EDUCATIONAL APPLICATIONS TO
IMPROVE
ASTHMA
AND
COPD
PATIENTS’ HEALTH OUTCOMES: A
SYSTEMATIC REVIEW
Word count: 7322
Paulien van der Wel
Student number: 01301387Supervisor: Prof. Dr. Apr. Lies Lahousse
A short dissertation Master of Teaching (9SP) submitted to Ghent University in partial fulfillment of the requirements for the degree of Master of Teaching in Health Sciences
ABSTRACT
Objective: Poor disease-management is common among asthma and Chronic Obstructive Pulmonary Disease (COPD) patients.(1) This poor management results in a major health- and economic burden for the patient and the society.(2, 3) Several interventions were developed in order to increase asthma and COPD patient’s self-management. Mobile health (m-Heath) is a recent development which fosters tools for self-management. The aim of this thesis was therefore to assess the impact of educational digital applications on asthma and COPD patients’ health outcomes.
Methods: A systematic review was performed. The search strategy was conducted in PubMed and Web of Sciences. Studies were included if the subjects were asthma and/or COPD patients and the intervention must be an educational application compatible with a mobile phone and/or tablet. The outcome of the included study must comprise at least one of the following health outcomes: disease control, therapy adherence, quality of life and/or inhalation technique. The reference lists of the included studies were screened for additional relevant studies. A quality assessment was performed on the included studies using the Effective Public Healthcare Panacea Project tool (EPHPP). The functions of the educational applications were categorized, as well as the examined effects.
Results: The used search strategy resulted in 193 publications on PubMed and 171 publications on the Web Of Science. After removing duplicates, applying in- and exclusion criteria and screening the reference lists, 12 studies remained. For the global rating on studies’ quality assessment only one study (8.3%) scored weak, 25.0% (n=3) scored moderate and 66.7% (n=8) scored strong. Nine studies examined disease control, two studies inhalation technique, four studies therapy adherence and six studies quality of life. All studies presented positive effects of the studied educational applications on health outcomes. Ten (83.3%) of the educational applications had at least three different functions. Respectively 66.7% (n=8), 41.7% (n=5) and 58.3% (n=7) of the applications had an informing, instructive and guiding function. Ten educational applications (83.3%) captured and saved patients’ data, 33.3% (n=4) displayed the entered data, five (41.7%) had a reminder function and five (41.7%) had a communication function. Almost half of the studies that recorded patients’ data did not mention any privacy policies (n=4, 40.0%). All studies that assessed patient’s perception on the educational application concluded that patients had a positive attitude towards the m-Health application (n=4).
Conclusion: The overall effect of m-Health educational applications on asthma and COPD patients’ health outcomes seemed positive, although several barriers were noticed during this review. More transparency is required concerning data privacy and the cost-efficiency of educational applications is unclear.
ABSTRACT
Objectief: Suboptimaal ziektemanagement is een veelvoorkomend probleem bij astma en COPD patiënten.(1) Dit resulteert in een zware last op economisch en op gezondheidsvlak voor zowel de patiënt als de maatschappij.(2, 3) Verschillende interventies zijn ontwikkeld om ziektemanagement van astma en COPD patiënten te verbeteren. Mobiele educatieve gezondheidsapplicaties (m-Health) zijn een van de meest recente ontwikkelingen. Het doel van deze thesis was om de impact van educatieve applicaties op astma en COPD patiënten hun gezondheidsuitkomst te evalueren.
Methoden: Een systematische review werd uitgevoerd. Er werd strategisch gezocht in PubMed en Web of Science. Studies werden geselecteerd als de doelgroep astma en/of COPD patiënten waren en de interventie moest een educatieve applicatie zijn die compatibel is met een smartphone en/of tablet. De onderzochte uitkomsten van de studie moesten minstens één van de volgende bevatten: ziektecontrole, therapietrouw, levenskwaliteit en inhalatietechniek. De bronnenlijsten van de geselecteerde studies werden gecontroleerd op andere interessante studies. De studies ondergingen een kwaliteitscontrole m.b.v. de EPHPP tool. De functies van de educatieve applicaties werden gecategoriseerd net zoals de onderzochte effecten.
Resultaten: De gehanteerde zoekstrategie resulteerde in 193 publicaties op PubMed en 171 resultaten op Web of Science. Na het weglaten van duplicaties, het toepassen van de in- en exclusie criteria en het controleren van de bronnenlijsten bleven er 12 studies over. Voor de algemene score bij de kwaliteitscontrole scoorde slechts één studie (8.3%) zwak, 25.0% (n=3) gemiddeld en 66.7% (n=8) sterk. In deze review onderzochten negen studies de ziektecontrole, twee studies de inhalatietechniek, vier studies de therapietrouw en zes studies de levenskwaliteit. Alle studies toonden een positief effect aan van de educatieve gezondheidsapplicaties op de gezondheidsuitkomsten. Tien educatieve applicaties (83.3%) hadden minstens drie verschillende functies. Respectievelijk hadden 66.7% (n=8), 41.7% (n=5) en 58.3% (n=7) van de applicaties een informatieve, instructieve en begeleidende functie. Tien educatieve applicaties (83.3%) houden patiënten data bij, vier (33.3%) geven ingegeven data visueel weer, vijf (41.7%) hebben een herinneringsfunctie en vijf (41.7%) hebben een communicatiefunctie. Bijna de helft van de studies waarbij patiënten data werd opgeslagen vermeldde niets over hun privacy beleid (n=4, 40.0%). Alle studies die de patiënt zijn mening evalueerden over de educatieve applicatie stelden vast dat patiënten een positieve attitude hadden naar de m-Health applicaties toe (n=4).
Conclusie: In het algemeen lijkt het dat m-Health educatieve applicaties een positief effect hebben op de gezondheid van astma en COPD patiënten. Er is echter nood aan meer transparantie over het beheer van patiënten data. Ook de kosten-effectiviteit is niet eenduidig.
I would like to thank all those whose assistance and support helped me in achieving this thesis. In particular my family for the assistance and my friends for the moral support. But my special thanks go to my promotor Prof. Dr. Apr. Lies Lahousse who allowed me to continue on the subject of my previous thesis. This opportunity made the research into this subject even more exciting to me.
Preamble concerning COVID-19
COVID-19 preambule
Aangezien deze thesis een literatuuronderzoek is, waren er geen complicaties wegens COVID-19.
TABLE OF CONTENTS
1 INTRODUCTION ... 1
1.1 Chronic diseases: A major health burden ... 1
1.2 Asthma and COPD, two common chronic respiratory diseases ... 3
1.3 Interventions to improve therapy adherence ... 3
1.3.1 Optimization of treatment ... 3
1.3.2 Cognitive behavioral interventions ... 4
1.3.3 Adherence aids ... 4
1.3.4 Adherence assessments ... 4
1.3.5 Patient education ... 4
1.4 Impact of patient education on health outcomes in asthma and COPD patients ... 5
1.5 Educational mobile applications for asthma and COPD patients ... 5
1.6 The My Puff application ... 7
2 OBJECTIVE ... 8
3 METHODS ... 9
3.1 Search protocol ... 9
3.2 Selection of the literature ... 9
3.2.1 Inclusion criteria ... 9
3.2.2 Exclusion criteria ... 10
3.3 Data analysis ... 10
3.4 Quality assessment of the included studies ... 11
4 RESULTS ... 12
4.1 Literature search results ... 12
4.2 Characteristics of the included studies ... 14
4.3 Quality assessment of the included studies ... 19
4.4 Description of the interventions ... 19
4.5 Effect of the interventions on the health outcomes ... 20
5 DISCUSSION ... 22
5.1 Discussion of results ... 22
5.2 A comparison with My Puff... 23
5.3 Limitations ... 24
6 CONCLUSION ... 25
7 REFERENCES ... 26
8 APPENDIX ... 30 8.1 Effective Public Health Practice Project – Quality assessment tool for quantitative studies . 30
ABBREVIATION LIST
ACQ Asthma Control Questionnaire
ACT Asthma Control Test
CARAT Control of Allergic Rhinitis and Asthma Test
CAT COPD Assessment Test
COPD Chronic Obstructive Pulmonary Disease
CRQ Chronic Respiratory Disease Questionnaire
EPHPP Effective Public Healthcare Panacea Project
FEV1 Forced Expiratory Volume in 1 second
FVC Forced Vital Capacity
HCP healthcare provider
KASE-Q Knowledge, Attitude and Self-Efficacy
Questionnaire
MARS Medication Adherence Report Scale
Mini-AQLQ Mini-Asthma Quality of Life Questionnaire
PACQLQ Pediatric Asthma Caregiver Quality of Life
Questionnaire
PAQLQ Pediatric Asthma Quality of Life
Questionnaire
QoL Quality of Life
1 Introduction
The introduction of this study describes the broader concept of the impact of chronic diseases and the different strategies to improve health outcomes for chronic patients. But the focus of this thesis is on two common chronic respiratory conditions: asthma and chronic obstructive pulmonary disease (COPD).(4, 5) As for the different strategies to improve health outcomes, patient education and more precisely educational applications, will be the focus point of this study.
1.1 Chronic diseases: A major health burden
Chronic diseases are the cause of more than 70% of the total deaths worldwide.(6) A chronic disease can be described as ‘a physical or mental health condition that lasts more than one year and causes functional restrictions or requires ongoing monitoring or treatment’.(7) Chronic diseases include conditions such as heart disease, stroke, cancer, diabetes, respiratory conditions and arthritis.(6) For each of these conditions adequate long-term therapies are available. These medicines diminish or even suppress the symptoms and therefore increase the quality of life of these patients. In order to obtain this objective, it is necessary to adhere to the prescribed dose regimen.(8) Only a correct and regular medication administration can obtain a good disease control. Despite having the possibility of achieving a good disease control by a good therapy adherence, it was estimated by the World Health Organization that only 50% of the patients with chronic conditions were adherent to their long-term therapies. This number is even lower for developing countries.(9)
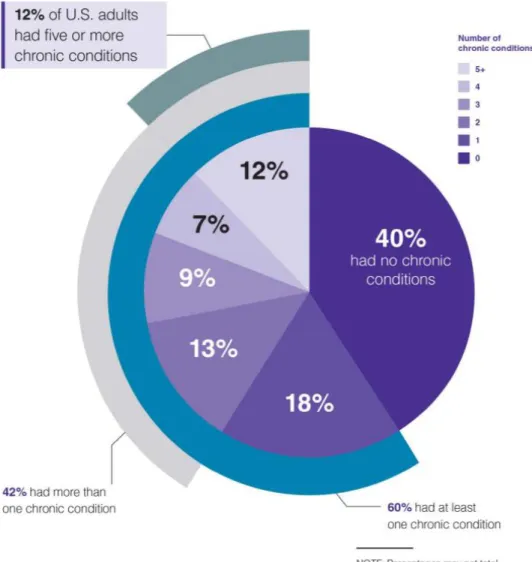
Figure 1 The prevalence of chronic diseases in the US (2017).(10)
Low adherence has several possible consequences for the patient. The patient may experience a deterioration of the symptoms. This deterioration can lead to exacerbations and thereby increase the risk of hospitalization. This poor disease control possibly affects the patient’s quality of life and can affect the patient’s mental health status.(9, 11) Poor therapy adherence does not only affect the individual itself but it affects the society as well. Chronic diseases are a major health- and economic burden. Healthcare costs increase due to the increased hospital admissions, comorbidities, add-on therapies,...(2, 3) For example the CODE-2 study examined the healthcare costs for the treatment of patients with type 2 diabetes. This study concluded that the direct healthcare costs of complications was 3-4 times higher for type 2 diabetics who are poorly controlled compared to patients with a good disease control.(12)
Poor adherence is a complex multifactorial problem. A patient’s poor adherence may be intentional or unintentional. The lack of adherence is often caused by several reasons such as age, attitude towards the therapy, complexity of the dose regimen. Besides the previous
reasons, the patient’s lack of knowledge of the disease and therapy is a common reason for low adherence.(2) This lack of knowledge may result from a poor health literacy.(13) Health literacy can be described as patient’s ability to collect, communicate and understand basic health information in order to make appropriate decisions concerning his/her health.(14) In order to achieve a good health literacy, education is needed.
1.2 Asthma and COPD, two common chronic respiratory diseases
Asthma and COPD are chronic respiratory diseases.(4, 5) Chronic respiratory diseases belong to the top ten of the most common chronic diseases worldwide.(1) More than 300 million people worldwide suffer from asthma.(15) As for COPD, the Global Burden of Disease Study reports a prevalence of 251 million cases globally in 2016.(16)
Asthma affects all ages and often has an early onset. Asthmatic patients suffer from chronic inflammation of the lungs with a variable course of symptoms. This condition leads to reversible bronchoconstriction and bronchial hyperreactivity. Typical symptoms of this condition are wheezing, coughing, dyspnea after exercise and chest tightness.(4)
Whereas asthma occurs at all ages, COPD has a late onset. COPD will occur after a long-term exposure to toxic components such as tobacco. COPD is a progressive lung disease with the following typical symptoms: partly reversible bronchoconstriction, dyspnea during exercise, excess of mucus and chronic coughing.(5)
The cornerstone treatment for both conditions includes inhalation therapy.(4, 5) The therapy may consist out of one or multiple (types of) inhalers and can be supplemented with oral medication. The therapeutic agent of the inhalation therapy will be a drug or multiple drugs of following therapeutic classes: beta-agonist, antimuscarinics, corticosteroids. The baseline treatment for asthma includes a corticosteroid.(4) For COPD a bronchodilator such as a beta-agonist or antimuscarinic covers the baseline treatment.(5) Add-on oral medication could be mucolytics, phosphodiesterase-4-inhibitors, antibiotics, corticosteroids or leukotrienes.(4, 5)
1.3 Interventions to improve therapy adherence
As mentioned before, good therapy adherence leads to good disease control.(2, 9) Therefore several interventions have been developed in order to improve patient’s therapy adherence and thereby achieving a good disease control.
1.3.1 Optimization of treatment
A complex dose regimen can lead to poor therapy adherence, hence the requirement of a simple medication schedule.(2, 17, 18) Therefore the reduction of the frequency of
administration is desired, as is the replacement of a single drug by a combination of preparations.
1.3.2 Cognitive behavioral interventions
It has been noted that patient’s perception and satisfaction towards the treatment is an essential factor for good therapy adherence.(19) Different approaches have been developed such as motivational training sessions or problem-solving sessions conducted by a trained counselor. During these sessions the patient addresses reasons for non-adherence and subsequently the counselor will suggest possible solutions or will give new insights regarding the non-adherence.(17, 18)
1.3.3 Adherence aids
If poor adherence is caused by forgetfulness, adherence aids can be used. The aim of these adherence aids is to remind the patient to administer their medication. Possible tools used for this purpose are text messages, electronic monitored pill boxes and telephone calls.(18, 20, 21)
1.3.4 Adherence assessments
The level of adherence can be determined by direct and indirect methods as well. As for direct methods, blood sampling or urine sampling can be used. Direct methods determine the concentration of drug or its metabolite in patient’s blood or urine. Indirect methods are more commonly used for adherence assessments. Monitoring patient’s pharmacy dispensing records may be considered as an indirect method for measuring the level of adherence.(17, 22)
1.3.5 Patient education
Increasing patient’s knowledge about the disease has proven to increase patient’s level of adherence.(23) Improving patient’s health literacy and knowledge about the condition is possible by education. Education can take many forms, hence various approaches are available such as information leaflets or instructive lessons. Instructive lessons can be elaborated in different ways such as education sessions conducted by trained health educators or as a consultation with the patient’s community pharmacist.(17, 18) The past decades, technology has gained its place more and more in the world of education. Nowadays it is possible to educate patients by using videos, computer-based learning or smartphone applications.(24) Digital technology broadens the range of educational possibilities.
1.4 Impact of patient education on health outcomes in asthma and COPD
patients
Health outcomes can be described as measuring instruments that assess the change in a patient’s or specific group’s health status. A change in health status can be attributed to an intervention.(25) In this case the patient education is considered as the intervention. Health outcomes can be observed or self-reported. Possible health outcomes for asthma and COPD may be: Health-related Quality of Life, therapy adherence, exacerbations, number of emergency department visits or worsening lung function parameters.
Overall there is a positive relation between education and asthma or COPD patient’s health outcomes.(18, 26, 27) Although this allegation must be specified. Education is a broad term since many different forms have been applied for patient education. For example there are several education programs conducted with positive tendencies. However, even among these education programs, different approaches (e.g. cognitivism and constructivism) and structures (e.g. individual or group sessions and the number of sessions) were used.(27) Next to the classical teaching, there are written action plans that have proven to be beneficial for asthma patients. A written action plan can be universal or individualized. This action plan is a set of instructions in order to manage deterioration of the symptoms and how to act on exacerbations.(28)
Since technology is developing quickly, the number of technological possibilities is increasing rapidly as well. There are many digital possibilities such as computer-based learning, health text messaging, video-monitoring or mobile applications.(24, 29) Due to the rapid development of technologic tools, clinical trials cannot keep pace. By consequence, a gap appears between digital educational tools and the necessary evidence of their efficiency.(30)
1.5 Educational mobile applications for asthma and COPD patients
The past decade the number of health-related mobile applications (m-Health) has increased rapidly. Nowadays, over 500 asthma and/or COPD m-Health apps exist.(31) These m-Health applications can be categorized into three groups: Reminder applications, digital based inhaler applications and educational applications.(29, 31, 32)
For the first group of applications, the main focus lies on reminding the patient to administer their medication by using pop-ups, alarms or other forms of notice.(33) As for the digital based inhaler applications, the patient’s inhaler will be digitally connected to a mobile application. Thus the application will register when the inhaler is used.(31) Educational applications will attempt to educate the patient. The subject of education may vary, for example, many
educational applications will encourage patients to demonstrate a correct inhalation technique while other applications will focus on educating a patient to a healthy life style concerning respiratory diseases.(29)
A review of Blakey et al. summarized the potential benefits of digital technologies for respiratory diseases as followed: precision, penetration, personalization and prediction.(29, 31) Mobile applications give the opportunity to provide long-term data of patient’s condition. Thereby healthcare providers (HCP) could monitor the patient’s real life adherence more precisely and the HCP could manage poor adherence with an adjusted approach. Penetration refers to the possibility to reach every social-economic class, in particular the patients in low-income settings. Wireless networks give the opportunity to reach these patients without an increase of patient’s costs. m-Health applications give an insight in the patient’s lifestyle. For example, several apps track patient’s daily activity, medication adherence or the patient’s beliefs and experiences on the treatment. This information provides a personalized view on the patient. A personalized approach by the application itself (by sending personalized reminders or by providing extra information) or by the HCP is also possible. The last potential benefit is prediction, the review of Blakey et al. suggests that in the future tools for data analysis and risk stratification tools could be incorporated. By consequence, exacerbations could be predicted and subsequently prevented. Conclusion: m-Health applications have multiple possible benefits for patient’s health outcomes.
Although m-Health applications give multiple opportunities regarding patient’s health, several burdens have been noticed as well. Among the great offer of m-Health applications, only a few are developed by professionals and even less are examined properly for effectiveness. (29, 31, 34) As for privacy only, 30% of the commonly used m-Health applications have privacy policies.(35) Privacy loss is a major concern for patients. Healthcare providers are hesitating to recommend m-Health applications without the insurance of guaranteed privacy. Regulation on data privacy from m-Health applications is necessary and currently absent.(31, 34) Therefore the quality and safety of multiple m-Health applications can be questioned.
In 2015, Apple® attempted to improve the consistency and quality of m-Heath applications by introducing ResearchKit®. ResearchKit® is an open source framework developed for iOS® devices. It provides the possibility for researchers to create applications for medical research. The framework consists out of three modules: 1. Informed consent, 2. Surveys, 3. Active tasks. The developer can choose to use one or multiple modules and can customize each module.(36, 37) The fact that Apple® provides a section for an informed consent is a step in the right direction concerning patient ethics, transparent data collection and patient’s privacy. However, it is still not mandatory to use an informed consent when developing an application
using ResearchKit®.(37) ResearchKit® is a user friendly tool, which means that the threshold to develop a m-Health application is even lower than before. ResearchKit® is not only very useful for qualified researchers but for every other interested person as well. Android® has developed a similar concept that is compatible on Android devices: ResearchStack®.(38)
1.6 The My Puff application
The setup of this paper originated out of one particular application: the My Puff app. The My Puff app is an educational application that teaches patients with chronic respiratory diseases a correct inhalation technique. The patient can choose its own inhalation device and will be guided and informed with written information and videos on how to use their inhalator correctly. The effectiveness of the application has been examined in an open randomized controlled trial over a three month period. In order to measure the effectiveness, the following health outcomes were chosen: disease control and inhalation technique. The study population consisted out of asthma and COPD patients. After recruitment, the subject was assigned to either the intervention or the control group. Both groups received standardized inhaler care. For continued education, the intervention group used the My Puff application and the control group received an information leaflet. For all patients with follow-up, the mean score on inhalation technique improved after three months. However, the inhalation technique of patients in the intervention group did not improve significantly compared to the control group. Conversely, the odds of achieving a clinically important improvement in disease control was three times higher for the leaflet group compared to the app group.
This randomized controlled trial emphasizes the importance of face-to-face pharmaceutical care programs to improve inhaler technique and disease control at the community level. More efforts are needed to develop successful educational materials or health applications meeting patients context, (digital) skills and needs for continued home education, since the study observed that the My Puff app was hardly used in daily practice, even after the guided instructions of the pharmacist.
2 Objective
Asthma and COPD are two common chronic respiratory diseases. Among asthma and COPD patients, the therapy adherence is low and the inhalation technique is poor.(4, 5) Poor therapy adherence and poor inhalation technique leads to a poor disease control. By consequence poor disease control leads to an increase in healthcare costs and a decrease of patient’s quality of life.(39, 40) Therefore different interventions were developed in order to improve patient’s health outcomes, e.g.: optimization of therapy, instructive sessions by a HCP or text messages as a reminder to administer the medication. The effectiveness of these interventions were thoroughly examined.(17, 18) Even though several of the current interventions seem effective, asthma and COPD patients’ health outcomes remains low.(9) By consequence there is a major need for new effective interventions.
In the past years technology evolved quickly. It found applications in diverse domains of the healthcare system and therefore also in the treatment of asthma and COPD. Technology provides the opportunity to develop new types of interventions. One of those new interventions are m-Health applications. m-Health applications are becoming more and more accessible for asthma and COPD patients as 45% of the population has a smartphone nowadays.(41) However m-Health applications are being developed at a much faster rate than researchers can conduct studies on the effectiveness and quality.(30) This difference in pace results in a gap between the development of m-Health applications and adequate studies upon their effectiveness and quality. The result is a discrepancy: many different m-Health applications are available for asthma and COPD but only a few have been examined upon their effectiveness.
There is need for a transparent overview of different m-Health applications for asthma and/or COPD patients regarding the quality, the safety and in particular the effectiveness. Transparency may ensure the HCP to recommend certain applications and patients may feel more comfortable using them. This thesis will attempt to give an overview of the effectiveness of several Health applications for asthma and COPD patients. The main focus lies upon m-Health applications aiming patient education. This thesis wants to give an answer on the following question: Does the use of educational applications improve asthma and/or COPD patient’s health outcomes?
3 Methods
The broader target of this study is to examine whether educational applications for asthma and/or COPD patients are effective or not. This section will clarify the in- and exclusion criteria, the used search protocol and the interpretation method of the collected data.
3.1 Search protocol
This thesis will be conducted as a systematic review. The databases PubMed and Web of Science were used in order to collect appropriate publications. The search terms used were: (education OR instruction) AND (asthma OR COPD) AND (digital OR technology OR mobile OR application) AND (adherence OR QoL OR inhalation technique OR disease control). For the used terms wildcards were applied to broaden the search field. Two filters were applied: publications in any other language than English were excluded from the search and the subject of the publications was narrowed to humans only. For all articles obtained, the reference lists were also checked for potential additional publications. The systematic search and the article selection were conducted by one researcher.
3.2 Selection of the literature
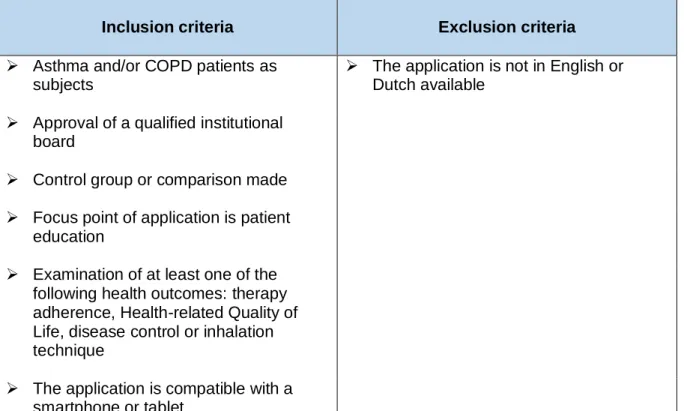
The aim is to select studies that examine the health outcomes of asthma and/or COPD patients using an educational application. In order to select adequate studies and be consistent as well, in- and exclusion criteria were established. The criteria were listed and subsequently used as guideline during the search and selection. Table 1 gives an overview of the selected in- and exclusion criteria.
3.2.1 Inclusion criteria
The patient population of interest of the application must be patients with asthma and/or COPD. The educational applications may have as target people of all ages. The study has to be approved by a qualified institutional board. As for the study design, the only requirement is the presence of a control group or the possibility to compare the outcome pre- and post-intervention. The study size is not considered as a criterion. It is mandatory that the main focus of the application is patient education. The application must educate the patient about his/her disease (management), inhalation technique or health literacy. In addition to education, the application may contain other functions as well e.g. administration reminders, diary function,… Studies were selected on the basis of the use of certain health outcomes to determine the effectiveness of the intervention. An educational application was considered as effective if the studied health outcome(s) had a positive (significant) result. The selected health outcomes were therapy adherence, Health-related Quality of Life, disease control and inhalation
technique. There are different methods possible to assess the chosen health outcomes, e.g. self-reported, questionnaires, checklists, evaluation by professionals,… As far as inclusion is concerned, examining at least one of the selected health outcomes is obligatory. Furthermore the application must be compatible with a smartphone or a tablet.
3.2.2 Exclusion criteria
The application must be intended for asthma and COPD patients in particular. Additional intended users are allowed as well (e.g. clinicians, pharmacists,…). Although, educational applications are excluded if they are exclusively intended for users other than the asthma and COPD patients. The applications are excluded as well if one of the possible languages is not English or Dutch. Whether an application is paying or for free has not been taken into account. Table 1 gives an overview of the selected exclusion criteria.
Table 1. An overview of the eligibility criteria.
Inclusion criteria Exclusion criteria
➢ Asthma and/or COPD patients as subjects
➢ The application is not in English or Dutch available
➢ Approval of a qualified institutional board
➢ Control group or comparison made ➢ Focus point of application is patient
education
➢ Examination of at least one of the following health outcomes: therapy adherence, Health-related Quality of Life, disease control or inhalation technique
➢ The application is compatible with a smartphone or tablet
3.3 Data analysis
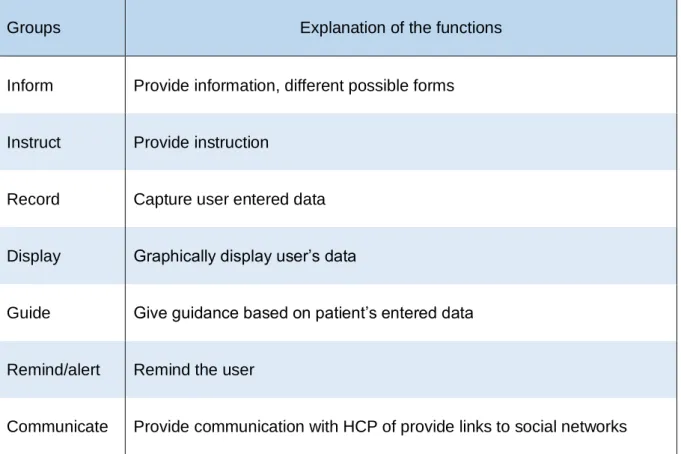
A systematic review is conducted using a descriptive analysis. The studies’ interventions are categorized into seven groups by functionality (inform, instruct, record, display, guide, remind/alert, communicate). Table 2 provides a further clarification of the used groups. The used categorization is derived from a previous study.(42) It is possible for an intervention to belong to multiple groups. Because the study of educational applications is the objective of this paper, studies should at least be categorized in at least one of the following groups: inform, instruct or guide.
Table 2. Explanation of the different groups of the functionality categorization.(42)
Groups Explanation of the functions
Inform Provide information, different possible forms
Instruct Provide instruction
Record Capture user entered data
Display Graphically display user’s data
Guide Give guidance based on patient’s entered data
Remind/alert Remind the user
Communicate Provide communication with HCP of provide links to social networks
The effect of the interventions is classified as well. The categories are statistically significant positive, statistically significant positive, statistically significant negative, statistically non-significant negative.
3.4 Quality assessment of the included studies
In order to determine the quality of the included studies, the quality assessment tool of the Effective Public Healthcare Panacea Project (EPHPP) is used.(43-45) (See Appendix) The EPHPP tool is suited for the evaluation of several different study designs. This tool can be considered as a checklist that consists of six components: selection bias, study design, confounders, blinding, data collection methods and withdrawals or dropouts. Each component comprises several questions. Using the associated dictionary, each section is scored and a global scoring is given as well. Assisted by the dictionary, each section is rated as strong, moderate or weak by the researcher. The global score was considered to be strong if there were no sections with a weak rating, moderate if there was only one weak rated section and weak if there were two or more weakly rated sections.(46) The quality assessment is executed by one researcher.
4 Results
4.1 Literature search results
Two databases were used for the study collection. The used search strategy resulted in 193 publications on PubMed and 171 publications on the Web Of Science. After screening out duplications and unsuitable publications, 26 studies remained. Thereafter, in- and exclusion criteria were applied. Ten of the 26 studies met eligibility criteria. The reference lists of the collected studies were checked for other possible studies. This search resulted in two extra studies, which brings the total number of included studies to 12. See the flowchart below for an overview (Figure 2).
There were different reasons to exclude several studies. The encountered reasons are listed below. The study did not examine one of the selected health outcomes (n=4). Two studies examined an application that was not compatible with a smartphone and/or tablet. The intervention of five studies were compatible with a smartphone, but were no digital application. The interventions were web-based (n=2), video-based (n=1) or sending informative/instructive text messages (n=2). One study examined the effectiveness of a digital monitored inhaler device linked to an application, but the application only registered the administrations without further functions. Another study’s subject was an application with solely a reminder function, educational features were absent. Finally, several studies did not restrict the subjects to asthma and COPD patients only (n=3). These three studies examined other populations as well but the results for asthma and COPD patients were stratified, therefore it was possible to examine these studies.
4.2 Characteristics of the included studies
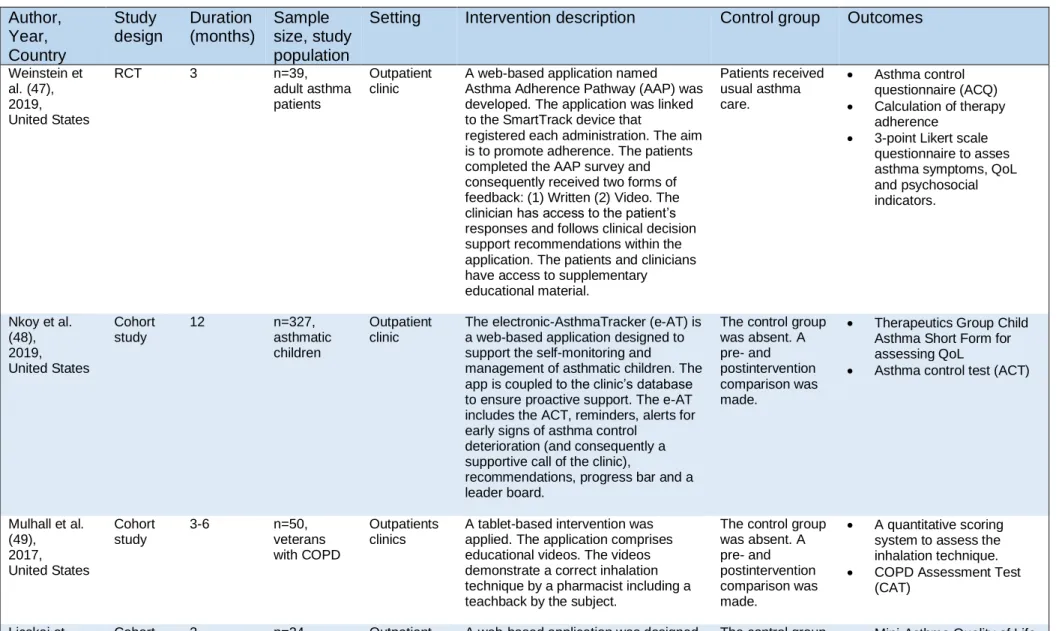
Five of the twelve included studies were conducted in the United States, two other studies in the United Kingdom. The remaining five studies took place in one of the following countries: Taiwan, Ireland, The Netherlands, China or Switzerland. The studies were executed during the period of 2011 until 2019. Five studies used a randomized controlled trial (RCT) as study design, whereas the other studies used a cohort study design (n=5) or cohort analytic study design (n=2). The duration of the study period varied from one day to one year. The majority of the studies lasted three to six months (n=8). Nine studies included solely asthma patients as subject, two included solely COPD patients and one study included asthma and COPD patients. Two studies exclusively included children (2-17 years) with asthma, one study included adolescents (12-18 years) with asthma, one study included adolescents and adults with asthma. The remaining studies exclusively included adult patients (n=8). For some studies the condition was specified in the selection criteria: poorly controlled asthma (n=3), cough variant asthma (n=1). The study size ranged from 22 to 327 participants (mean: 70 participants). Of the intended outcomes for this review nine studies examined disease control, two studies inhalation technique, four studies therapy adherence and six studies quality of life. Disease control was examined by questionnaires, e.g.: Asthma Control Questionnaire (ACQ) and COPD Assessment Test (CAT). The assessment tools for patient’s quality of life were questionnaires as well, e.g.: Mini-Asthma Quality of Life Questionnaire (mini-AQLQ) and Pediatric Asthma Quality of Life Questionnaire (PAQLQ) The inhalation technique was assessed by a composed checklist. Therapy adherence was assessed by a questionnaire (e.g.: Medication Adherence Report Scale (MARS)), self-reported, calculated or the method was not specified. In addition to these outcomes several studies examined other outcomes as well, for example pulmonary tests, FeNO and blood cell counts. The main characteristics of the included studies are summarized in the table below (Table 3).
Almost all included mHealth applications (except for two) recorded patients’ data. Four of the ten studies did not mention any privacy policies concerning patients’ data. The remaining six studies specified the application’s data security. The majority of the applications encrypted personal data, required a login with password to access the data and/or provided a secured server to store the data. Four of the included studies examined patient’s perception on the educational application. All four studies concluded that patients had a positive attitude towards the m-Health application.
Table 3: Main characteristics of the included studies. Author, Year, Country Study design Duration (months) Sample size, study population
Setting Intervention description Control group Outcomes
Weinstein et al. (47), 2019, United States RCT 3 n=39, adult asthma patients Outpatient clinic
A web-based application named Asthma Adherence Pathway (AAP) was developed. The application was linked to the SmartTrack device that
registered each administration. The aim is to promote adherence. The patients completed the AAP survey and consequently received two forms of feedback: (1) Written (2) Video. The clinician has access to the patient’s responses and follows clinical decision support recommendations within the application. The patients and clinicians have access to supplementary educational material. Patients received usual asthma care. • Asthma control questionnaire (ACQ) • Calculation of therapy adherence • 3-point Likert scale
questionnaire to asses asthma symptoms, QoL and psychosocial indicators. Nkoy et al. (48), 2019, United States Cohort study 12 n=327, asthmatic children Outpatient clinic
The electronic-AsthmaTracker (e-AT) is a web-based application designed to support the self-monitoring and
management of asthmatic children. The app is coupled to the clinic’s database to ensure proactive support. The e-AT includes the ACT, reminders, alerts for early signs of asthma control
deterioration (and consequently a supportive call of the clinic),
recommendations, progress bar and a leader board.
The control group was absent. A pre- and postintervention comparison was made.
• Therapeutics Group Child Asthma Short Form for assessing QoL
• Asthma control test (ACT)
Mulhall et al. (49), 2017, United States Cohort study 3-6 n=50, veterans with COPD Outpatients clinics
A tablet-based intervention was applied. The application comprises educational videos. The videos demonstrate a correct inhalation technique by a pharmacist including a teachback by the subject.
The control group was absent. A pre- and postintervention comparison was made. • A quantitative scoring system to assess the inhalation technique. • COPD Assessment Test
(CAT)
2013, United Kingdom
adult asthma patients
management. The features of the app include: AQHI Forecast for Tomorrow, Breathing Test, daily symptom registration pre- and postintervention comparison was made. • Questionnaires evaluating self-management,
understanding of the action plan, symptom control and health care utilization. Rassouli et al. (51), 2018, Switzerland (and Germany and Austria) Cohort study 2 n=34, COPD patients Real life setting
Kaia COPD is a mobile educational application that promotes self-management and exercise training for COPD patients. After downloading the app the patient’s health status and physical limitations are evaluated and integrated. Consequently the
application provides personalized exercises. The exercises include: (1) videos with physical exercises, (2) patient education, (3) mindfulness technique for disease coping and (4) an individualized asthma action plan.
The control group was absent. A pre- and postintervention comparison was made.
• The COPD Assessment Test (CAT)
• The Chronic Respiratory Disease Questionnaire (CRQ) Kosse et al. (52), 2018, The Netherlands RCT 6 n=234, adolescent asthma patients Primary care setting. (Community pharmacies)
The application’s aim is to improve patient’s adherence. The app includes: (1) CARAT test, (2) reminders, (3) study peers chat function, (4) pharmacist chat function, (5)
educational and motivational videos, (6) two questions every two weeks to monitor non-adherence. The intervention was interactive, the pharmacist could monitor results and send additional information.
The control group received usual asthma care.
• Medication Adherence Report Scale (MARS) • Asthma control assessed
with Control of Allergic Rhinitis and Asthma Test (CARAT)
• Pediatric Quality of Life Questionnaire (PAQLQ) Cook et al. (53), 2016 United States Cohort study 4 n=60, adult patients with poorly controlled asthma Real life setting
The educational mobile application aims to improve patients asthma control by sending alerts and requests to assess patient’s self-management and patient’s knowledge regarding asthma selfcare. The previous entries results in an individualized coaching. The app features educational materials as well in written or video format.
The control group was absent. A pre- and postintervention comparison was made.
• Asthma Control Test (ACT) • Forced Expiratory Volume
Shields et al. (54), 2018, Ireland Cohort analytic study 6 weeks n=22, children with poorly controlled asthma Real life setting
This mobile based application
instructed the parents or subjects (if old enough) to capture the inhalation technique twice daily on camera. Consequently the video was uploaded and evaluated by a researcher. The clinical team contacted the participants by telephone for instructive support, if they noticed a poor inhalation technique.
The control group was absent. A pre- and postintervention comparison was made.
• FeNO and spirometry measures
• Self-reported Medication Adherence Report Scale (MARS)
• Pediatric Asthma Quality of Life Questionnaire
(PAQLQ) or Pediatric Asthma Caregiver Quality of Life Questionnaire (PACQLQ)
• Asthma Control Test (ACT) • Inhalation technique checklist Press et al. (55), 2017, United States Cohort analytic study 1 day n=90 subjects, adult asthma and COPD patients Outpatient clinic
The application focuses on an amelioration of inhalation technique. The applied method is a Virtual Teach-To-Goal adaptive learning (V-TTG). Patients complete a series of multiple choice questions about correct inhalation technique, followed by an explanatory video. Afterwards patients receive the same survey with a video of an incorrect inhalation technique. If the patient did not succeed in answering correctly, the patient was required to review the video and complete the survey again up to three times.
The control group was absent. A pre- and postintervention comparison was made. • Inhalation technique checklist
• Online survey based on the Bloom’s learning theory assessing confidence, mastery and self-efficacy.
Cao et al. (56), 2018, China RCT 3 n=80, adult asthma patients with the cough-variant asthma Real life setting
For this study the mobile application WeChat was used. On this application a platform containing educational articles and videos about asthma was developed. Messages, voices and videos can be left on the platform and be replied to by healthcare providers. The app also included a timer and reminder to administer the medication.
The control group received usual asthma care.
• (Forced Expiratory Volume in 1 second) FEV1 and Forced Vital Capacity (FVC)
• Asthma Control Questionnaire (ACQ) • Asthma Quality of Life
Questionnaire (AQLQ) • Leicester cough
questionnaire
• Blood cell counts, total IgE and FeNO
• ED visits Ryan et al. (57), 2012, United Kingdom RCT 6 n=288, adolescent and adult patients with poorly controlled asthma Real life setting
The t+ Asthma application was developed in order to improve patient’s asthma management. The symptoms, drug use and peak flow must be reported twice on a daily basis. The results are displayed as traffic lights. Red or orange zones alert the healthcare provider who is to contact the patient the next day.
The control group was asked to keep a paper diary twice daily.
• Asthma Control Questionnaire (ACQ) • Knowledge, Attitude and
Self-Efficacy Questionnaire (KASE-Q)
• Mini-Asthma Quality of Life Questionnaire (mini-AQLQ) • Adverse occurrences
obtained from practice records
• Prescriptions of asthma drugs recorded in the patients’ healthcare record Liu et al. (58), 2011, Taiwan RCT 6 n=89, adult asthma patients Outpatient clinic
The application is developed to improve asthma control. The patients must report their asthma symptoms daily, inhaler use, PEFR and PEFR variability. According to the given answers patients received the
assessment of their asthma status and the corresponding management advice.
The control group was asked to keep a paper diary daily and received an individualized written asthma action plan.
• Short Form (SF)-121 questionnaire for assessing QoL
• Episodes of acute exacerbation and
medication used for asthma control
• Forced Expiratory Volume in 1 second (FEV1)
• Forced Vital Capacity (FVC)
• Asthma symptom score • ED visits
4.3 Quality assessment of the included studies
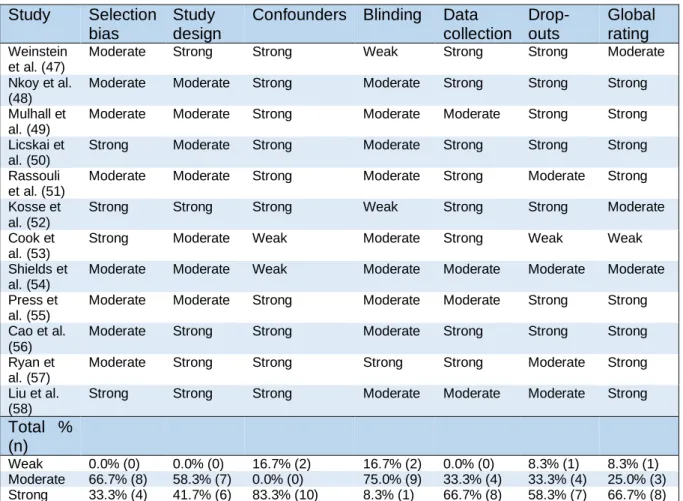
Table 4 gives an overview of the quality assessment of the included studies. 66.7% (n=8) and 33.3% (n=4) scored respectively moderate and strong on selection bias. As for the study design 58.3% (n=7) has a moderate study design and 41.7% (n=5) a strong study design. 16.7% (n=2) is rated weak considering confounders whereas none of the studies is rated moderate. For the section blinding 16.7% (n=2) scored weak, 75.0% (n=9) scored moderate and 8.3% (n=1) scored strong. 66.7% (n=8) scored strong for data collection and 33.3% (n=4) scored moderate. The final section, drop-outs and withdrawals rated weak, moderate and strong respectively 8.3% (n=1), 33.3% (n=4) and 58.3% (n=7). For the global rating only one study, 8.3% scored weak. 25.0% (n=3) scored globally moderate and the majority (66.7% (n=8)) scored globally strong on the quality assessment.
Table 4: Quality assessment of the included studies
Study Selection bias
Study design
Confounders Blinding Data collection Drop-outs Global rating Weinstein et al. (47)
Moderate Strong Strong Weak Strong Strong Moderate
Nkoy et al. (48)
Moderate Moderate Strong Moderate Strong Strong Strong
Mulhall et al. (49)
Moderate Moderate Strong Moderate Moderate Strong Strong
Licskai et al. (50)
Strong Moderate Strong Moderate Strong Strong Strong
Rassouli et al. (51)
Moderate Moderate Strong Moderate Strong Moderate Strong
Kosse et al. (52)
Strong Strong Strong Weak Strong Strong Moderate
Cook et al. (53)
Strong Moderate Weak Moderate Strong Weak Weak
Shields et al. (54)
Moderate Moderate Weak Moderate Moderate Moderate Moderate
Press et al. (55)
Moderate Moderate Strong Moderate Moderate Strong Strong
Cao et al. (56)
Moderate Strong Strong Moderate Strong Strong Strong
Ryan et al. (57)
Moderate Strong Strong Strong Strong Moderate Strong
Liu et al. (58)
Strong Strong Strong Moderate Moderate Moderate Strong
Total % (n)
Weak 0.0% (0) 0.0% (0) 16.7% (2) 16.7% (2) 0.0% (0) 8.3% (1) 8.3% (1)
Moderate 66.7% (8) 58.3% (7) 0.0% (0) 75.0% (9) 33.3% (4) 33.3% (4) 25.0% (3)
Strong 33.3% (4) 41.7% (6) 83.3% (10) 8.3% (1) 66.7% (8) 58.3% (7) 66.7% (8)
4.4 Description of the interventions
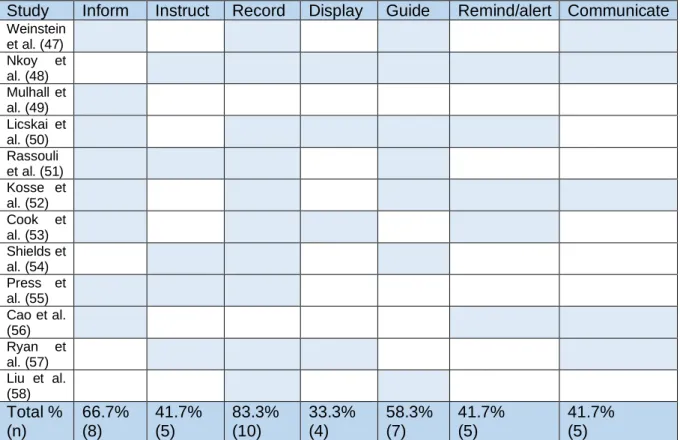
Table 5 represents a summary of the classified functions of the educational applications. Respectively 66.7% (n=8), 41.7% (n=5) and 58.3% (n=7) of the applications had an informing, instructive and guiding function. Ten of the 12 applications (83.3%) captured and saved
patients’ data. One third (33.3%; n=4) displayed the entered data by using graphs or color codes. Five educational applications (41.7%) had a reminding function as well. The function of the reminder differed between the applications, it was used as a reminder to enter the daily data, a reminder for medicine administration or as an alert to point out early signs of disease deterioration. Almost half of the applications (41.7% (n=5)) had the possibility to contact or to be contacted by HCP (n=5) and/or other participating peers (n=1). One application had only an informing function, another application had two functions: Data recording and patient guiding. All the other applications had at least three functions. One application had six out of seven functions.
Table 5: Description of the interventions
Study Inform Instruct Record Display Guide Remind/alert Communicate
Weinstein et al. (47) Nkoy et al. (48) Mulhall et al. (49) Licskai et al. (50) Rassouli et al. (51) Kosse et al. (52) Cook et al. (53) Shields et al. (54) Press et al. (55) Cao et al. (56) Ryan et al. (57) Liu et al. (58) Total % (n) 66.7% (8) 41.7% (5) 83.3% (10) 33.3% (4) 58.3% (7) 41.7% (5) 41.7% (5)
4.5 Effect of the interventions on the health outcomes
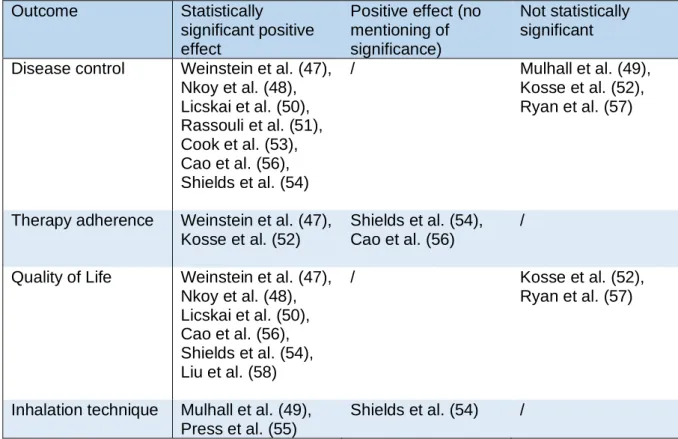
Table 6 only displays the outcomes examined in this review. There were no outcomes with a statistically significant negative effect or with a statistically non-significant negative effect. Disease control was examined in ten studies. Seven out of ten had a statistically significant positive effect, the effect of the other three studies were not significant. Four studies assessed therapy adherence, all with a positive effect as result. Half of the studies resulted in a positive effect on adherence without any mention of statistical significance. The other half of the studies had a positive statistically significant result on adherence. As for quality of life eight studies examined this outcome, whereof six studies had statistically significant positive results and two
studies had non-statistically significant effects. Inhalation technique was assessed by three studies, all studies had positive results whereof one study had a positive effect without specifying the significance.
Table 6: Effect of the interventions on the health outcomes
Outcome Statistically
significant positive effect
Positive effect (no mentioning of significance)
Not statistically significant Disease control Weinstein et al. (47),
Nkoy et al. (48), Licskai et al. (50), Rassouli et al. (51), Cook et al. (53), Cao et al. (56), Shields et al. (54) / Mulhall et al. (49), Kosse et al. (52), Ryan et al. (57)
Therapy adherence Weinstein et al. (47), Kosse et al. (52)
Shields et al. (54), Cao et al. (56)
/
Quality of Life Weinstein et al. (47), Nkoy et al. (48), Licskai et al. (50), Cao et al. (56), Shields et al. (54), Liu et al. (58) / Kosse et al. (52), Ryan et al. (57)
Inhalation technique Mulhall et al. (49), Press et al. (55)
5 Discussion
5.1 Discussion of results
Overall the studied outcomes seemed positive. None of the studies had an outcome with a negative effect, whether or not statistical significant. Although several studies had a positive effect without specifying the statistical significance or had a non-significant effect. Regarding disease control, seven of the ten studies showed a positive significant result whereas the three remaining studies had a non-significant positive effect. Of the three non-significant studies, one study included COPD patients, the other two studies included asthma adolescents. Previous research suggests that therapy adherence is low during adolescence.(59, 60) Poor therapy adherence worsens patient’s disease control.(9) As for therapy adherence all four studies that examined this outcome were positive. Quality of life improved significantly for all studies that examined this topic, except for two, where the significance was not specified. The two studies included adolescent asthma patients. Adolescents may struggle with the social-stigma of their condition which affects the adolescent’s mood and self-confidence.(59) Only three of the included studies examined patient’s inhalation technique. All three studies showed positive results whereof one study did not indicate if it was statistically significant. The total result may indicate that educational mobile applications have a positive effect on disease control, therapy adherence, quality of life and inhalation technique in asthma and COPD patients.
The different functions of the educational applications were categorized into seven groups. All studies with a positive significant effect examined educational applications with more than three different functions except for one study that examined an application with two different functions. One application only had an informing function. The study of this application resulted in a non-significant effect on disease control and a significant positive effect on inhalation technique. An educational application with multiple functions seems beneficial for the patient’s disease management. A previous review of Hui et al. indicates the advantage of multiple functions as well.(61)
Almost half of the m-Health applications that record data did not mention any privacy policies concerning patients’ data. A previous study stated that only one third of the available m-Health applications have privacy policies.(35) It is important to ensure patients’ privacy and data safety. Only then, HCPs will feel comfortable to recommend m-Health applications to their patients. The lacking of a clear policy results in ignorance concerning patients’ privacy which in turn causes hesitation among HCPs to recommend and among patients to use m-Health
applications.(31, 34) It emphasizes the need for consistent privacy regulations for m-Health applications.
It must be mentioned that the use of educational applications can be cost and time saving. Several of the included studies noted that the use of a m-Health application caused a decrease in emergency department visits. Other studies opted for a communication function in the application. This function gives patients the possibility to contact the HCP or vice versa. In this way the HCP can monitor the patient more thoroughly and the number of checkup healthcare visits can be reduced. Several of the included studies mentioned the cost saving advantage but also acknowledge the need for cost-efficiency studies. Ryan et al. conducted a cost efficiency study. According to this study, the total healthcare costs were similar for the intervention and control group. Another study noted that cost-efficiency can have conflicting results.(61) This irregularity stresses the need for more cost-efficiency studies for each type of educational application. Each m-Health application has different points of focus and approaches and therefore differs in costs and effects as well.
Patients have a positive attitude towards the m-Health applications according to the studies that assessed patient’s perception. A high degree of satisfaction was captured. This is an important determination as patient’s perception is an important factor for a good therapy adherence.(2)
5.2 A comparison with My Puff
The added value of My Puff could not be determined in contrast to the majority of the studies included in this review. There are several possible causes but the lack of positive attitude towards My Puff was remarkable. Only 25% installed the application whereof 13% actively used the application. My Puff comprises informational and instructional functions. Maybe the lack of individualized information or the lack of the possibility to communicate with the HCP is the cause. As mentioned earlier, multiple functions in an educational application may have beneficial effects.(61) The population is an important factor as well. The My Puff study included asthma and COPD patients. COPD has a late onset and typically consists of elderly patients. Digital skills are often suboptimal among elderly patients.(62) Three of the examined studies included COPD patients as well with mostly positive effects as a result. One study guided the patients in the correct use of the educational application and was next to mobile devices compatible to desktop computers as well. Another study was tablet-based whereby the HCP guided the patient for the correct utilization. The latter study had as inclusion criterion digitally skilled users. All three studies anticipated on patients’ (possible poor) digital skills in contrast to the My Puff study. The My Puff study acknowledged that more efforts are needed to develop successful educational materials that fulfill patients’ preferences.
However, it must be mentioned that the study concerning My Puff demonstrates the efficacy of pharmaceutical care. This emphasizes the fact that all educational applications may have an additional value for patients’ disease management but are not developed to replace the traditional (and already proven effective) healthcare.
5.3 Limitations
This review has several limitations. First of all, the review was conducted by only one researcher. Therefore there was no control by another researcher during study selection, data collection and quality assessment of the selected studies. The study selection was restricted by use of only two databases. It is possible that several relevant studies are therefore not included. This issue was tended to overcome by screening the reference lists of the included studies for other relevant studies. In addition to the number of databases, the chosen language was a limitation as well. Possibly relevant studies missed out because the article was published in another language than English. Although the literature search was conducted in a structured way by entering in- and exclusion criteria, the selected studies varied in design and methods. Due to this difference, it was difficult to compare the selected studies. Therefore this review provides a descriptive overview. Lastly, it must be noted that this review has a very contemporary topic. Technology is developing very fast, and thus likewise for educational applications. Therefore it is possible that several studies were missed during selection because they are not completed yet or are completed, but have not been published yet.
6 Conclusion
The overall effect of m-Health educational applications on asthma and COPD patients’ disease control, therapy adherence, quality of life and inhalation technique seemed positive.
However, several barriers need to be overcome before a full integration in the healthcare system is possible. There is need for more transparency concerning data privacy. Almost half of the m-Health applications that record data did not mention any privacy policies. Both the patients and HCPs are demanding for guaranteed privacy.(31, 34) Mandatory regulations on data privacy may give the necessary consistency. In addition to privacy regulations, cost-efficiency of m-Health applications is unclear. Several included studies mentioned the possible time and cost saving effect of educational applications but acknowledge the need for cost-efficiency studies.
Digital educational training in the use of educational applications seems beneficial for asthma and COPD patients. However patients’ perception and preferences must not be underestimated to integrate these applications in real life practice. Previous research already stressed the importance of patient’s attitude towards therapy.(2) This review had similar findings. Patients had a high degree of satisfaction towards the concerning educational applications and positive outcomes were captured. Whereas the My Puff application did not capture beneficial outcomes and patients had a more negative attitude towards the application. Next to patients’ preference, multiple functions on educational applications seemed beneficial as well.
Educational m-Health applications are very contemporary and quickly evolving. It seems digitalized education is beneficial for patients, but m-Health applications have not found their place in the healthcare system yet. Therefore close collaboration with all stakeholders during development and follow-up and monitoring of their potential effects are needed. Only then, mHealth evolution and barriers can be identified and consequently researchers and app -developers can anticipate. In this way, optimized and individualized m-Health applications that fulfill all patients’ and HCPs’ requirements can be developed.
m-Health educational applications seem a promising addition to traditional healthcare to support patient’s disease knowledge and management. However, future research investigating their use in real life practice and cost-efficiency should show their merit.