of a Fumigated Diesel-Methanol Dual-Fuel Engine
Academic year 2019-2020
Master of Science in Electromechanical Engineering
Master's dissertation submitted in order to obtain the academic degree of
Counsellor: Ir. Jeroen Dierickx
Supervisors: Prof. dr. ir. Sebastian Verhelst, Dr. ir. Louis Sileghem
Student number: 01507430
Jens Peeters
of a Fumigated Diesel-Methanol Dual-Fuel Engine
Academic year 2019-2020
Master of Science in Electromechanical Engineering
Master's dissertation submitted in order to obtain the academic degree of
Counsellor: Ir. Jeroen Dierickx
Supervisors: Prof. dr. ir. Sebastian Verhelst, Dr. ir. Louis Sileghem
Student number: 01507430
Jens Peeters
The reader should be aware of the circumstances under which this dissertation was written to fully understand the structure and the content. On the first meeting with dr. ir. L. Sileghem, he warned me that an experimental disseration may come with a lot of struggles, never could he be more right. In the beginning of the research, I started exploring the possibility of in-stalling a common-rail on a diesel-methanol dual-fuel engine. After a few months, it became clear that this was too difficult within a master thesis as the geometry of the injectors were to dissimilar. So my research was reoriented to a study of the effects of injecting hydrous methanol in a diesel-methanol dual-fuel engine. In the first semester I made a literature re-view on the effects of both diesel-methanol dual-fuel as on the effects of water injection and I changed the methanol-fuel system to cope with hydrous methanol.
During the second semester there was a worldwide outbreak of a coronavirus. As this global COVID-19 pandemic obstructed the experimental research with hydrous blends of methanol completely, the goal of this dissertation had to be changed again. Due to these extraordi-nary circumstances this dissertation became a capita selecta about some unexplored research questions in the field of diesel-methanol dual-fuel engines.
Even without the addition of experimental data, the literature study about water injection remains relevant, hence it is kept unchanged in the dissertation. Measurements from the preparation phase and from previous years were used for the experimental research and data analysis. This way a detailed description of the combustion phenomena was given for higher fractions of methanol than in the reports in literature. As well as a quantitative analysis of the NOx and soot emissions, focusing on the contributions of different influences. Furthermore,
parameters indicating the limiting phenomena and pre-ignition were examined.
Although the pandemic influenced my dissertation, it cannot be compared to the impact it had on other peoples life. So most importantly, I would like to express my deepest respect to the people who worked in the front line and to anyone who lost a loved one during this crisis.
Jens Peeters
First of all, I would like to express my sincere thanks and gratitude to my supervisor ir. Jeroen Dierickx. He was, without any doubt, the person who helped me the most to complete my dissertation. Furthermore I would like to thank him for the good talks we had and I wish him the best of luck with his PhD. Secondly, I would like to thank my two promoters prof. dr. ir. Sebastian Verhelst and dr. ir. Louis Sileghem for allowing me to work on the subject and for their wise academic advice.
Koen Chielens, the engine technician, also deserves a special acknowledgement for all the help and useful tips considering the practical side of this dissertation. Although my first welds reminded him to his children spreading their toasts with Nutella (and he did not bother laughing with me), I would like to thank him for teaching me the basics of welding. Moreover, I would like to gratefully acknowledge all other staff members of the research group Sustainable Thermo-Fluid Energy Systems (STFES) who made this dissertation possible.
Besides the people from the research group STFES, I would like to thank all members of the ”reading committee”, informally known as my mom, my dad and my brother, for dotting the i’s and crossing the t’s. I would also like to show my appreciation to my parents for their mental, practical and financial support during my studies.
Finally, a recognition to my friends. Thank you Sander Devriese, for always being there for me and countlessly reminding me that a dissertation is not a sprint but a marathon. Thank you Jonas Houf, for the good laughs in the lab and the assistance with hoisting. A final big thank you to all my other friends for the pleasant times when I was not working on this dissertation.
The author gives permission to make this master dissertation available for consultation and to copy parts of this master dissertation for personal use. In the case of any other use, the copyright terms have to be respected, in particular with regard to the obligation to state expressly the source when quoting results from this master dissertation.
Ghent, June 2020
The author
Abstract: Analysis of the Combustion
Behaviour and Emissions of a Fumigated
Diesel-Methanol Dual-Fuel Engine
By: Jens Peeters
Supervisors: Prof. dr. ir. Sebastian Verhelst, dr. ir. Louis Sileghem, ir. Jeroen Dierickx
Master’s dissertation submitted in order to obtain the academic degree of Master of Science in Electromechanical Engineering
Ghent University
Faculty of Engineering and Architecture (FEA)
Department of Electromechanical, Systems and Metal Engineering (EMSME) Research group of Sustainable Thermo-Fluid Energy Systems (STFES)
In the past decades, many alternatives have been proposed to improve the sustainability of the transportation sector. Based on a vast amount of selection criteria, one of the po-tential options, especially valuable for the shipping industry, is the fumigation of methanol in diesel-methanol dual-fuel (DMDF) engines. The first part of this dissertation is a litera-ture review that studies the effects of the fumigation of pure methanol and it indicates the potential effects of fumigating hydrous methanol. Furthermore, the normal and abnormal combustion behaviour as well as some of the notable trends in the emissions of a DMDF engine are studied based, on experimental research. A prolonged ignition delay mainly char-acterises the low load operation (resulting in a relative decrease of the efficiency of -29.7 %), while an increased intensity characterises the high load operation (efficiency remains constant at 41.0 %). The trends in the total NOx emissions can be explained by incorporating reaction kinetics of the Zeldovich mechanism, and the conversion of NO to NO2 depends both on the
amount of methanol fumigation as on the oxygen availability. There appears to be a third order decrease of the specific soot emissions when increasing the amount of methanol fumiga-tion. Apart from the low tendency of methanol to form soot and the increase of the amount of diesel that burns premixed, the increasing amount of fuel bound oxygen, which enhances the soot oxidation, is suggested as a third important factor. Above in-cylinder temperatures of 985 K, a pre-ignition may occur, which gives rise to knocking conditions, limits the maximum amount of diesel substitution and increases the soot emissions.
Keywords: Internal Combustion Engine, Alternative Fuel, Diesel, Methanol, Dual-Fuel, Combustion, Emissions, Pre-ignition, Water
Emissions of a Fumigated Diesel-Methanol Dual-Fuel Engine
Jens Peeters
Supervisor(s): Prof. dr. ir. Sebastian Verhelst, Dr. ir. Louis Sileghem, Ir. Jeroen Dierickx
Abstract—In the past decades, many alternatives have been proposed to improve the sustainability of the transportation sector. One of the potential options, especially valuable for the shipping industry, is the fumigation of methanol in diesel-methanol dual-fuel (DMDF) engines. A literature review studies the effects of fumigating pure methanol and indicates the potential effects of fumigating hydrous methanol. Furthermore, the normal and ab-normal combustion behaviour as well as some of the notable trends in the emissions are studied based on experimental research.
Keywords— Internal Combustion Engine, Alternative Fuel, Diesel, Methanol, Dual-Fuel, Combustion, Emissions, Pre-ignition, Water
I. INTRODUCTION
Although shipping is the most energy-efficient mode of mass transport of cargo, international shipping is respectively respon-sible for 2.6 %, 15 % and 13 % of the global anthropogenic CO2, NOxand SOxemissions [1]. The rising awareness of
cli-mate change and local pollution imposes demanding challenges to the shipping industry, but due to the exceptional requirements of shipping, the era of the internal combustion engine is not over yet [2]. However, even under the most optimistic predictions concerning improvements in efficiency and handling emissions, no significant downward trend in emissions can be met if fossil fuels remain dominant [1]. Because of this, there is a rising in-terest to use alternative fuels in the shipping sector. Methanol proves to be one of the potential alternative fuels, as it is a liq-uid, clean burning fuel that can be made in a renewable way [3]. Downsides are the high cost of renewable methanol and the fact that currently most methanol is made in a non-renewable way.
II. FUMIGATING METHANOL INCIENGINES
The shipping industry is most familiar with compression ig-nition (CI) engines and the rules and regulations primarily aim at them. The use of methanol in a conventional CI engine on the other hand is not straight forward due to the fuel characteristics (Cetane number = 2). The fumigation concept, where methanol is injected in the intake, is the cheapest and least complex so-lution to use methanol in CI engines [3]. Within this disserta-tion the combusdisserta-tion characteristics, operating range, efficiency and emissions of a diesel-methanol dual-fuel (DMDF) engine are described, based on the available literature. Followed by an experimental research and data analysis of the normal and ab-normal combustion behaviour, the efficiency and the emissions. A. Literature review
Many different aspects influence the combustion of a fumi-gated DMDF engine. The high heat of vaporization and the in-crease of the heat capacity dein-crease the in-cylinder temperature during the compression stroke. Around top dead center (TDC) a pilot injection of diesel (acting as a liquid spark) ignites the homogeneous methanol-air mixture, which burns through flame
propagation, while the fraction of diesel that did not burn pre-mixed, burns by diffusion combustion. The lower in-cylinder temperature, due to the methanol evaporation, leads to an in-creased ignition delay and hence more fuel burns premixed.
Partial burn, misfire, roar combustion and knock limit the maximum amount of diesel that can be substituted by fumi-gating methanol. At lower loads the maximum methanol en-ergy fraction (MEF) increases when increasing the load, while at higher loads the maximum MEF decreases again when in-creasing the load.
When varying the MEF within the operating range of the DMDF engine, the change of combustion behaviour results in a change of the overall efficiency. At lower loads the efficiency decreases while at higher loads it may increase. As methanol has a higher hydrogen to carbon ratio than diesel it offers the possibility of a CO2reduction. But as efficiency also influences
the specific CO2emission, no general conclusion can be taken
on the specific CO2 emissions. Note that the CO2emission is
almost zero when using renewable methanol.
Using DMDF fumigation also influences the other emissions. There is a general consensus in literature that DMDF decreases the NOx, NO and PM emissions but the NO2, HC and CO
emis-sions increase.
Although quite some research has been done on the effects of DMDF, some pecularities remain. Many researchers only eval-uated a low diesel substitution, they only examined low break mean effective pressure (BMEP) or their research lacks a pro-found quantitative analysis of the occurring phenomena. By analysing self-performed measurements and measurements of previous years, this dissertation fills some of the research gaps. B. Experimental research and data analysis
The engine used for the DMDF research is a Volvo Penta D7C-B TA high speed marine engine, which is converted to a DMDF engine, and which is fully equipped with a data acquisi-tion system. Table I lists the main specificaacquisi-tions of the engine.
TABLE I: Main specifications of the Volvo Penta D7C-B TA. Engine layout 4-stroke 6 cylinder in-line Aspiration Turbocharger + aftercooler Compression ratio 19.0
Bore / stroke 108 mm / 130 mm Displacement 7.15 l
Diesel injection system Cam-driven injection pumps Diesel injection pressure 1200 bar
Maximum torque / speed 904 Nm / 1500 rpm Rated power / speed 195 kW / 2300 rpm
B.1 Normal combustion behaviour and efficiency
A profound analysis of two operating points at 65 % MEF compares DMDF at two loads. A prolonged ignition delay char-acterises the low load operation (BMEP = 3.5 bar) while an in-creased intensity characterises the high load operation (BMEP = 12.3 bar). These differences result in different effects on the pressure, temperature and efficiency. At low load DMDF causes a decrease of the efficiency (-29.7 %) due to the less isochoric expansion while at high load the efficiency remains the same. These conclusions are in line with reports in literature at differ-ent loads and at lower substitution levels [3, 4, 6–8].
B.2 Emissions in normal operation
When measuring the NOx and NO emissions over a wide
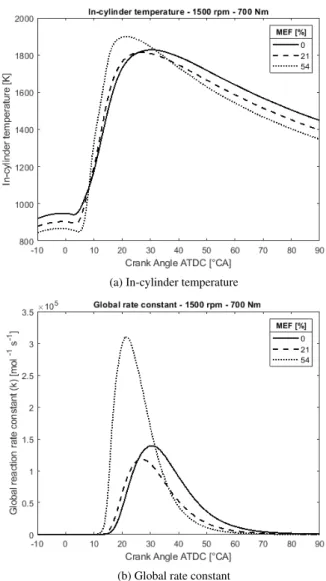
range of MEF a parabolic trend (with a local minimum) can be noticed at the higher loads [4]. The dissertation further investi-gates the parabolic behaviour by incorporating reaction kinetics of the Zeldovich reaction mechanism. Figure 1 shows the effect of DMDF on the development of the in-cylinder temperature and the resulting rate constant of the NO production. There is a positive correlation between the development of the global rate constant and the total NOxformation (R = 0.60-0.75), and this
way the parabolic trend can be explained.
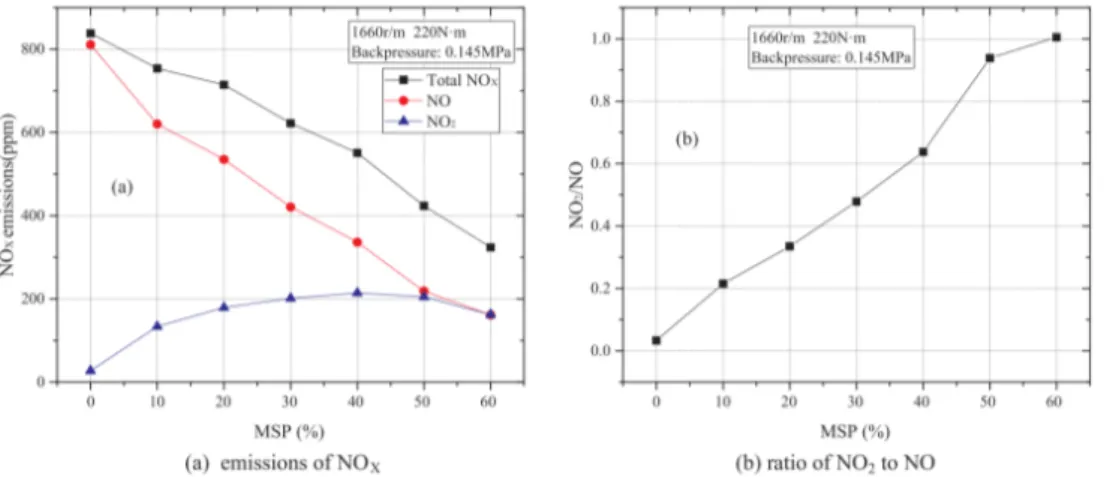
As some part of the NO converts to NO2, the total amount
of NOxconsists of a blend of NO and NO2. The oxidation of
methanol leads to higher concentrations of HO2radicals, which
promote the conversion of NO to NO2. Furthermore, the
ra-tio of NO2/NO also depends on the oxygen availability. The
ratio seems to depend approximately linearly on the MEF and quadratically on the air-fuel equivalence ratio (R=0.893). Fig-ure 2 emphasizes the dependency of the ratio of NO2/NO on the
MEF and the air-fuel equivalence ratio (i.e. oxygen availabil-ity). The contour lines follow a trend as described above till a certain MEF, but for higher substitution levels the effect of the MEF diminishes, for a ratio NO2/NO of one this occurs above
40 % MEF.
In DMDF operation there appears to be a third order decrease of the specific soot emissions for an increasing MEF, see Figure 3. The low tendency of methanol to form soot (due to its molec-ular structure) and the increasing amount of fuel burned in the premixed phase are widely accepted explanations. A quantita-tive analysis indicates that these explanations still underestimate the substantial decrease of the soot emissions. The increasing amount of fuel-bound oxygen in methanol, which enhances the soot oxidation after being formed, is suggested as an extra rea-son for the considerable decrease of the soot emissions. B.3 Abnormal combustion behaviour
To fully profit the advantages of methanol, only just as much pilot injection of diesel should be used as required to ignite the methanol-air mixture. Different parameters are evaluated on their capability to determine and predict the upper limits of the MEF described above. But no parameter is found to adequately predict them. Depending on the load, several parameters may be used to detect the limit, but no precise values are found to rig-orously determine it, as no measurements are done beyond the operating limit of the engine.
(a) In-cylinder temperature
(b) Global rate constant
Fig. 1: In-cylinder temperature and global rate constant at high load (BMEP = 12.31 bar), all data from Verbiest et al. [5].
Fig. 2: Molar ratio of NO2/NO emissions as a function of the
MEF and the air-fuel equivalence ratio for different loads at 1500 rpm, all data from Verbiest et al. [5].
Fig. 3: Specific soot emissions at 1500 rpm.
The ignition of methanol before the injection and auto-ignition of diesel is called pre-auto-ignition. As it gives rise to knock-ing conditions, limits the maximum MEF and increases the soot emission, it should be avoided. Pre-ignition did not occur if the in-cylinder temperature before the start of combustion remained below 985 K. There is a relation between the in-cylinder tem-perature around TDC and the intake temtem-perature, but the rela-tion depends on the engine, the operating point and the MEF. Future research is recommended to examine the influence of the air-methanol ratio on the limiting temperature for pre-ignition.
III. HYDROUS METHANOL INDMDFENGINES
During the production of pure methanol, the dehydration step accounts for a considerable part of the overall cost and energy requirement [9]. Moreover, water injection (WI) in the intake of CI engines also leads to NOxreductions. So fumigating hydrous
methanol seems interesting from an economical, an energetic and from an emission point of view.
Therefore, a literature review elaborates about the effects of WI in CI engines and the use of hydrous methanol in DMDF engines. A short overview of the potential injection locations is given, followed by the effects of WI on the combustion charac-teristics, the operating range and the emissions.
The effects of WI on the combustion behaviour can be split up in three groups: the thermal effects, the chemical effects and the dilution effects. Mainly because respectively: the heat of evaporation and the increase of the specific heat capacity cause a cooling effect, a small part of the water molecules dissociate in H and OH radicals and the remaining part can be considered as inert and hence dilutes the mixture.
As water is a knock suppressant, WI in DMDF engines may increase the high load operating limit, but the decrease of reac-tivity may decrease the substitution limit at the partial burn and misfire boundaries.
WI does not influence the efficiency unless an excessive amount of water is injected, and it does not cause a considerable difference in CO2 emissions. Due to the thermal and dilution
effects, WI reduces the NOxemissions but there is a trade-off
relation with other emissions such as PM, NO2, HC and CO.
The most interesting characteristics of WI in a DMDF engine are the NOxreduction and the possible increase of the
substitu-tion limit at high load. Unfortunately, no experimental research could be done on the effects of fumigating hydrous methanol due to the global COVID-19 pandemic.
IV. CONCLUSIONS
• Based on a vast amount of selection criteria, methanol proves
to be a valuable alternative fuel, principally because it is a liquid, clean burning fuel that can be made in a renewable way.
• A prolonged ignition delay mainly characterises the low load
operation while an increased intensity characterises the high load operation.
• At low load DMDF causes a decrease of the efficiency (-29.7
%) due to the less isochoric expansion while at high load the efficiency remains the same.
• The parabolic trend in the NOx and NO emissions (with a
local minimum) at the higher loads can be explained by incor-porating reaction kinetics of the Zeldovich mechanism.
• The ratio of NO2/NO emission depends both on the MEF as
on the oxygen availability.
• There appears to be a third order decrease of the specific
soot emission for an increasing MEF. This may be explained by the low tendency of methanol to form soot, the increase of the amount of diesel that burns premixed and the increasing amount of fuel-bound oxygen which enhances the soot oxidation.
• Above an in-cylinder temperature of 985 K, a pre-ignition
may occur, which gives rise to knocking conditions, limits the maximum MEF and increases the soot emissions.
• Based on a literature review, the fumigation of hydrous
methanol seems interesting to reduce the NOxproduction and
to increase the substitution limit at high loads. ACKNOWLEDGMENTS
The author would like to acknowledge the suggestions of his supervisor ir. J. Dierickx and his promoters prof. dr. ir. S. Verhelst and dr. ir. L. Silghem. Koen Chielens, the engine tech-nician, also deserves a special acknowledgement for all the help and useful tips considering the practical side of this dissertation.
REFERENCES
[1] IMO, ”IMO Greenhouse Gas Study 2014”, Tech. rep. IMO (2014), pp 1-4. [2] M.D.R. Darley, ”Global marine fuel trends 2030”, In: IASH 2015 - 14th International Symposium on Stability, Handling and Use of Liquid Fuels (2015), pp. 1–60
[3] C. Yao et al, ”Methanol fumigation in compression-ignition engines: A critical review of recent academic and technological developments”, In: Fuel 209 (2017), pp. 713-732.
[4] J. Dierickx et al. ”Efficiency and Emissions of a High Speed Marine Diesel Engine converted to Dual-Fuel Operation with Methanol”, In: Cimac, Van-couver (2019), pp. 1-14.
[5] J. Verbiest and T. Janvier. ”Optimisation of a methanol-diesel dual-fuel marine engine”, Master thesis, Ghent University (2019), pp. 1-119. [6] Z. H. Zhang et al. ”Influence of fumigation methanol on the combustion and
particulate emissions of a diesel engine”, In: Fuel 111 (2013), pp. 442-448. [7] J. Liu et al. ”Effects of injection timing on performance and emissions of a
HD diesel engine with DMCC”, In: Fuel 134 (2014), pp. 107-113. [8] J. Coulier and S. Verhelst. ”Using Alcohol Fuels in Dual Fuel Operation
of Compression Ignition Engines: A Review”, In: Cimac, Helsinki (2016), pp. 1-13.
[9] S. Verhelst et al. ”Methanol as a fuel for internal combustion engines”, In: Progress in Energy and Combustion Sience 70 (2019), pp. 43-88.
I Literature Review 1
1 Introduction 2
1.1 Criteria to select an alternative fuel . . . 3
1.1.1 Availability and production . . . 3
1.1.2 Economic aspects . . . 4
1.1.3 Environmental aspects . . . 5
1.1.4 Storage . . . 6
1.1.5 Safety . . . 6
1.2 Conclusion and goal statement . . . 7
2 Diesel-Methanol Dual-Fuel 11 2.1 Injection locations for DMDF engines . . . 12
2.2 Combustion characteristics of DMDF engines . . . 13
2.3 Operating range of DMDF engines . . . 18
2.4 Efficiency of DMDF engines . . . 21 2.5 Emissions of DMDF engines . . . 22 2.5.1 NOx emissions of DMDF engines . . . 22 2.5.2 PM emissions of DMDF engines . . . 24 2.5.3 SOx emissions of DMDF engines . . . 25 2.5.4 HC emissions of DMDF engines . . . 26 2.5.5 CO emissions of DMDF engines . . . 27 2.6 Conclusion on DMDF . . . 27
3 Water Injection in Compression Ignition Engines 30 3.1 Water injection locations . . . 31
3.1.1 Water injection in the intake - fumigation . . . 32
3.1.2 Direct injection of water in the combustion chamber . . . 34
3.1.3 Injection of a water-diesel emulsion . . . 34
3.1.4 Conclusion on the injection location of water . . . 34
3.2 Effect of WI on the combustion characteristics . . . 35
3.3 Effect of WI on the operating range of a DF engine . . . 39
3.4 Effect of WI on the efficiency of diesel engines and DF engines . . . 40
3.5 Effect of WI on the Emissions . . . 42
3.5.1 NOx emissions with WI . . . 42
3.5.2 PM emissions with WI . . . 43
3.5.3 HC and CO emissions with WI . . . 44
3.6 Conclusion on WI . . . 45
II Experimental Research and Data Analysis 47 4 Data Collection and Processing 48 4.1 Experimental setup . . . 48
4.1.1 Hardware . . . 48
4.1.2 Adapted methanol fuel system . . . 50
4.1.3 Mixture formation . . . 52
4.1.4 Measurement matrix to investigate the effects hydrous methanol . . . 53
4.2 (Post-)processing of the data . . . 55
4.2.1 Heat release rate . . . 56
4.2.2 Combustion timing . . . 57
4.2.3 In-cylinder temperature . . . 57
4.2.4 Emissions . . . 57
4.3 Optimisation of the data collection and (post-)processing . . . 58
4.4 Summary of the data collection and processing . . . 62
5 Combustion Behaviour of DMDF Engines 63 5.1 Normal combustion behaviour and performance . . . 63
5.1.1 Effects of DMDF on the heat release rate and combustion timing . . . 64
5.1.2 Effects of DMDF on the in-cylinder pressure . . . 66
5.1.3 Effects of DMDF on the in-cylinder temperature . . . 67
5.1.4 Effects of DMDF on the efficiency and air-fuel equivalence ratio . . . . 69
5.1.5 Comparison with literature . . . 70
5.1.6 Summary of the normal combustion behaviour and performance . . . 71
5.2 Abnormal combustion . . . 72
5.2.1 Parameters indicating partial burn and misfire . . . 73
5.2.2 Parameters indicating roar combustion and knock . . . 74
5.2.3 Pre-ignition . . . 77
5.2.4 Summary of the abnormal combustion behaviour . . . 80
6 Notable Trends in the Emissions of a DMDF Engine 83
6.1 Influence of DMDF on the NOx emissions . . . 83
6.1.1 Parabolic behaviour of NOx and NO for an increasing MEF . . . 83
6.1.2 Ratio NO2/NO . . . 88
6.2 Influence of DMDF on the soot emissions . . . 90
6.2.1 Soot formation in DMDF operation . . . 90
6.2.2 Soot formation in case of pre-ignition . . . 93
6.3 Conclusion on the notable trends in the emissions of a DMDF engine . . . 95
7 Conclusion 96
III Appendices 102
A Volvo Penta D7 operating procedures 103
B Elementary calculations on the temperature of the in-cylinder mixture 106
C Rapid combustion fraction 110
D Handling the bung barrel 113
List of Figures 115
List of Tables 118
AFR Air-Fuel Ratio AFRd Air-Fuel Ratio diesel
AFRm Air-Fuel Ratio methanol
AFRst Air-Fuel Ratio stoichiometric
BMEP Break Mean Effective Pressure BSFC Break Specific Fuel Consumption BTE Break Thermal Efficiency
CA Crank angle
CA10 Crank angle at wich 10 % of the total heat is released CA50 Crank angle at wich 50 % of the total heat is released CA90 Crank angle at wich 90 % of the total heat is released CI Compression Ignition
CN Cetane Number
CNG Compressed Natural Gas CO Carbon monoxide
CO2 Carbon dioxide
COV Coefficient Of Variation CTV Crew Transfer Vessel DAQ Data Acquisition DF Dual-Fuel
DI Direct Injection
DMDF Diesel-Methanol Dual-Fuel DO Diesel only
DSR Diesel Substitution Ratio DWT Deadweight Tonnage ECA Emission Control Area ECU Engine Control Unit
EEDI Energy Efficiency Design Index EGR Exhaust Gas Recirculation EOC End of combustion
EVO Exhaust valve opening H Hydrogen (radical) H2 Hydrogen (gas)
H2O Water
HAM Humid Air Motor HC Hydrocarbons HO2 Hydroperoxyl
HFO Heavy Fuel Oil HRR Heat Release Rate IC Intercooler
ICE Internal Combustion Engine
IGF International code of safety for ships using Gases and other low-flashpoint Fuels IMO International Maritime Organisation
IMEP Indicated Mean Effective Pressure LCA Life Cycle Analysis
LHV Lower Heating Value LNG Liquid Natural Gas
MARPOL Convention on Maritime Pollution MON Motor Octane Number
MBT Minimum spark advance for Best Torque MEF Methanol Energy Fraction
MMF Methanol Mass Fraction MPI Multi Point Injection
MSP Methanol Substitution Percent NOx Nitrogen oxides
NO Nitrogen monoxide NO2 Nitrogen dioxide
NTP Normal Temperature and Pressure (293.15 K, 101 325 Pa) OH Hydroxy
PCP Peak Cylinder Pressure PFI Port Fuel Injection
PLIF Planar Laser-Induced Fluorescence PM Particulate Mater
PPRR Peak Pressure Rise Rate RBF Rapid Burning Fraction RCF Rapid Combustion Fraction RI Ringing Intensity
RMD Ratio Methanol Diesel RON Research Octane Number
SECA Sulphur Emission Control Area
SEEMP Ship Energy Efficiency Management Plan SFC Specific Fuel Consumption
SI Spark Ignition SOx Sulfur oxides
SO2 Sulfur dioxide
SO3 Sulfur trioxide
SOC Start of combustion SOLAS Safety Of Life At Sea SPI Single Point Injection Sr Substitution rate SS Stainless Steel
STFES Research group of Sustainable thermo-fluid energy systems STP Standard Temperature and Pressure (273.15 K, 100 000 Pa) TDC Top Dead Centre
WI Water Injection WFR Water-Fuel Ratio
α Dilution factor AF Rd Air-Fuel Ratio diesel
AF Rm Air-Fuel Ratio methanol
AF Rst Air-Fuel Ratio stoichiometric
β Rapid combustion fraction BM EP Break Mean Effective Pressure BSF C Break Specific Fuel Consumption
CAp,min Crank angle of the local minimum in pressure
CAp,peak Crank angle of the maximum in pressure
cp Heat capacity
cp,air Heat capacity of air at constant pressure
cp,water Heat capacity of water at constant pressure
COVIM EP Coefficient of variation of the indicated mean effective pressure
E Energy
Ea Activation energy for the Zeldovich reaction mechanism
Econs Energy consumption
ηe Effective efficiency
ηi Indicated efficiency
ηm Mechanical efficiency
γ Specific heat capacity ratio h Specific enthalpy
H Enthalpy
Ha Absolute humidity of the inlet air
HRRmax Maximum heat release rate
IM EP Indicated mean effective pressure
k Global rate constant of the Zeldovich reaction mechanism kint Crank angle integral of k
KE Kinetic energy
λ Air-fuel equivalence ratio λm Air-methanol equivalence ratio
LHVd Lower heating value of diesel
LHVm Lower heating value of methanol
LHVf uel Lower heating value fuel
m Mass
mmix Mass of mixture in one cylinder
˙
mair,dry Mass flow of dry air
˙
mair,wet Mass flow of humid air
˙
md Mass flow of diesel
˙
md,DO Mass flow of diesel in diesel only operation
˙
md,DF Mass flow of diesel in dual-fuel operation
˙
mf uel Mass flow of fuel
˙
mm Mass flow of methanol
˙
mm,DF Mass flow of methanol in dual-fuel operation
˙
mwater Mass flow of water
M EF Methanol Energy Fraction M M F Methanol Mass Fraction M SP Methanol Substitution Percent p Pressure
patm Atmospheric pressure
pav In-cylinder pressure averaged over different cycles
psat,inl Saturation pressure of the inlet air
Pb Brake power
P CP Peak cylinder pressure Pe Effective power
P E Potential energy Pi Indicated power
P M EP Pumping mean effective pressure P P RR Peap pressure rise rate
Q Heat
R Universal gas constant R Correlation coefficient
Ra Relative humidity of the inlet air
Rexh Specific gas constant of the exhaust gasses
RI Ringing intensity
RP Me Exact amount of rotations per minute
T Mean in-cylinder temperature Te Exact breaking torque
TaIC Air temperature after intercooler
Tatm Atmospheric temperature
TEV O In-cylinder temperature at the exhaust valve opening
Tmax Peak in-cylinder temperature
TT DC In-cylinder temperature at top dead center
u Specific interal energy U Internal energy V Cylinder volume Vs Total swept volume
W Work
Wi Indicated work per cycle
Literature Review
Introduction
The shipping industry must reduce its emissions
Shipping is the most energy-efficient mode of mass transport of cargo. Which leads to the fact that the shipping industry transports 90 % of all cargo by volume [1]. On the other side of the coin, international shipping annually emits more than a billion tons of CO2,
more than twenty million tons of NOx and more than eleven million tons of SOx, which
respectively corresponds to 2.6 %, 15 % and 13 % of global anthropogenic emissions [1]. Due to a raising awareness of global warming and local pollution, several measures have been taken to reduce these emissions. Under the maritime pollution convention (MARPOL), measures as the Energy Efficiency Design Index (EEDI) and the Ship Energy Efficiency Management Plan (SEEMP) aim to reduce the CO2 output of ships, while measurements as the Tier regulations
and (Sulphur) Emission Control Areas ((S)ECA) aim to reduce the NOx and SOx emissions
[2]. These regulations become gradually more strict and they drive the shipping industry to improve efficiency and decrease emissions.
The era of the ICE is not over
Internal combustion engines (ICE) are, by far, the most used power generation method for shipping in the last century and predictions indicate that the era of the ICE is not over yet [3]. The main advantages of the ICE are the fact that they are easy to produce (using abundantly available and recyclable materials) and the high power density. Besides that, people have a lot of trust and knowledge in handling them. Taking into account the expected growth of the shipping industry, even under the most optimistic predictions concerning improvements in efficiency and handling emissions, no significant downward trend in emissions can be met if fossil fuels remain dominant [1]. Because of this, there is a rising interest in the use of alternative fuels in the shipping sector.
1.1
Criteria to select an alternative fuel
An alternative fuel must be compatible with existing and future technology for the engines, the storage and the handling. One of the possible alternative fuels is methanol, a liquid, clean burning fuel that can be made in a renewable way. Many academic research has been done on the use of methanol in ICE and several running projects indicate that there are no problems sailing on methanol as a fuel [4, 5]. As methanol is among the top five most traded chemicals no technical problems considering handling are met as well [6]. Besides these, many other selection criteria exist to evaluate potential alternative fuels, which results in the fact that different authors use different selection criteria for alternative fuels. The next paragraphs give a summary of these selection criteria and verifies them with the properties of methanol.
1.1.1 Availability and production
A valuable alternative fuel needs to be available, both in the short term as in the long term. On the short term, there must be a global network of bunkering facilities for the fuel [3, 7]. While in the long term, the fuel needs to be derived from abundantly available materials on earth [8].
Short term availability
Currently methanol is among the top five most widely traded chemicals in the world [6]. Worldwide 97 ports confirmed the availability of methanol, directly in the port or through chemical suppliers [4]. These two facts indicate that methanol is and will be available in the near future.
Conventional methanol production
Various methods exist to produce methanol. In fact methanol can be produced from anything that is or ever was a plant. The first step to produce conventional methanol consists of the gasification of a fossil fuel to create synthesis gas. Then, the synthesis gas is converted to hydrous methanol, having a purity of about 90 %. In the third step, hydrous methanol is dehydrated to produce pure methanol [9]. While presently most methanol is produced by catalytic conversion of synthesis gas originating from fossil fuels, i.e. natural gas and coal, much research has been done on the production from renewable and sustainable resources.
Renewable methanol
Renewable methanol is made by combining carbon dioxide, captured from the air, in com-bination with green hydrogen, i.e. hydrogen made by electrolysis of water using renewable energy. According to Bozzano et al. [9], nowadays this can be considered a mature technology. Brynolf et al. [10] partly support this claim as they report that several demonstration-scale
facilities have been developed. But on the other hand, they point out the fact that there are still some aspects that need to be clarified in order to understand the potential role of methanol in the future transportation sector. Currently, the technology readiness level (TRL) of renewable methanol is estimated around seven [11–13], meaning that the production of re-newable methanol is on the tipping point between pilot scale demonstration projects and industrial installation. At present, plans are made to produce green hydrogen and renewable methanol in the harbours of Ostend and Antwerp [14–16]. When making renewable methanol one mole of water is produced for every mole of methanol, this corresponds to a 30 % volume fraction water [6]. Thus similarly as for convention methanol, a dehydration step is required to produce pure methanol.
Long term availability
In addition to the production from fossil fuels or in a renewable way, methanol can also be produced as bio-methanol from both primary- and secondary feedstock [7]. Both renewable methanol and bio-methanol are made from the abundantly and even excessively available carbon dioxide in our atmosphere, combined with water. Thus renewable methanol or bio-methanol can be long term options as an alternative fuel.
Hence, methanol will be available in the near future and it might be available in a renew-able way in the long term. But in order to be a valurenew-able alternative fuel, an alternative fuel should not only by available, it should be available at a reasonable cost. Therefore the economic aspects of using methanol as an alternative fuel in the shipping industry need to be evaluated.
1.1.2 Economic aspects
The cost is one of the main drivers/barriers for uptake of any alternative fuel [17]. Both the capital expenditures as the operational expenditures influence the overall cost. When analysing the cost, several boundary conditions have to be specified. For example, a distinc-tion can be made between ships sailing within or outside (S)ECA areas and a distincdistinc-tion can be made considering the origin of the fuel.
Investment cost
The investment cost of retrofitting or installing a new engine is mostly neglectable compared to the operational costs [7]. But when taking the investment cost into account, the cost for a methanol retrofit is in the same range as the cost for installing an after treatment system for heavy fuel oil (HFO) and it is lower than the cost of retrofitting a liquid natural gas (LNG) system [18–20].
Operational cost
In the past, several studies pointed out that the operational cost of a ship sailing on methanol outside the SECA area was higher than with conventional fuel [7]. But from the 1st of January 2020 the sulphur cap of 0.50 % mass in marine fuels over the whole world will influence the results of the comparison in favour of methanol [21, 22]. The operational costs using methanol are lower compared to LNG or ethanol [19, 23]. Due to the fact that renewable methanol is only produced on pilot scale, the cost of producing renewable methanol is not competitive yet. A recent (2019) life cycle analysis (LCA) indicates that, with current technology and market prices, the operational cost of using renewable methanol is higher than when using diesel [24].
Both for the conventional production of methanol as for the production of renewable methanol, the dehydration step takes an important part of the overall cost and energy requirement. Therefore it is interesting to investigate the use of hydrous methanol to reduce the cost and decrease energy requirements during the production [6].
Using conventional methanol as an alternative fuel is economically feasible, but the current cost of renewable methanol is too high. In a transition period, conventional methanol can be used until scale effects and maturity of the technology reduce the cost of renewable methanol. Without any doubt, an economic intervention of regulating authorities would aid the transition towards a more sustainable shipping industry. The economic in-tervention is advisable because methanol possesses promising environmental and sustainable characteristics.
1.1.3 Environmental aspects
To discuss the environmental aspects a distinction should be made between normal operation and the risk in case of leakage. In normal operation an alternative fuel should be sustainable while the risk of mayor marine pollution by a leakage should be limited.
Emissions during normal operation
As examined more profoundly in Chapter 2 and 6, reductions in the NOx, SOx and soot
emis-sions are possible when sailing on methanol. Using conventional methanol, a small reduction on CO2 emission is possible because of the lower carbon to hydrogen ratio of methanol,
compared to conventional fuels. When using renewable methanol, the CO2 emitted during
combustion was first captured and it is used as an energy carrier. Because of this, the net CO2 emission is almost zero and thus way lower than the CO2 emission of conventional
fu-els. These emission reductions make methanol particularly interesting as a sustainable alternative fuel.
Risks in case of leakage
Ship accidents as collisions, groundings and other leakages cause a potential risk for the marine environment. These potential risks need to be determined to prevent major damage to the ecosystems. Methanol spills migrate quickly, due to the solubility of methanol in water. Consequently, high concentration occurrence of methanol only last for a short period of time in case of a spill [25]. Moreover, methanol is of low toxicity to aquatic and terrestrial organisms [26]. And above that, methanol is biodegradable in soil, sediments and in water, both under aerobic as anaerobic conditions [26]. Because of these reasons, the International Maritime Organization (IMO) does not classify methanol as a marine pollutant, so it may be carried in tanks next to the hull [7]. This is in great contrast with conventional fuels requiring a double hull between the fuel and the seawater, because they are marine pollutants. The following section further describes the storage of methanol.
1.1.4 Storage
A fuel must first be bunkered until the moment it is used. On this aspect, methanol excels compared to other alternative fuels. Based on the molecular weight of methanol (32.04 g/mol) one could expect methanol to be gaseous. But because of the hydrogen bonds formed by the alcohol group, methanol is the simplest carbonaceous molecule that is liquid at standard temperature and pressure (STP) [6]. This increases the ease of storage a lot. As men-tioned in the previous section, there is no need to store methanol in a double hulled tank. In contrast to methanol, other alternative fuels require more care. LNG needs to be stored cryogenic and CNG and hydrogen gas are stored in pressurized tanks.
Yet, it should be noted that the volumetric energy density of methanol is only half the volumetric energy density of gasoline and diesel [9, 17]. Assuming a same efficiency, this im-plies that 2.0 to 2.5 times as much bunker space is needed to have the same operating range.
In other words, the the fact that methanol is liquid at STP makes it a particularly attractive alternative fuel, but compared to diesel, twice as much bunker space is needed. Moreover, the use of methanol as a fuel in the shipping industry comes with some safety issues.
1.1.5 Safety
To protect sailors, a risk analysis should be done to identify and limit possible risks of using any alternative fuel. Every ship sailing in international waters should comply with the Safety Of Life At Sea (SOLAS) regulations. The main risks when using methanol as a fuel are the fact that humans, and non-human primates, are uniquely sensitive to the toxic effects of methanol and the low flashpoint of methanol [26, 27].
Toxicity to humans
When taking some almost obvious measures, the risk related to the toxicity of methanol can be confined quite well. Direct contact and internal ingestion needs to be avoided but this is also the case for gasoline and diesel [28]. Just as with gasoline, inhalation of vapours also need to be avoided. The requirements for the storage of methanol as a fuel are the same as for the storage of methanol as bulk. As methanol is already so widely shipped this does not rise any major problems [18].
Explosion risk
A somewhat more complex concern results from the low flashpoint of methanol (11°C), which is below the range of normal operation conditions in a ship [18]. Because of this, ships using methanol need to comply with the international code of safety for ships using gases or other low-flashpoint fuels (IGF) of IMO. Just as methanol, hydrogen and LNG also need to comply with the code. One of the main consequences of the IGF is that methanol needs to be transported in double walled piping in the engine room and a leakage detection system needs to be present [20, 27]. Existing projects indicated that non of these problems are insurmountable with a reasonable investment cost [20, 27].
1.2
Conclusion and goal statement
Table 1.1 summarises this concise overview of the most important aspects of an alternative fuel. Methanol clearly has some potential as an alternative fuel in the transportation sector, and in the shipping sector in particular. The most important advantages are the ease of storage (liquid at STP), the fact that it can be produced in a renewable way and the emission reductions. Downsides of methanol are the high operational cost (compared to conventional marine fuel) and the fact that currently most methanol is made in a non-renewable way.
Stating that methanol is by far the most appropriate fuel for the future shipping sector would underestimate the complexity of the problem. But methanol does have some poten-tial, and the choice of an appropriate propulsion system needs to be done case by case. A crew transfer vessel, having a deadweight tonnage (DWT) of 100 tonnes, doing multiple short transports a day, cannot be compared with a 200 000 DWT container vessel, sailing from Western Europe to Eastern Asia. Depending on their own specific boundary conditions they require an appropriate solution. Therefore, it is really important to know the characteristics of each solution, and this requires research on all aspects of different alternative fuels.
Table 1.1: Selection criteria for alternative fuels and verification for methanol.
Availability Short term World wide traded chemical & production 97 ports confirm availability
Long term Renewable methanol Bio-methanol
Economic Capex = after treatment system for HFO < LNG system
Opex Outside SECA: > convention fuel Inside SECA: competitive
< LNG or ethanol
Cost renewable methanol is too high Environmental Normal operation NOx, SOx and soot reduction possible
Renewable methanol: CO2 ≈ 0
Risks for leakage Spills migrate quickly
Low toxicity to aquatic & terrestrial organisms Biodegradable, not a marine pollutant
No double hulled tank required
Storage Liquid at STP
1/2 volumetric energy density gasoline/diesel Safety Toxicity to humans Avoid inhalation, contact & internal ingestion
Similar as gasoline or diesel
Explosion risk Low flashpoint; IGF code (cf. H2 & LNG)
Double walled piping in the engine room Leakage detection system
Original goal statement
Within this dissertation, research is done on the use of methanol in a high speed dual-fuel compression ignition engine. Previous research focused on the efficiency and emissions, on the injection position of methanol and on low load throttling [24, 29]. This research examines the effects of water injection in a dual-fuel engine. Water injection in a dual-fuel engine can reduce NOx emissions, shifts the operating range and can be economically advantageous [20]. But
more research is needed to fully quantify the possible benefits together with the disadvantages. First a literature review studies both the effects of using methanol as a fuel in a duel-fuel engine as the effects of water injection in diesel engines and in dual-fuel engines. This is followed by an experimental investigation on the maximum attainable methanol fraction, the efficiency and the emissions when using hydrous blends of methanol in a dual-fuel engine.
Revised goal statement
The circumstances created by the worldwide COVID-19 pandemic prohibited experimental research on the effects of water injection in the dual-fuel engine. The literature study of the dissertation remains unchanged as the relevance persists. The scope of the experimental research was changed to a capita selecta about some of the unresolved questions on the fumigation of methanol. The research is based on tests done in the preparation phase and in previous research by Dierickx et al. [29] and Verbiest et al. [24]. A first chapter about the combustion behaviour evaluates the combustion in normal conditions, assesses the limits of the normal combustion behaviour and analyses the pre-ignition phenomenon. This is followed by a chapter which investigates some remarkable trends in the NOxand soot emissions of a
Figure 1.1: Ov erview of the con ten t of the dissertation.
Diesel-Methanol Dual-Fuel
Fuel characteristics of methanol
Methanol can be used in both spark ignition (SI) as in compression ignition (CI) engines. The use of methanol in a SI engine is quite straight forward while the use of methanol in a CI engine is not. Due to the high resistance against auto-ignition of simple alcohols, they are principally considered SI engine fuels [6]. Both the research octane number (RON) (109) as the motor octane number (MON) (92) of methanol are about 10 % higher than those of typical gasoline (RON: 95, MON: 85). Methanol can be burned both purely as in a blend in a SI engine. The high resistance against auto-ignition allows higher compression ratios and enables minimum spark advance for best torque over a wider range of operation points. Even when knock does not form a problem, the elevated burning velocity and wider flammability limit open some alternative options for load control [6].
Methanol in CI engines
As the shipping industry is so familiar with CI engines, suggesting the use of a SI engine could prevent uptake. On top of that, the rules and regulations primarily aim at CI engines. Furthermore, the use of SI engines would increase the complexity of retrofits on existing ships. Because of these three reasons, it is interesting to look into the possibilities of using methanol in a CI engine. The high octane numbers of methanol indicate a very low cetane number (CN). The CN of methanol is so low that it cannot be measured directly, but needs to be determined by extrapolation. Pure methanol has a CN of 3 and methanol with 10 % water content has a CN of 2 [6]. Because of the very low CN, methanol is not suited for a conven-tional CI engine. Additives can be used to increase the CN close to the level of diesel, but these additives are toxic, carcinogenic and expensive. Moreover, diesel fuels also lubricate the engine fuel pumps and injectors. As methanol is a low viscous liquid this also causes problems, but again this could be solved with lubricity improvers [30].
It is clear that the use of methanol in a conventional CI engine is not so straight forward. To use methanol in a CI engine, dual-fuel (DF) operation can be used. The following section explains the different existing injection locations for the second fuel. This is followed by an analysis of the combustion characteristics, the efficiency and the emissions of diesel-methanol dual-fuel (DMDF), each with a focus on the fumigation concept.
Indicators for the substitution ratio
In literature around DMDF there is a wide variety of indicators for the ratio between methanol and diesel. Table 2.1 gives an overview of some of the frequently used parameters to indicate the diesel-methanol fraction.
Table 2.1: Overview of parameters used to indicate the methanol and diesel fraction [24].
Methanol substitution percent MSP = m˙d,DO− ˙md,DF
˙ md,DO
Methanol mass fraction MMF = m˙m,DF
˙
mm,DF+ ˙md,DF
Methanol energy fraction MEF = m˙m,DF·LHVm
˙ mm,DF·LHVm+ ˙md,DF·LHVd Ratio methanol-diesel RM D = ˙ mm,DF ˙ md,DF
2.1
Injection locations for DMDF engines
Methanol can be injected at several different injection locations in a DMDF engine, each having their own properties. A brief overview describing the different injection strategies is given below and illustrated in Figure 2.1 [5].
Port fuel injection (PFI) of methanol - Fumigation
The PFI strategy (fumigation), injects methanol in the intake, so a methanol-air mixture enters the combustion chamber during the suction stroke. Methanol can be injected both in a single point, in the intake, or at multiple points, with injectors directed to the intake valve. As the methanol is injected in the intake line, a low pressure injection system can be used, which is an advantage for the cost of the concept. A pilot injection of diesel, sometimes referred to as a liquid spark, ignites the mixture around top dead center (TDC).
Injecting a mixture of diesel and methanol
A second strategy injects a mixture of diesel and methanol. An advantage is that this only requires one injector per cylinder and that the conversion is quite straightforward. A disadvan-tage is that the substitution ratio cannot be changed instantaneously. Another disadvandisadvan-tage is that diesel and methanol are not very good miscible and additives are required.
Direct injection of methanol using a separated or a split injector
With this concept the substitution ratio can be changed instantaneously. But the concept requires an extra injector per cylinder or the use of a more complex injector, implying modi-fications to the cylinder head when doing a retrofit.
Compared to the fumigation concept, the installation of the other concepts is more com-plex. Therefore, the fumigation concept is the most appropriate as a retrofit solution. The fumigation concept preserves the possibility to switch back to 100 % diesel operation in case of a problem with methanol or in case no methanol supplier is found in a harbour. This benefits possible uptake by the conservative shipping industry [24]. Fore these reasons, this dissertation focuses on the fumigation concept to inject methanol.
2.2
Combustion characteristics of DMDF engines
A profound understanding of the combustion behaviour in DMDF mode is important to un-derstand the efficiency and the emission formation, which in turn requires an unun-derstanding of the fuel characteristics of methanol. Therefore, the most important fuel properties are listed and the combustion behaviour of a DMDF four stroke engine is explained.
Table 2.2 lists the properties of methanol compared to those of diesel and gasoline. The most important properties for the combustion in DF engine using the fumigation concept are the high heat of vaporization, the low volumetric energy content, the low adiabatic flame temperature and as discussed before the RON, MON and CN.
Table 2.2: Physical and chemical properties of diesel, gasoline and methanol [5, 6, 30–34].
Property Diesel Gasoline Methanol Chemical formula av C14H30 av C8H15 CH3OH
Density @ STP [kg/m3] 856 740 790 Molecular weight [kg/kmol] 198.4 107.0 32.0 Heat of vaporization [kJ/kg] 620 180-350 1 100
Flash point [°C] 52 -45 11
Lower heating value [MJ/kg] 41.7 42.9 20.1 Volumetric energy content [MJ/m3] 35 695 31 746 15 871 Stoichiometric AFR [kg/kg] 14.7 14.7 6.5 Adiabatic flame temperature [K] 2 305 2 275 2 143 Research Octane Number <0 95 109
Motor Octane Number <0 85 92
Cetane Number 45-50 8-14 3
Laminar flame speed @ NTP, λ=1 [cm/s] 30.0 33.0 40.0 Auto-ignition temperature [K] 523 604 738
When using the fumigation concept for a DMDF engine, methanol is injected in the intake. Thus, the engine sucks a methanol-air mixture in during the intake stroke. Due to the high heat of vaporization of methanol, the temperature of the intake air drops. the cooling effect increases the volumetric efficiency of the engine [6]. On the other hand, the evapo-rated methanol also takes in some volume which cannot be occupied by air, thus lowering the volumetric efficiency. These two effects almost level out, the evaporation of methanol causes a decrease in the intake temperature, but it does not significantly affect the volumetric efficiency of the engine. This conclusion can be taken by analysing the measurement results of Verbiest et al. [24].
After the intake stroke, the compression stroke compresses the methanol-air mixture which has a lower temperature than an air mixture in diesel operation. In case of an incomplete evaporation of methanol before the intake valve closes, the remaining liquid methanol droplets evaporate during the compression stroke.
Around TDC, the pilot injection of diesel occurs and the combustion proces starts. While the combustion process of a common diesel engine can be split up into three phases [35]:
1. The ignition delay period
2. The premixed combustion period 3. The diffusion combustion period
The combustion process of a DF engine is more complex. The first phase is the same for a DF engine but with an increase in the fumigation level, the duration of the ignition delay increases. Around TDC the pilot diesel is injected, once the auto-ignition of the pilot diesel occurs, the premixed combustion starts. During the premixed combustion phase, the combustion of the premixed diesel and some part of the methanol in close proximity occurs. After the premixed combustion period, the remaining diesel fuel burns during the diffusion combustion period. Simultaneously with the diffusion combustion of the remaining diesel, the homogeneous methanol-air mixture burns through flame propagation. Because of this combustion behaviour, a fumigation DF engine has the combined characteristics of a CI and a SI engine [35]. There are two main reasons for the longer ignition delay. On the one hand, chemical changes occur in the radical pool when methanol is added and on the other hand the lower temperature also increases ignition delay [5]. The increased ignition delay with an increased methanol fraction has been experimentally confirmed [24].
Li et al. [36] defined a rapid combustion fraction (β) to quantify the percentage of heat release during the premixed combustion. Equation 2.1 displays the definition of the rapid combustion fraction. Figure 2.2 indicates the demarcation points between the premixed com-bustion phase and the diffusion comcom-bustion phase. This way, Li et al. measured a clear increase in the fraction of heat release during the rapid combustion phase when increasing the methanol ratio. They attribute this to the fact that the methanol is already mixed with the air, but also to the fact that a bigger fraction of diesel burns in the premixed combustion mode due to the longer ignition delay.
β = R2 1 HRR dφ R3 1 HRR dφ (2.1)
Liu et al. [37] measured the in-cylinder pressure and heat release rate at each crank angle for different methanol-diesel ratios. The definition of their methanol substitution rate (Sr) is the same as MEF defined in Table 2.1. Figure 2.3 shows their results. For an increasing amount of methanol the increased ignition delay is clearly visible. There is an increase in the maximum peak pressure, an increase in the maximum heat release rate and an increase in the pressure increase rate. Although the combustion starts later, the end of combustion is at the same moment because an increasing methanol fraction decreases the combustion duration [29].
Figure 2.3: In-cylinder pressure and heat release rate for different methanol energy fractions [37].
Zhang et al. [35] have done a more intensive analysis of the pressure and heat release rate. Figure 2.4 presents the results of their measurements. It should be noted that the higher increased ignition delay at low loads compared to the less increased ignition delay at high loads, reported in the paper and in the text below is not visually clear on the figure. Yet, other research also reports a higher increased ignition delay at low loads [24]. At low loads, Zhang et al. notice a decrease of the maximum peak pressure when increasing the methanol content, while at high loads, the maximum peak pressure increases when increasing the methanol content. At lower loads, the in-cylinder gas temperature is low and reduces even further when increasing the methanol content. On top of that the mixture is also leaner than at high loads. Hence a high ignition delay, which results in a heat release further away from TDC and a resulting lower peak cylinder pressure. At higher loads the methanol-air mixture becomes richer, the in-cylinder gas temperature is higher and thus the ignition delay does not increase as much as at low loads when increasing the methanol content. As the mixture is richer at high loads, the mixture burns faster. This yields a higher maximum peak pressure.
Figure 2.4: In-cylinder pressure and heat release rate for different methanol mass fractions at different loads [35].
For all loads they notice an increase in the maximum heat release rate for an increased methanol content. This can be explained by the fact that the premixed phase burns more diesel and because the methanol is already premixed with air.
As the adiabatic flame temperature of methanol is 7.5 % lower than the adiabatic flame temperature of diesel and because of the higher heat capacity of the burned gas, the temper-ature of the exhaust gases reduces [5, 6]. But as methanol burns premixed while some part of the diesel burns in the diffusion flame, there is no one-on-one relation between the adiabatic flame temperature and the actual flame temperature. Other factors than the adiabatic flame temperature, such as the combustion duration and the local air-fuel ratio (AFR) also influ-ence the flame temperature. Both fuels burn with an other local AFR, both different from the stoichiometric AFR.
Due to the fumigation of methanol in the intake, there is another combustion process com-pared to a conventional diesel engine. A sufficient understanding of these combustion charac-teristics is important to understand the influence on the operating range, the efficiency and the emissions. The most important differences are the lower in-cylinder temperature, the increased ignition delay and the fact that more fuel is burned in the premixed combustion phase. The combustion behaviour of a DMDF engine is further studied in Chapter 5. The combustion process described in this section cannot occur with any arbitrary ratio between methanol and diesel, there are limits on the operating range of DMDF engines.
2.3
Operating range of DMDF engines
To fully profit the advantages of methanol, only just as much pilot injection of diesel should be used as required to ignite the methanol-air mixture. In reality, four boundaries limit the maximum amount of methanol burned in the DF fumigation concept:
1. Partial burn 2. Misfire
3. Roar combustion 4. Knock
Wang et al. [38] experimentally determined and described the boundaries of the operating range of a six cylinder 7.14 l engine at an engine speed of 1400 rpm. However, the boundaries between different limiting factors are not always clear. Figure 2.5 graphically presents their results. Table 2.1 gave the definition of their methanol substitution percent (MSP). Apart from their paper, no other reports quantitatively assess and discuss the operating limits of a DMDF engine.
Figure 2.5: Experimental determined operating range of a 7.14l DMDF engine at 1400 rpm [38].
Partial burn
At low loads, partial burn limits the maximum attainable MSP. The maximum MSP in-creases almost linearly with an increasing load. When a partial burn occurs, some part of the methanol-air mixture escapes combustion due to flame quenching on the walls or the effects of crevices. Partial burn is determined if the engine output torque stops increasing when increasing the amount of methanol. When this happens, it can be assumed that only a fraction of the methanol burns and the excess methanol is emitted into the exhaust pipe. Partial burn can also be detected by observing the exhaust temperatures of the cylinders [29].
Misfire
As the load increases to medium loads, the misfire bound is reached, and their MSP reached it maximum value of 76 %. Misfire occurs when the pilot injection of diesel is unable to initiate the propagation flame through the methanol-air mixture. According to Wang et al., a roar or a knocking cycle follows after a misfire cycle. Leading up to the misfire, the coefficient of variation (COV) increases. Similarly as partial burn, misfire can be detected with the pressure analyser or by observing the exhaust temperatures of the cylinders [29].
Roar combustion
As the load increases even further, high pressure rises and loud noise occur, which limit the MSP. This is defined as the roar combustion bound [5]. The maximum attainable MSP decreases again with an increasing load. Roar combustion is determined by an excessive increase in the peak pressure rise rate (PPRR). Wang et al. [38] set the PPRR limit for their engine at 1.5 MPa/°CA.
Knock
At the highest loads, knock limits the maximum MSP. Knock occurs if the premixed methanol auto-ignites before the pilot injection of diesel ignites the mixture (pre-ignition) or if the end-gas ignites instead of being burned by flame propagation. If the methanol auto-ignites before the diesel injection, both fuels almost combust separately, Figure 2.6 shows this behaviour and Section 5.2.3 intensively discusses it. Knock is determined by an excessive peak cylinder pressure (PCP). A maximum PCP of 15 MPa was used by Wang et al.
Figure 2.6: Comparison of combustion characteristics between diesel and DMDF mode at pre-ignition condition [38].
In other words, at low loads the maximum MSP increases with an increase in the load, as partial burn or misfire, due to the low temperature and lean mixture, limit further increase of the MSP. However, at high loads the maximum MSP decreases again with an increase in the load, as roar combustion and knock, due to a higher degree of premixed mixture in combination with the high compression ratio of a diesel engine, limit further increase of the MSP. These boundaries limit the operating range of a DMDF engine, within the operating range the MSP and load may vary. But note that changes in load and MSP also influence the efficiency of a DMDF engine.
2.4
Efficiency of DMDF engines
Break thermal efficiency
The change of combustion behaviour described in Section 2.2 results in a change of the overall efficiency when varying the methanol substitution within the operating range of the DMDF engine. Equation 2.2 defines the break thermal efficiency (BTE).
BT E = Pe
˙
md,DF· LHVd+ ˙mm,DF· LHVm
(2.2)
Coulier et al. [39] combines the results of other researches and concludes that at low and medium load the efficiency decreases while at higher loads the efficiency may in-crease. The decrease at lower loads is attributed to the longer ignition delay, yielding com-bustion further away from TDC and a lower burning velocity due to the leaner and colder mixture. The increase in efficiency at high load is attributed to the faster combustion, in richer mixtures at higher temperatures, which is closer to a thermodynamic ideal isochoric combustion. The rapid energy release also reduces the heat loss to the cylinder walls.
At all loads, the longer ignition delay due to the fumigated methanol can be compensated by advancing the injection timing of the pilot diesel causing an increase in efficiency [37, 40]. At low loads the efficiency decreases because of the reduced temperature of the mixture, but this effect can be compensated by intake preheating [41].
CO2 emissions
When burning a carbonaceous fuel the emission of CO2 is inevitable. CO2 contributes to
global warming so excessive emission should be avoided. But as CO2is a product of complete
combustion and therefore inevitable it should not be considered as a noxious emission. When evaluating the CO2 emission of a ship, it is compared to the economic value created. In the
definition of specific CO2 the break power reflects the engine economic value. The IMO uses
the EEDI which compares the equivalent CO2 emission with to the transport work. In both
definitions the emission of CO2 and the efficiency are important.
Methanol has a higher hydrogen to carbon ratio than diesel and gasoline. In case of an ideal combustion, each kilogram of diesel fuel generates 3.150 kg of CO2, each kilogram of
methanol fuel generates 1.375 kg of CO2[42]. When taking into account the LHV from Table
2.2 this results in a CO2 production of 75.5 g/MJ for diesel and 68.4 g/MJ for methanol,
a relative difference of 9.4 %. For methanol and gasoline the relative difference is 7.0 % in favour of methanol [6].
According to Coulier et al. [39], no general conclusion considering the CO2 emission can be
taken based on literature. Yet methanol clearly offers the possibility of a reduction in CO2
emissions due to the lower hydrogen to carbon ratio, but efficiency determines whether there is an actual weighted reduction. It should be noted that the explanation above is correct in case of burning conventional methanol. Even then, a LCA should take into account the pro-duced CO2 during each of the production steps. But in case of burning renewable methanol,
a LCA should take into account the amount of CO2 extracted from the air in the production
process. This way, the LCA can evaluate based on a net CO2 emission.
When operating an ICE the exhaust gasses do not only consist of the products of a com-plete combustion as CO2 and H2O, but other substances are also emitted. The next section
evaluates the emission of those hazardous substances when operating a DMDF engine.
2.5
Emissions of DMDF engines
2.5.1 NOx emissions of DMDF engines
The term nitrogen oxides (NOx) is a generic name mainly consisting of the combination of
nitrogen monoxide (NO) and nitrogen dioxide (NO2). These two gases contribute to the
formation of acid rain, smog and they affect the ozone layer. In other words, emission of these gases should be avoided or limited as much as possible. This section discusses the NOx
formation based on reports in literature, Section 6.1 further examines the NO formation and the ratio of NO2/NO by analysing measurements of Verbiest et al. [24].
The (extended) Zeldovich mechanism describes the production of NO which mainly depends on three factors (for a more detailed description, see Section 6.1) [43]:
1. High temperature (”thermal NOx”)
2. High oxygen concentration 3. Long residence time
Some part of the produced NO converts to NO2, predominantly through the following
reac-tion: NO + HO2 −→ NO2 + OH. This reaction is promoted by hydrocarbons that produce
reactive radicals (e.g. OH, O), which lead to an additional hydroperoxyl (HO2) production
[44]. The inverse reaction ( NO2 to NO) only occurs if there is a long residence time at high
temperature.
Using PLIF imaging, Dec et al. [45] were able to locate the NO formation in a diesel engine. They conclude that NO is not produced by premixed combustion (which is fuel-rich). They observed that the major fraction of NO is produced at the lean side of the diffusion flame,
![Figure 2.1: Overview of different injection strategies, adapted from Dierickx et al. [29].](https://thumb-eu.123doks.com/thumbv2/5doknet/3274788.21406/32.892.130.775.809.1051/figure-overview-different-injection-strategies-adapted-dierickx-et.webp)
![Table 2.2: Physical and chemical properties of diesel, gasoline and methanol [5, 6, 30–34].](https://thumb-eu.123doks.com/thumbv2/5doknet/3274788.21406/34.892.156.740.194.564/table-physical-chemical-properties-diesel-gasoline-methanol.webp)
![Figure 2.3: In-cylinder pressure and heat release rate for different methanol energy fractions [37].](https://thumb-eu.123doks.com/thumbv2/5doknet/3274788.21406/36.892.210.679.383.708/figure-cylinder-pressure-release-different-methanol-energy-fractions.webp)
![Figure 2.4: In-cylinder pressure and heat release rate for different methanol mass fractions at different loads [35].](https://thumb-eu.123doks.com/thumbv2/5doknet/3274788.21406/37.892.219.693.156.993/figure-cylinder-pressure-release-different-methanol-fractions-different.webp)
![Figure 2.5: Experimental determined operating range of a 7.14l DMDF engine at 1400 rpm [38].](https://thumb-eu.123doks.com/thumbv2/5doknet/3274788.21406/39.892.206.709.166.567/figure-experimental-determined-operating-range-dmdf-engine-rpm.webp)
![Figure 2.6: Comparison of combustion characteristics between diesel and DMDF mode at pre-ignition condition [38].](https://thumb-eu.123doks.com/thumbv2/5doknet/3274788.21406/40.892.256.646.561.829/figure-comparison-combustion-characteristics-diesel-dmdf-ignition-condition.webp)
![Figure 3.2: Air and water flow in and out of the humidification tower of a HAM [60].](https://thumb-eu.123doks.com/thumbv2/5doknet/3274788.21406/52.892.239.679.629.869/figure-air-water-flow-humidification-tower-ham.webp)