aquatic environments from human activities has increased over the past century to such a degree that it is now beyond natural fixation, resulting in a more than doubled global cycling of nitrogen (anthropogenic N production, 210 Tg N per year; natural N production, 203 Tg N per year) (Stevens, 2019). In many parts of the world, due to this increase in nitrogen fixation, air, water and soil pollution has become an important aspect of concern.
Nitrogen use is, however, substantially disproportionate worldwide. In countries outside the Organization for Economic Co-operation and Development (OECD) and major emerging economies, the amount of nitrogen taken up by the crops remains low. Not only are there insufficient fertilizers, the available nutrients are also inefficient in usage. Sub-Saharan Africa provides an appropriate example. In 2012, nutrient-poor soils yielded an average of 1 metric ton (MT) ha−1 for grain crops, with fertilizer use averaging 9 kg ha−1 of the cultivated soil (Stevens, 2019). On the other hand, Asia, where there are major emerging economies, has achieved crop yields exceeding 4.5 MT ha−1 with fertilizer applications of 96 kg ha–1. Lack of nitrogen obviously contributes to significant problems in fulfilling people's nutritional requirements. These problems are just as difficult to address as the problems that nitrogen pollution causes in other parts of the world.
For the preparation of chemical fertilizers, the Haber Process, also called the Haber-Bosch Process is employed. It is a complex chemical procedure which uses nitrogen from the air and combines it with hydrogen to produce ammonia under high pressures and temperatures. Today, this ammonia is the basis of the widely used synthetic nitrogen fertilizer worldwide (Gellings and Parmenter, 2016). Haber processes produce approximately 500 million tons of fertilizer per year (453 billion kilograms). This fertilizer helps feed about 40% of the population of the planet. Even after so many advantages, on the curse side, we have several socio-environmental issues with the production of nitrogen fertilizers using this technology (Gellings and Parmenter, 2016). First, this is a high energy process and takes up about 1 – 2% of the world’s energy supply every year (Harford, 2017). According to 2017 statistics, this number corresponds to around 500 TWh. Furthermore, fertilizers seep into rivers which causes algae blooms. In turn, bacteria feed on the dead algae using up oxygen in the water, as a result killing aquatic animals. This further contributes in causing ocean dead zones.
6 2.3 Relevance of P and its availability in the ecosystem
Phosphorus is the 11th most common element found on Earth with a concentration in the crust
of about 1 g/kg and it is safe to say that is fundamental to all biotic species (Tiessen 2008). It is important for creating DNA, cell membranes, and also for the creation of human bones and teeth. This is important for food production because it is one of three nutrients used in commercial fertilizer (nitrogen, potassium, and phosphorus). It is not present free in nature but is widely dispersed, generally as phosphates, in many minerals. Inorganic phosphate rock partially consisting of apatite is the source of most of the world’s phosphorus up to date (Tiessen 2008).
In agriculture and food production, around 90% of the world's mined phosphate rock is used, more as fertilizer, less as animal feed and food additives (FAO 2020). Moreover, the market polarity makes this resource more inaccessible due to political or geographical reason. According to the US Geological Survey (USGS), about 50% of the global reserves of phosphorus are with the Arab nations. Major apatite deposits are also found in China, Russia, Morocco and USA (in Florida, Idaho, Tennessee and Utah). Moreover, the phosphorus that is available in the nature cannot be extracted up to the demands of the modern world due to physical, economic, energy or legal constraints (USGS 2020). In addition to this, majority of this extracted phosphorus is lost in the processing. About 30 – 40% is lost during mining and processing and another 50% is wasted in the complexity of the food chain (Renee, 2013). Just 20% of phosphorus in phosphate rock enters global food consumption. Much of the excess phosphorus from farm or fertilizer runoff or from phosphates in detergent and soda washed into drains reaches our rivers, lakes and oceans and results in eutrophication (Renee, 2013). It is a severe form of water contamination in which algae blooms produce, then die, consume oxygen, and create a "dead zone" in which nothing can survive. There are over 400 dead coastal areas at river mouths that are rising at a rate of 10% per decade (Renee, 2013).
The current extractable phosphorus reserves, according to the reliable estimates show the capability of phosphate rock resources to last an additional 300 – 400 years. With a world population predicted to hit 9 billion by 2050 and needing 70% more food than we currently generate, and a rising global middle class that consumes more meat and milk, phosphorus is crucial to global food security (Renee, 2013). Yet there are no international bodies or laws that control capital from global phosphorus. In these crucial times, the experts are looking at the other sources of phosphorus which can be used to meet the global P demand in the future.
7 Animals and people excrete nearly all of the phosphorus they consume in food. For ages, animal manure has been applied to soil for improving the nutrient composition of soil, aiding crop production. Even today, in some parts of the world (e.g. Vietnam), manure is directly applied to the soil as a fertilizer (Shrivastava et al, 2019). However, this practice is mostly done in developing countries or in others with fragile or undrafted legislation.
In the context of the EU countries, phosphate rock is classified as a critical raw material by the European Commission (European Commission, 2019). The phosphorus is completely imported to serve the necessary demands therefore making it vulnerable to market fluctuations in fertilizer and mineral P prices (The World Bank, 2020). Many studies have been done in recent years to understand the P-flow analysis (PFA) that provide insight into how people use and reuse P and how P on various spatial scales is lost to the environment (Chowdhury et al, 2014). PFA findings indicate that significant amounts of P are lost outside agriculture, by wastewater and biodegradable solid waste, in manufacturing, consumption and waste handling sectors. National PFA studies show that there are significant variations in phosphorus flow within countries and within regions. However, the research on the extraction of phosphorus from different sources is developing rapidly. Municipal wastewater, sewage sludge and animal manure are some of the promising sources of P recycling to replace P derived from phosphate rocks (Ehmann et al, 2017).
2.4 Concerned legislation
Due to the environmental threats (such as organic micropollutants and pathogens) associated with sewage sludge, its agricultural application is prohibited by legislation or is not practiced in many European countries. Owing to these possible environmental and health threats, acceptance of direct applications and thus direct P recovery in many European countries is poor or declining (Ott and Rechberger, 2012). For the latest alternative methods of handling waste, such as cement co-incineration, caloric power plants and waste incinerators, P is irreparably lost. Moreover, one of the biggest flaws in the current EU legislation regarding the soil application of manure is its “status”. The manure, even after any kind of treatment or digestion is particularly considered as waste, which further limits its application as a fertilizer alternative. However, this legislation is under revision currently at the EU, increasing the chances of more usage of manure in the future applications.
One way to reduce the direct risk of P losses to surface waters is to restrict the P application by setting maximum application levels. In addition, general legislation regarding the use of manure or fertilizers can indirectly restrict the use of P in agriculture. Manure typically has a
8 N/P ratio of 2 to 8, as opposed to the N/P ratio of 7 – 11 required by crops for efficient growth (Amery, 2014). This results in an excess of P if the crop's N requirement is met using only manure. The application of manure in (NVZ) is limited to 170 kg N/ha/yr or a higher value in case of derogation. It helps to restrict the application of P to soil, depending on the N/P ratio of the manure applied (Amery, 2014). The limits may have a standard value (which are established according to local legislations) but may also be distinguished with respect to crop type, soil phosphorus status or even crop yield. Most laws apply to all the types of (acceptable) phosphorus application but often only the use of manure or inorganic fertilizer is regulated. If the case of Belgium, a new Manure Decree comes into effect in Flanders every four years as a means of enforcing the Nitrates Directive. This new Manure Decree (Anon, 2011) tackles the distribution of phosphorus and nitrogen. The current application standards generally fluctuate between 65 and 95 kg P2O5/ha/yr depending upon the type of crop. The average application
levels for phosphorus are around 5 kg P2O5/ha/yr less than the crop's general uptake of
phosphorus, resulting in a low, negative injection of phosphorus into the soil (Amery, 2014). Phosphorus can be supplied to the soil in the form of compost, organic materials or natural fertilizers (only chemical P fertilizers cannot be used by derogation farms). Table 1 shows the directive principles for various crops.
Table 1. Phosphorus (total) application standards in Flanders region (Amery and Schoumans, 2014)
Crop
Application limits (kg P2O5/ha/y)
2011-2012
2013-2014 2015-2016* 2017-2018*
Grassland (only mowing) 95 95 95 90
Grassland (not only mowing) 90 90 90 90
1 cut grass/rye + maize 95 95 95 90
Maize 80 80 75 70
Winter wheat - triticale 75 75 70 70
Other cereals 75 70 70 70
Other crops 75 65 55 55
* limits for 2015-2018 are indicative
Just 40 kg of P2O5/ha/yr can be added to soils found in saturated phosphate areas. No
phosphorus can be used on soils located in security zone category 1 i.e. the drinking water extraction areas. No phosphorus can be added to these agricultural soils in vulnerable natural areas (Anon, 2011).
9 In case of United States, the legislations define the limits in the form of Animal Units. The overall application levels of manure on all soils are limited by crop-available nitrogen. Nevertheless, criteria for the phosphorus-based rate must also be met in certain specific circumstances (MPCA, 2019). Table 2 shows the summary of nutrient application rate requirements for manure.
Table 2. Nutrient application rate requirements for manure (MPCA, 2019)
Nutrient type Application rate requirement
Nitrogen (N) a) Cannot exceed crop N needs for non-legumes.
b) Cannot exceed crop N removal for legumes.
Phosphorus (P) a) No long-term soil P build-up near waters.
b) Manure management plan with P management strategy required if applying to extremely high P soils and facility is over 300 animal units.
Potassium (K) No restrictions in rule.
There is no legislation in India governing the use of P fertilizer, as a result of which the soil check for P showed a moderate status in cultivated soils whereas the P deficiency in soils was maximum two decades earlier. The excess N-P-K complex fertilizer is more of a compulsion than an exception in most irrigated soils due to extensive ads from chemical fertilizer manufacturers and traders to improve their industry with complete disregard for soil and water quality and the related environmental pollution problems. Sadly, soil testing for P or any nutrient for that matter is not needed to decide on crop need or otherwise fertilization. Thanks to the increase in chemical fertilizer prices that ultimately swayed farmers from opting for organic fertilization such as composting, vermicomposting, green manure, green leaf manure, oil cakes etc. (Reddy, 2015). Legislation to encourage the use of chemical fertilizers and pesticides, herbicides are imperative for every nation, if not the planet, to ameliorate the quality of natural resources and the life of all Earth's species.
2.5 Use of manure for agriculture
As per 2017 census, over 115 million tons of nitrogen (Mt N) has been supplied as an input into agricultural soils by animal manure globally (FAO, 2019). 75 % (88 Mt N) of this animal manure is left permanently in paddocks and pastures. One quarter (27 Mt N yr-1) was treated in manure management systems and applied as fertilizer; and another one
10 third of N deposited or applied to soils (34 MtN) was lost to leaching (FAO, 2019). More than 50% of all animal manure was produced by cattle. The overall livestock manure N production increased by 0.6%, compared with the mean growth of 0.9% for previous ten-year cycle (2008-2017). The average cumulative growth rate was 1.3% per ten-year, between 1961 to 2017. In 2017, the production of cattle manure was 75% higher than in 1961. In 2017, Brazil (7.9 Mt N from beef cattle), the United States of America (3.0 Mt N from beef cattle) and China (2.0 Mt N from goats) reported the highest N inputs of pasture by country and animal type (FAO, 2019). Furthermore, the largest inputs of manure treated and applied to soils by country and animal type were in China (1.4 Mt N from pigs), India (1.0 Mt N from buffalo) and again China (0.9 Mt N from chicken) (FAO, 2019).
Table 3. Manure production (liquid content) and nutrient content for major livestock species (Shrivastava et al, 2019).
Animal Manure Produced
(kg yr-1) Nutrient Concentration (kg 1000 l-1) Total N Total P NH3-N Swine (Farrow-finish) 17007 3.4 1.3 1.9 Dairy (Cow) 24490 3.7 0.8 0.7 Dairy (heifer) 11338 3.8 0.7 0.7 Beef (Cow) 13605 2.4 0.8 0.8 Poultry (layer) 59 6.8 2.7 4.4 Poultry (broiler) 38 7.6 2.1 1.6 Poultry (turkey) 128 6.4 2.1 1.9 2.6 Heavy metals
Pig manure is a sustainable nutrient source that has been commonly used as organic fertilizer in agriculture. Nevertheless, the presence of antibiotic residues and heavy metals (HM) in pig manure restricts its direct land use (Wang et al, 2016). Trace elements may be taken up from the soil by plants or biota and cycle in biological tissues before ultimately returning to the soil through decaying biological remains (Tack, 2010). Under nominal concentrations, HM such as Cu, Zn, Se, etc. are important for the plant life cycle, but the metals become toxic to the edaphic organisms and crops above different threshold ranges. HM are absorbed by the root and are translocated to the shoot by different transport mechanisms that mediate the movement of non-essential HMs such as Pb and Cd in certain circumstances (Holzel et al, 2012). Trace metals like Cu and Zn (Cd appears as a contamination in Zn feed) are used globally to prevent
11 infectious diseases in intense animal breeding and as promoters of growth (Cang et al, 2004; Mccarthy et al, 2013). Mineral feed contributes to emissions of Cd Pb, As and Hg. Other potential sources of HMs, such as Cu, Cd, Cr, Zn and Ni, are added for the prevention of corrosion in metal structures, ash alteration of manures for odour control and lime alteration for disinfection purposes (Anonymous, 2004). Such heavy metals can accumulate in soil, up taken by plants and affect animal and human health through the food chain (Buelna et al, 2008; Qureshi et al, 2008). The prolonged application of HM into the soil can also reduce the soil buffering capacity, and thus contribute to permanent soil and groundwater pollution. While certain mechanisms of heavy metal tolerance may be linked to mechanisms of antimicrobial resistance (Akinbowale et al, 2007; Bass et al, 1999), heavy metals may select bacteria that are resistant to antibiotics. This was observed in the case study done by Berget et al (2005), where copper-resistant soil-isolated bacteria that were substantially more resistant to antibiotics (e.g. ampicillin, tetracycline, sulfone-amides, chloramphenicol) than copper-sensitive strains. For the remediation of heavy metals from pig slurry, composting and anaerobic digestion approaches have typically been employed (Marcato et al, 2008; Lu et al, 2015), however these demonstrate other limits, such as the heavy metals can only be fixed and the total heavy metals cannot be reduced. Organic modifications such as effective application of cattle manure have also been reported to regulate heavy metals (HM) content in soil (Wei et al, 2015). Nonetheless, applying organic matter (OM) as soil enhancers is an effective technique for heavy metal immobilization by complexion components, thus potentially decreasing plant uptake.
Pig manure has a higher Cu and Zn content than cattle manure in general. After they have been applied to the soil, HMs are subject to reactions that alter the plants' bioavailability. Moreover, adsorption on to clay and/or organic, precipitation as insoluble salts (carbonate and phosphate) and changes in the oxidation state of HM affects its solubility (Provolo et al, 2018).
2.7 Factors affecting composition of pig manure
The composition of manure from livestock and within different lots of a specific domestic animal varies greatly. Azevedo & Stout (1974) has confirmed that certain variations in manure nutrient concentrations have been caused due to factors such as digestion processes and feed preferences of various animal species (Table 4). For instance, due to the ability of the ruminants to extract the organically bound P from plant feeds, its manure generally has lower P value in comparison to poultry and swine manure. Based on overall composition, liquid fraction of swine manure contains on an average of 9% of total N by mass and 4% dry matter in comparison to 7% and 10% is case of liquid fraction in cattle manure (Evans et al, 1977).
12 Furthermore, as per the data collected by Azevedo and Stout stated in Table 3, 10 hogs produce as much as 80% of manure N as two beef cows. The concentration of different dietary salts and other mineral additives (Cu, As and other minerals) in swine manure are directly affected by the concentration of the aforementioned minerals in swine rations (Sutton et al, 1976; Brumm & Sutton 1979; Sutton et al, 1984a). Thus, high use of manure from such sources can therefore be harmful to plants and can reduce productivity of the soil.
It is stated by Sutton et al (1984a) that swine manure from 0.5% salt averaged diet is more than twice the sodium content in comparison with diet containing 0.2% salt. The use of manure, however, did not contribute to soil pollution or phytotoxicity. Wallace et al (1960) reported addition of dietary copper to swine rations at levels of 125 mg/L to 250 mg/L to stimulate growth. However, a 16 to 32-fold increase in Cu concentration in manure has been observed in the study done by Kornegay et al (1976); where swine fed with a 250–300 mg/L Cu diet. The housing system, the manure collection, storage, and handling process may also influence the manure composition (Szoegi, Vanotti and Hunt, 2015). Usually 50% of N in pig manure slurry is present in the form of ammonium nitrogen and the remaining 50% in the form of organic nitrogen. (Choudhary et al, 2015). The organic nitrogen constitutes microbial N, labile organic N and stable organic. Beauchamp (1983) determined that approximately 20% of organic N will be mineralized and usable throughout the growing season, and that 25% of ammonia will be volatilized, resulting in a net supply of approximately 50% of N used for crop growth. According to the work done by Nasiru et al (2014), the method of storage of manure and the treatment process contributes to about 10 – 30% ammonia nitrogen losses from manure. Moreover, manure storage leads to gases such as ammonia, nitrous oxide, and methane in the atmosphere (Kulling et al, 2001). The amount of gas emissions depends on the digestibility of feed and the general output of animals. The lowest loss was from closed shelters such as aerated storage and the maximum loss was the open storages like feed-lot surfaces and anaerobic lagoons. Al-Kanani et al (1992) reported that use of sphagnum peat moss reduced NH3 losses
by 75%. Nasiru et al (2014) suggested the use of vermicast for the reduction of ammonia nitrogen losses. Losses relating to P and K are mostly minimal only contributing to 5 – 10%. However, these losses increase significantly in case of open-lot or lagoon handling systems where 40 – 50% of P can be lost to runoff and leaching (Sutton et al, 1984a).
The rate, time and method of applying manure varies, including weathering, soil characteristics, crop type and mineralization rate of nutrients. When integrated immediately after land application to mitigate loss of nutrients, the maximum nutrient gain from swine
13 manure is obtained. For minimizing the localized salt concentration, the uniform application of manure must be practiced, especially in the case of Na which can significantly reduce germination and crop yields. In case of severe odour problems resulting because of manure, knifing the manure into the soil is recommended. However, this procedure also limits the rate of manure application. In the study performed by Dickey and Vanderholm (1975), 30 – 90% losses of NH3− N from manure were observed with ploughing down respectively.
Table 4. Quantity of nutrient elements excreted in manure of various animals during reproduction cycles*
Number and type of animal, length of production period, and animal weight during production period
Element excreted (kg) N P K 1000 broilers: 10 weeks: 0-1.8 kg 70 14 23 100 hens: 365 days: 2.3 kg 57 20 19 10 hogs: 175 days: 14-91 kg 52 13 15 10 beef: 365 days: 181-500 kg 64 13 66 2 dairy cows: 365 days: 544 kg 64 13 66 *Adapted from Webber et al (1968) as in Azevedo & Stout (1974)
In case of the areas with high rainfall and highly permeable soils, the nutrient availability of the crop could be maximized by application of manure close to the planting date. However, germination and growth in the planting process may be reduced immediately after application of heavy manure due to the accumulation of salt. In the areas with low temperature climates, application of manure may not be possible in early spring, because of the possibility to be frozen in the pit, particularly when it is stored in open lagoons. The fall–winter application may cause a decrease of 25 – 30% N from the manure, a longer field duration will allow soil micro-organisms to decompose the manure and make the nutrient more available to spring seeded crops (Madison et al, 1995). The loss of N from fall–winter application of manure can be minimized either by injecting it into the soil or by addition of a nitrification inhibitor. To obtain the best possible results from manure application, the rate should be such that the amount of available nutrients is equal to the amount required by the crop. However, a problem is encountered as only 45% of N is mineralized in the first year of application.
A study done by Larson (1991) also shows that even after the period of 5 years, only 80% of the total N is mineralized from the manure. Alternatively, almost all the P and K present in manure are available at the time of application. Low permeability for heavy-structured soils and low decomposition levels further corresponds to lower rates of manure application in comparison to highly permeable coarser textured soils, promoting faster decomposition of
14 manure. However, high levels of application of manure in coarse soil textured can pollute groundwater by nutrient release, while high application rates of manure on heavy textured soil may be beneficial because of the high nutrient holding capacity of these soils. Manure should not be applied to snow or frozen land, particularly if the soil is subjected to accelerated spring runoff. Furthermore, the leaching of N contaminating the groundwater can be prevented by restricting on use of the heavily manured fields during summers.
2.8 Valorization technologies
2.8.1 Hydrothermal carbonization
Hydrothermal carbonization (HTC) was first introduced by Friedrich Bergius in 1913. It is often used to convert waste feedstock/biomass (f) into a solid fuel of relatively high calorific value as compare to brown coal (Oliveira et al, 2013). The process of HTC involves the temperature treatment of organic matter in saturated conditions, generally rich in moisture content, at around 180-250℃ (Román et al, 2012) and 2-10 MPa of pressure for a certain time. Thus, HTC is a combination of processes such as dehydration, hydrolysis, polymerization and decarboxylation (Román et al, 2012). Although, the rudimentary resemblance of HTC lies with torrefication (dry pyrolysis), but, in comparison to torrefication, it requires less time and can be employed for wide range of biomass types. In general, this process leads to an increase in carbon content of the matter while lowering its oxygen and hydrogen share and the final solid product is termed as hydrochar. The hydrochar can be used as soil amendment whereas the liquid products of HTC are usually acidic and contain organic and inorganic compounds (e.g., nitrogen, phosphorus and some mineral matter originated from raw material) (Oliveira et al, 2013). The key advantage of the HTC is its ability to transform wet biomass into highly useful and carbonaceous solid fuel without depending upon the initial drying (Román et al, 2012). However, the installation cost and sophisticated maintenance requirements are among the main concerns of this technology.
2.8.2 Ammonia stripping
Ammonia stripping (AS) works on the principle of mass transfer, where wastewater is brought in contact with air to strip the ammonia gas present in it. Ammonia in wastewater is found in the form of ammonium ions and ammonia gas and their relative concentration depends upon the pH and temperature of the wastewater (Wang, 2018) The ammonia stripping is carried out in two steps. First, the influent, containing high nitrogen content, is ammonified and anaerobically predigested (3 – 5 days). In this step the ammonification bacteria break down
15 proteins and ammonium ions (NH4+) are formed. The second step is stripping, ammonium ions are transformed into ammonia (NH3) by adjusting the pH up to 10 (Guštin and Marinšek-Logar,
2011). The increase in pH gives rise to increase in ammonia gas formation, therefore lime is typically used to raise the pH value of the wastewater prior to stripping step (Wang, 2018). Along with raising the pH of the influent, lime (calcium oxide) also generates calcium carbonate which act as a coagulant for particulate matter. Ammonia is then recovered in the form ammonium sulphate [(NH4)2SO4] by scrubbing with acidic solution.
2.8.3 Crystallization
Struvite precipitation is a well-recognized process for the recovery of phosphorus. This method not only solves the problem of environmental pollution caused by the discharge of phosphorus into natural streams but can also be utilized as a useful resource in the form of fertilizer (Suzuki et al, 2007). Numerous studies have been carried out on the topic of struvite precipitation of swine wastewater and it has been identified that phosphate recovery depends on the concentration of magnesium, ammonium and phosphate ions as well as pH, N/P ratio and influent flow rate (Kozik et al, 2013; Desmidt et al, 2013; Tansel, Lunn and Monje, 2018). The addition of magnesium salts along with pH adjustment for struvite precipitation is among the most commonly employed approach (Suzuki et al, 2007; Kruk, Elektorowicz and Oleszkiewicz, 2014).
2.8.4 Pyrolysis
Pyrolysis (PL) is a process of thermal decomposition where lignocellulosic biomass is degraded under oxygen-deficient conditions and inert environment. At around 350 – 550℃ the organic matter in the biomass starts to degrade and can persist until 700 – 800℃ in the absence of oxygen (Bridgwater, Meier and Radlein, 1999; Bridgwater and Peacocke, 2000). The end products of this process include biochar, bio-oil and gases (e.g., CH4, H2, CO2 and CO and their relative proportion depends upon various parameters such as the temperature, heating rate, residence time, pressure, types of precursors and reactor configuration. Depending upon the form of final products, pyrolysis can be categorized into two types; (i) slow pyrolysis (yields heat and biochar) and (ii) fast pyrolysis (yields bio-oils along with biochar) (Zaman et al, 2017). Unlike pyrolysis, the gasification process allows the biomass to react with a regulated oxygen amount with steam or air (Brewer 2012; Mohan et al, 2014). In gasification, the biomass is heated to a temperature higher than 800°C with an average residence time ranging between several seconds to multiple hours (Inyang and Dickenson 2015).
16 Biochar may be defined as “the product of thermal degradation of organic materials in the absence of air (pyrolysis) and is distinguished from charcoal by its use as a soil amendment” (Lehmann and Joseph, 2009). It could be used for soil improvement, due to its water holding properties and the improvement of nutrient content, thus increasing the overall soil fertility and crop productivity.
Biochar has gained significant recognition in recent years for its multi-functionality including carbon sequestration and soil fertility enhancement (Laird et al, 2010), bio-energy production (Field et al, 2013), and environmental remediation via adsorption process (Mohan et al, 2014).It also provides a stable pool of organic carbon for extended periods, contributing to the beneficial effects of organic matter in soils (Tack and Egene, 2019). This may therefore decrease the solubility of pollutants, mobilize and leach from soil solids, and minimize the movement of pollutants to other compartments of the environment and dispersion into adjacent areas (Tack and Egene 2019). The production of biochar from biomass (agriculture residues, forest waste, manure, food waste etc.) can substantially reduce the quantity of waste produced in the environment (Ahmad et al, 2013). Furthermore, greenhouse gases emissions are reduced by biochar application due to its composition as a high carbon content material (Lehmann et al, 2007, Liu et al, 2014). Biochar could also be used as a sorbent for contaminants, including organic and inorganic contaminants, due to its high surface area and large pore volume (Zhang and Ok, 2014).
Lehmaan and Joseph (2009) performed experiments using manure-based biochar has obtained high degree of usable nutrients that can increase crop productivity. The increased crop productivity is also attributable to the reduction of nutrient leaching and the increase in microbial activities following the use of biochar for soil application (Verheijen et al, 2010, Lehmann et al, 2011). However, when applying a biochar form such as solid waste-derived biochar, careful consideration should be taken as the heavy metal contents or toxic compounds can severely inhibit the mechanisms of soil organisms (Ahmad et al, 2014a).
Biochar is typically formed by slow pyrolysis, rapid pyrolysis and gasification, differentiated with heating, temperature and residence time (Ronsse et al, 2013, Inyang and Dickenson, 2015). In slow pyrolysis, biomass is heated at a slow pace from 5 to 30 min in the absence of oxygen to 400 to 500oC (Mohan et al, 2014). This process leads to secondary reactions (between the vapor and solid phase) that can allow the production of carbonated solids to increase or optimize the production of biochar (Brewer, 2012). In comparison, during fast pyrolysis, the biomass is heat-degraded for about 2 seconds during a short residence time
17 (Inyang and Dickenson, 2015). It aims to rapidly extract vapors and aerosol components in order to maximize the output of liquid (bio-oil) (Brewer, 2012).
2.8.5 Mechanical Solid-liquid separation
When the liquid and solid fractions of raw manure or digestate are separated through mechanical separation, it results into the enrichment of nitrogen (as well as potassium) in the liquid phase and the enrichment of phosphorus and organic matter in the solid phase. This way, a high amount of phosphorus can be concentrated in a small volume. This technique is generally applied as a pre-treatment step in nutrient recovery processes. However, this technique can also help in substantial manure management. Separation can be performed by various technologies such as screw press, centrifuge of belt press (Hjorth et al, 2010). The N-containing liquid fraction can be applied on cultivable land which helps to reduce the usage of mineral fertilizer. Whereas the P-rich solid fraction can be employed in regions where P-content in soil is low or carbon demand is high.
2.8.6 Biological Treatment
2.8.6.1 Algal uptake
Nutrient uptake by microalgae from organic waste is another potential technique of nutrient recycling from manure. This a sustainable and environmentally friendly technique where nutrient assimilation into algal biomass give rise to high quality fertilizers. Manure digestate is typically a favorable medium to cultivate microalgae for the production of biofertilizers because it has less contamination compared to raw manure and has comparatively high N and P concentrations. Similarly, this technique can also be used for nutrient extraction from the liquid fraction of digestate. In the recent past, several studies have demonstrated the potential of dry algal biomass, generated from the treatment of anaerobically digested manure, as a probable fill-in for commercial fertilizers (Mulbry et al, 2005; Veronesi et al, 2015; Uggetti et al, 2014).
2.8.6.2 Composting
Composting is defined as the biological degradation process of heterogeneous solid organic materials under controlled moist, self-heating, and aerobic conditions to obtain a stable material that can be used as organic fertilizer (Lobo and Dorta, 2019). The cycle involves decomposing organic material into a humus-like substance which could act as a nutrient source supporting plant growth (Lal, 2013). Composting includes the following three components: human
18 control, aerobic conditions, and internal biological heat production. For an effective working, it requires the counterbalance of four essential counterparts:
• Carbon — The microbial oxidation of carbon produces the heat. The compounds with high carbon content tend to be brown and dry.
• Nitrogen — for growing and replicating more carbon oxidizing species. The high nitrogen products, including fruit and vegetables, appear to be green (or colorful) and wet.
• Oxygen — for oxidizing the carbon, the decomposition process.
• Water — to maintain the activity of microorganisms without inhibiting aerobic conditions.
The composting is mostly preferred with an optimal C:N ratio of around 25:1. Rapid composting is encouraged by having a C/N ratio of approximately 30 or less (Lal, 2003). Above 30, the substrate is nitrogen starved, below 15 it is likely to outgas a portion of nitrogen as ammonia.
Composting is used as an important recycling tool for local-generated livestock waste and offers an environmentally friendly alternative method for organic waste disposal as it contributes to organic waste stabilization and usage. Many studies indicate that the application of mature compost to agronomic soils improves production because of its characteristics such as high plant nutrient content and retention of moisture. In a study performed by Huang et al (2004) reported that, at the initial C/N of 30, the co-composting of pig manure with sawdust provided a maturity for the compost after 49 days. With manual turning, the stable compost produced could be used for organic farming or as a soil amendment. However, treatment at a low initial C/N of 15 significantly influenced behaviours of many important parameters such as high dissolved organic carbon (DOC) and soluble NH4+ during the co-composting.
Moreover, the resulting compost's high electrical conductivity values must be reduced to levels that would not impose an inhibition on plant development.
2.8.6.3 Anaerobic Digestion
Anaerobic digestion of animal manure is an alternative way to treat large volumes of organic waste and its related issues in feeding lots and limited feeding operations (Ileleji, Martin and Jones, 2015). There are majorly four steps involved in the process of anaerobic digestion (figure 1):
19 • Hydrolysis - Biopolymers are decomposed to monomeric building blocks or other dissolvable basic products. Fats decompose to fatty acids, carbohydrates as for example polysaccharides are transformed in monosaccharides or oligosaccharides and the proteins in pectin respectively (Shrivastava et al, 2019).
• Acidogenesis - simple monomers are converted into fatty and carbonic acids, as for example butyric, propionic and acetic acid, then in lower alcohols - ethanol.
• Acetogenesis – A biological reaction where the lower fatty and carbonic acids as well as the lower alcohols are converted in acetic acid.
• Methanogenesis – A biological reaction where the acetic acids are converted into methane (Shrivastava et al, 2019).
Figure 1. Cycle for biogas generation and utilization (Shrivastava et al, 2019).
1. Digestate
Increasing numbers of anaerobic digestion plants in Europe have resulted in increased nutrient-rich digestate production with great potential as fertilizer for arable land. Anaerobic digestion of animal manure prior to being used as a fertilizer is usually considered favourable, since the digestate generated has greater proportions of mineralized, plant-available nutrients than untreated manure (Insam et al, 2015). Other environmental benefits such as increase in soil carbon and reduction in atmospheric carbon levels, soil erosion (runoff) and nitrate leaching
20 further makes it an attractive option (LPELC, 2020). The digestate nutrient composition varies with the substrate processed by the biogas plant and may contain compounds stimulating or inhibiting soil microbial activity (Risberg et al, 2017). The composition of the digestate depends on the origin of the ingoing substrate and on the management digestion cycle. Factors like animal form (omnivore, ruminant, etc.), sex, species, age, and diet, as well as geographic and environmental conditions strictly changes the composition of digestate. According to the study done by Arthurson (2009), the proportion of ammonium (NH4+) is generally higher in
digestate than in the organic substrate going into the anaerobic digestion (AD) process. Another study performed by Risberg et al (2017) displayed higher ammonium levels and lower organic carbon levels in digestates as compared with pig slurry and cattle manure. In addition, digestate usually induced increased potential for ammonium oxidation, while pig slurry and cattle manure caused substantially more respiration. This trend indicates that for heavy soils with a high level of clay and carbon, the digestate may be better alternative, whereas for lighter sandy soils with less organic carbon the slurry and cattle manure are more efficient.
2. Biogas
Biogas is a mixture of methane, carbon-dioxide, sometimes nitrogen, hydrogen, hydrogen-sulphide, ammonia, and other remainder-gases, which are produced by micro-organisms in anaerobic environment from organic materials. The biogas mainly constitutes of methane corresponding to around 45 – 70%, followed by CO2 between 35 – 50%. In a study done by
Nagy and Wopera (2012) at Hungary, the anaerobic digestion was carried out in a batch reactor with continuous mixer at a thermophilic temperature of 54°C. For a hydraulic loading of 100,000 m3 pig slurry/yr, the estimated biogas production was about 1,024 m3/day. The methane content of the produced biogas was normally 60%, which is proper for the work of gas engines. With the gas engines cogeneration can be achieved. Through the cogeneration on the one hand heat can be produced and on the other hand electricity can be generated. If this biogas is used to release heat by burning, a corresponding energy of 22,022 MJ/day will be obtained. The electrical equivalence of this heat will be 6,117 kWh/day (Nagy and Wopera, 2012). Moreover, for a higher biogas yield, the co-digestion (involving two or more type of organic matter) is mostly preferred to maintain the C/N ratio in the feedstock. In another study performed by Vasmara et al (2015), wheat straw was pre-treated with lignocellulosic fungi and then is co-digested with pig slurry. The co-digestion in this case helped to reduce the maximum methane yield time from 35 to 21 days with an average increase of 37% in biogas production.
21
Methodology
The meta-analysis of research studies was carried out according to the guidelines suggested by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (www.prismastatement.org). Moreover, the principles of random effects model were employed for this meta-analysis.
3.1 Data and literature sources
The studies were collected from various online databases such as Web of Science, Journal Storage (JSTOR), ScienceDirect and Google Scholar on the bases of relevance to the concerned topic. The approach for ferreting out the relevant studies from these databases was based on the subject headings such as resource recovery, manure management, etc. and the corresponding key words. To obtain more topic-oriented results, additional filters were involved in the methodology. These filters contained search terms such as (1) manure, (2) valorization and (3) nutrients. Furthermore, the manual selection of studies has also been done, however this did not lead to inclusion of any further studies.
3.2 Study selection
The selection of the articles/studies from the initial database for further assessment first proceeded via elimination of duplicates. For this purpose, studies were entered in a web application named Kopernio, which facilitates in the blinded screening. After removing the duplicates from the data, the remaining articles were first assessed based on the eligibility of titles and abstracts of these studies. This further helped in labelling the articles as “included” or “excluded”. Later on, the following conditions were selected and applied as a criterion to find out the appropriate studies for a meta-analysis.
a. The study had to refer to pig manure.
b. The study must report the initial and final concentrations of nutrients or a combination of either one of the above two values and the recovery efficiency. c. The language of study could be one of the following: English, Dutch, French,
German, or Spanish.
The research studies that did not meet the aforementioned inclusion criteria, were excluded from this study. The exclusion criteria adopted, for labelling a study as ineligible, comprised of the following points.
22 a. Manure Type: Any other source of manure (such as cattle, poultry) were not
considered
b. Studies reporting anaerobic digestion for biogas generation were excluded (as the primary focus of the study was nutrient recovery).
c. The studies dealing with piggery wastewater were not included.
d. Research articles available in only Japanese or Chinese languages (i.e. no translation available) were omitted.
e. Studies reporting only recovery/valorization efficiency were eliminated.
f. The studies for which full text could not be obtained from any source or the studies with irrelevant abstract were discarded.
3.3 Data extraction
The data extraction was started from the shortlisted papers for which a Microsoft Excel data sheet file was created for recording the results. The extracted data contained following information about the studies: (1) recovered nutrients - nitrogen, phosphorous, organic carbon or potassium, (2) technology implemented, (3) scale of implementation: lab scale, pilot scale and full scale, (4) final concentration of nutrients, (5) flow/volume of test, (6) nutrient recovery efficiency, (7) location of study, (8) duration of study and (9) authors names and DOI of study. 3.4 Data analysis
The dataset was sorted by nutrient extracted and filtered by valorization technique used. Using this, a database of six main treatment technologies were identified for inclusion: hydrothermal carbonization (HTC), ammonia stripping, pyrolysis, Crystallization, Biological processes and Separation techniques. For each valorization mechanism, the reported recovery efficiency (% recovery) was recorded. When percentage recovery was not directly given in the study, they were calculated from the influent and effluent concentrations where provided.
3.5 Strategy for data assessment
3.5.1 Qualitative assessment
The data mined from the eligible studies will be described in a narrative style. This will summarize the characteristics of the studies in terms of year wise trend in technological shift, country wise technological preference and nutrient wise distribution of studies. A 95% confidence interval is a range of values that you can be 95% certain contains the true mean of the population.