RIVM letter report 2020-0168 M. Knol et al.
RIVM letter report 2020-0168
Page 2 of 22
Colophon
© RIVM 2020Parts of this publication may be reproduced, provided acknowledgement is given to the: National Institute for Public Health and the Environment, and the title and year of publication are cited.
DOI 10.21945/RIVM-2020-0168 M. Knol (author), RIVM
H. de Melker (author), RIVM L. Sanders (author), RIVM
N. van Sorge (author), Netherlands Reference Laboratory for Bacterial Meningitis, Amsterdam UMC
Contact: M.J. Knol
Center Epidemiology and Surveillance of Infectious Diseases
mirjam.knol@rivm.nl
This investigation has been performed by order and for the account of the Ministry of Health, Welfare and Sport and the Dutch Health Council, within the framework of V/151103/20/RV, Surveillance of the National Immunization Program.
Published by:
National Institute for Public Health and the Environment, RIVM
P.O. box 1 | 3720 BA Bilthoven The Netherlands
Synopsis
Pneumococcal vaccination of the elderly Information for the Dutch Health Council
Pneumococcus is the main cause of severe infections such as pneumonia, septicaemia and meningitis. These infections are most common in older people with less resistance and in children under five years of age. In the elderly, the bacterium mainly causes pneumonia. In the Netherlands, about 10,000 patients a year are hospitalized with a pneumococcal infection. The elderly in particular have a higher risk (10-20 percent) of dying from this infection.
In February 2018, the Dutch Health Council recommended that people aged 60-80 years should be offered a pneumococcal vaccination (PPV23) every five years. The intention was that all people aged 60, 65, 70 or 75 would be invited to this in the fall of 2020.
Due to the COVID-19 pandemic, the Health Council advised in April 2020 to first offer the vaccination to the oldest age groups (70-79 year olds). They have a higher risk of severe pneumococcal infections as well as a severe course of COVID-19. Since there are insufficient vaccines to vaccinate all people aged 70 to 79 in 2020, people aged 73 to 79 will be invited first in the autumn.
In December 2020, the Health Council will advise which age groups are eligible for pneumococcal vaccination in 2021. In support of this advice, RIVM has now collected the available and relevant scientific information. This includes the number of people in the Netherlands who fall ill with pneumococci just before and during the COVID-19 pandemic. The number of patients with pneumococcal disease has been up to 80 percent lower since April 2020 than in previous years. This is probably because pneumococci spread less easily due to the measures against COVID-19. The report also contains information about new
pneumococcal vaccines that are currently under development.
Keywords: pneumococcus, pneumococcal disease, vaccination, burden of disease
RIVM letter report 2020-0168
Publiekssamenvatting
Pneumokokkenvaccinatie bij ouderen Informatie voor de Gezondheidsraad
De pneumokok is de belangrijkste veroorzaker van ernstige infecties als longontsteking, bloedvergiftiging en hersenvliesontsteking. Deze
infecties komen het meest voor bij ouderen met minder weerstand en bij kinderen onder de vijf jaar. Bij ouderen veroorzaakt de bacterie vooral longontsteking. In Nederland worden ongeveer 10.000 patiënten per jaar in het ziekenhuis opgenomen vanwege een
pneumokokkeninfectie. Vooral ouderen hebben een groter risico (10 tot 20 procent) om hieraan te overlijden.
In februari 2018 heeft de Gezondheidsraad geadviseerd om mensen van 60-80 jaar elke vijf jaar een pneumokokkenvaccinatie (PPV23) aan te bieden. Het was de bedoeling dat hiervoor alle mensen van 60, 65, 70 of 75 jaar in de herfst van 2020 zouden worden uitgenodigd.
Vanwege de COVID-19-pandemie heeft de Gezondheidsraad in april 2020 geadviseerd om de vaccinatie eerst aan te bieden aan de oudste leeftijdsgroepen (70-79-jarigen). Zij hebben zowel een grotere kans op ernstige pneumokokkeninfecties als op een ernstig verloop van COVID-19. Aangezien er onvoldoende vaccins zijn om alle mensen van 70 tot 79 jaar in 2020 te vaccineren, worden in het najaar eerst mensen van 73 tot 79 jaar uitgenodigd.
In december 2020 zal de Gezondheidsraad adviseren welke leeftijdsgroepen in 2021 voor de pneumokokkenvaccinatie in
aanmerking komen. Als ondersteuning van dat advies heeft het RIVM nu de beschikbare en relevante wetenschappelijke informatie
bijeengebracht.
Het gaat onder meer om het aantal mensen dat in Nederland ziek wordt van pneumokokken vlak voor en tijdens de COVID-19 pandemie. Het aantal patiënten met pneumokokkenziekte is sinds april 2020 tot 80 procent lager dan voorgaande jaren. Dit komt waarschijnlijk doordat pneumokokken minder worden verspreid door de maatregelen tegen COVID-19. Ook staat er informatie in het rapport over nieuwe
pneumokokkenvaccins die momenteel ontwikkeld worden.
RIVM letter report 2020-0168
Contents
1 Background — 9
2 Pneumococcal disease — 11
3 Burden of disease — 13
3.1 Invasive pneumococcal disease — 13 Incidence — 13
Serotype distribution — 14
Impact COVID-19 measures — 15 3.2 Pneumococcal pneumonia — 17
3.3 COVID-19 and pneumococcal disease — 17
4 New pneumococcal vaccines — 19
4.1 15-valent PCV — 19 4.2 20-valent PCV — 19
RIVM letter report 2020-0168
1
Background
In February 2018, the Dutch Health Council advised to offer pneumococcal vaccination with the 23-valent pneumococcal polysaccharide vaccine (PPV23) to people of 60 years and older to reduce the burden of pneumococcal disease among elderly (1). The 23-valent polysaccharide vaccine, covering 23 common disease-causing pneumococcal serotypes, requires repeated vaccination with 5-year intervals to maintain vaccine effectiveness. The advice was adopted by the Minister of Health. As of autumn 2020, all persons aged 60, 65, 70 and 75 years were to be offered PPV23 immunization, in order to cover all 60-79 year-olds over a period of 5 years. A revaccination with PPV 23 was to be offered every 5 years until age 80 years.
However, in February 2020, the first COVID-19 case was reported in the Netherlands. At the time, it was unclear how COVID-19 would impact the incidence of pneumococcal disease. A secondary pneumococcal infection might occur after a SARS-CoV-2 infection, as frequently observed following influenza (2). In addition, it had become clear that elderly people are at high risk of severe COVID-19 resulting in
hospitalization and intensive care admission. To reduce the burden of severe infectious diseases in the elderly, the importance of both
influenza and pneumococcal vaccination during the COVID-19 pandemic was stressed by several institutions (3-5). In April 2020, the Dutch Health Council advised to prioritize pneumococcal vaccination in the oldest age group, i.e. 70-79 years, as they are the most vulnerable to severe pneumococcal disease as well as severe COVID-19 (6). Due to limited vaccine availability, all 73-79 year olds are invited by their general practitioner to receive PPV23 vaccination together with the seasonal influenza vaccination in autumn 2020.
The Dutch Health Council will come with a new advice on the
implementation of PPV23 vaccination in elderly for 2021, based on the data currently available. In this report we provide information on the burden of pneumococcal disease, just before and during the COVID-19 pandemic. Information on the serotype distribution related to vaccine coverage is provided, also with respect to new pneumococcal conjugate vaccines.
RIVM letter report 2020-0168
2
Pneumococcal disease
The bacterium Streptococcus pneumoniae (pneumococcus) is a gram-positive diplococcus and is a commensal of the upper respiratory tract in humans. Based on the structure of the capsule, almost 100 different serotypes of the pneumococcus are discerned. Children under 5 years of age are the main reservoir of pneumococcus and primarily responsible for the transmission of pneumococcus in the population. They have the highest pneumococcal carriage rate at high carriage density, because of their immature immune system. Adults and elderly individuals are believed to contribute little to pneumococcal transmission due to their lower pneumococcal carriage rate and lower carriage density.
Pneumococcal carriage may lead to disease, which can either be non-invasive, e.g. otitis media, sinusitis or pneumonia, or non-invasive, e.g. septicaemia, meningitis and invasive pneumonia (in which case the pneumococcus is detected in the bloodstream). Pneumococcal disease is most prevalent in children below 2 years of age and in adults over 50-60 years of age. Non-invasive disease is far more common than invasive disease. In case of invasive pneumococcal disease (IPD), this mostly concerns invasive pneumonia in elderly, whereas in children septicaemia or meningitis are more common. Apart from children and older adults, a higher prevalence of pneumococcal disease is seen in persons with a primary or secondary immunodeficiency or other underlying conditions. In 2017-2019, the incidence of IPD was 15 per 100,000 per year, equal to ~2500 patients per year of which ~1800 were 60 years of age or older (7). In case of IPD, nearly all patients are hospitalized. The incidence of hospitalization for non-invasive all-cause pneumonia is estimated to be 10 times higher; in 2012-2014 this incidence was calculated to be 188 per 100,000 per year, adding up to 31,000
hospitalization per year, of which two-third concerned persons 60 years or older (n=22,000) (8). The fraction of non-invasive all-cause
pneumonia that can be attributed to the pneumococcus is estimated to be 20-30%, which means that around 7500 patients per year are admitted with non-invasive pneumococcal pneumonia, of which around 5500 persons of 60 years or older. The mortality of IPD and non-invasive pneumococcal pneumonia is 10-20% and also long-term mortality seems significantly higher in persons who have had pneumococcal disease.
RIVM letter report 2020-0168
3
Burden of disease
3.1 Invasive pneumococcal disease
3.1.1 Incidence
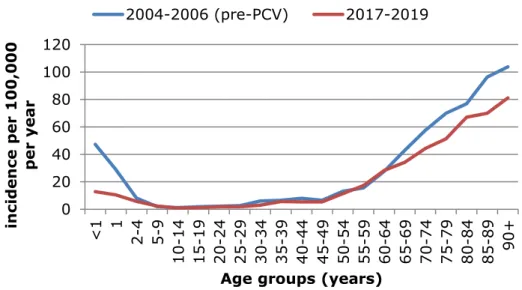
In epidemiological years (June to May) 2017-2018 and 2018-2019, the incidence of IPD in the Netherlands was 15 per 100,000 population per year, constituting 2580 patients per year. The incidence varies by age group with a relatively high incidence in children <2 years (Figure 1). The incidence increases strongly with increasing age from the age of 50 years. The absolute number of cases is highest between 60 and 80 years with 1300 cases (Figure 2). These data are very comparable with the data used for the 2018 advice of the Health Council on
pneumococcal vaccination for elderly (1, 8).
Figure 1 Incidence of invasive pneumococcal disease per 100,000 population per year by age group in epidemiological years 2004-2005 and 2005-2006 (before implementation of 7-valent pneumococcal conjugate vaccination (PCV7) in children) and epidemiological years 2017-2018 and 2018-2019
0 20 40 60 80 100 120 <1 1 2-4 5-9 10 -14 15 -19 20 -24 25 -29 30 -34 35 -39 40-44 45-49 50-54 55-59 60-64 65-69 70-74 75-79 80-84 85-89 90+ in ci de n ce pe r 100,000 p er y ea r
Age groups (years) 2004-2006 (pre-PCV) 2017-2019
RIVM letter report 2020-0168
Page 14 of 22
Figure 2 Absolute number of cases of invasive pneumococcal disease per year by age group in epidemiological years 2004-2005 and 2005-2006 (before
implementation of 7-valent pneumococcal conjugate vaccination (PCV7) in children) and epidemiological years 2017-2018 and 2018-2019
3.1.2 Serotype distribution
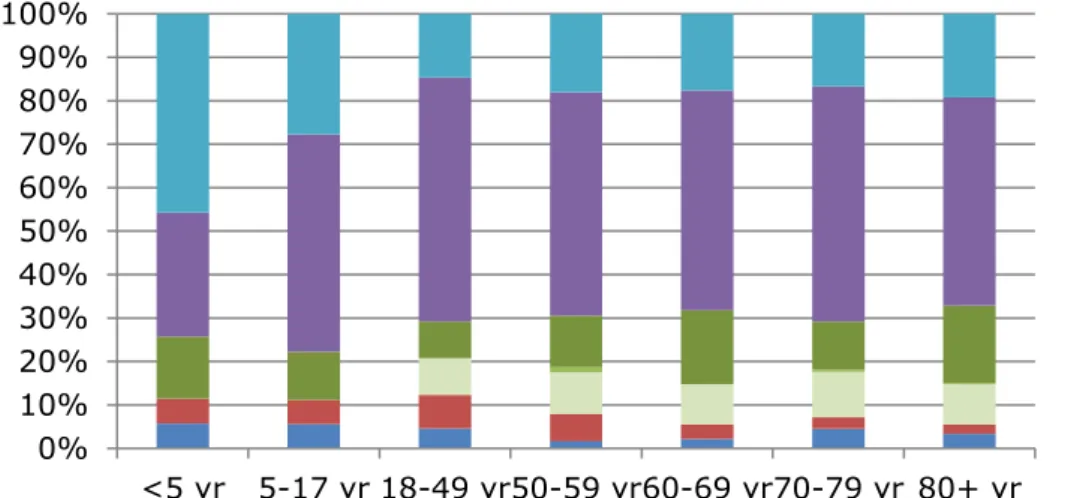
The proportion of IPD caused by serotypes covered by PPV23 is currently around 80% in adults (Figure 3). This is quite similar to the proportion at the time of the 2018 Health Council advice (1). The proportion of IPD caused by serotypes included in the 10-valent pneumococcal conjugate vaccine (PCV10; the vaccine currently in the National Immunization Program for children) further decreased over the last years from 12% in 2015-2017 to 6% in 2017-2019 in persons 50 years or older due to herd effects (Figure 3). The coverage of PCV13 (PCV10 plus serotypes 3, 19A and 6A) amounts to 30% in adults (Figure 3).
There are two new pneumococcal conjugate vaccines which are
expected to be registered in the next few years, a 15-valent vaccine of MSD and a 20-valent vaccine of Pfizer (Table 1). In conjugate vaccines, the pneumococcal capsule polysaccharides are conjugated to a carrier protein, and therefore pneumococcal conjugate vaccines (are expected to) have a longer duration of protection than pneumococcal
polysaccharide vaccines (data on PCV13 in (9, 10).
The two additional serotypes (22F and 33F) included in PCV15 (compared with PCV13) cause 11% of IPD in elderly, and the total coverage of PCV15 sums up to 42% in elderly (Figure 4). The five additional serotypes included in PCV20 (compared with PCV15, i.e. serotypes 8, 10A, 11A, 12F, 15B) cause 32% of IPD in elderly, summing up to a total coverage of PCV20 of 74% in elderly (Figure 4).
0 50 100 150 200 250 300 350 400 <1 1 2-4 5-9 10-14 15-19 20-24 25-29 30-34 35-39 40-44 45-49 50-54 55-59 60-64 65-69 70-74 75-79 80-84 85-89 90+ N u m b er o f ca se s p er ye ar
Age group (years) 2004-2006 (pre-PCV) 2017-2019
Figure 3 Serotype distribution of invasive pneumococcal disease by age group in epidemiological years 2017-2018 and 2018-2019 for registered vaccines
Table 1 Overview of new pneumococcal conjugate vaccines
Vaccine Included serotypes
PCV15 PCV13 serotypes + 22F and 33F
PCV20 PCV13 serotypes + 8, 10A, 11A, 12F, 15B, 22F, 33F
Figure 4 Serotype distribution of invasive pneumococcal disease by age group in epidemiological years 2017-2018 and 2018-2019 for registered (PCV10 and PCV13) and expected conjugate vaccines (PCV15 and PCV20)
3.1.3 Impact COVID-19 measures
The number of IPD cases suddenly dropped by 80% in April to June 2020 compared with the 5-year moving average (Figure 5). This
0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100%
<5 yr 5-17 yr 18-49 yr50-59 yr60-69 yr70-79 yr 80+ yr
non-PCV20 PCV20 extra PCV15 extra PCV13 extra PCV10 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100%
<5 yr 5-17 yr 18-49 yr50-59 yr60-69 yr70-79 yr 80+ yr
non-PPV23 PPV23 extra PCV13 extra - 19A PCV13 extra - 6A PCV13 extra - 3 PCV10 extra PCV7
RIVM letter report 2020-0168
Page 16 of 22
adults 18-59 years (Figure 6). This could be due to better adherence to the COVID-19 measures by the elderly compared with other age groups.
Figure 5 Number of cases of invasive pneumococcal disease by month in 2020 compared with the 5-year moving average and a threshold value
Number of cases is based on sentinel laboratory surveillance covering ~25% of the Dutch population.
Figure 6 Number of cases of invasive pneumococcal disease by month in 2020 compared with the 5-year moving average and a threshold value by age group
Age group 5-17 years is not included because of very low number of cases. Number of cases is based on sentinel laboratory surveillance covering ~25% of the Dutch population; for age group <5 years, data from all laboratories in the Netherlands is used.
0 20 40 60 80 100 120
Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec
N u m b er o f I P D c ase s (s en ti n el l ab s)
2020 5-year moving average threshold (+2sd)
0 2 4 6 8 10 12 14
Jan Feb Mar AprMay Jun Jul AugSep Oct Nov Dec
N u m b e r o f ca se s (a ll la b s) <5 yrs 0 5 10 15 20 25 30 35
Jan Feb Mar Apr May Jun Jul AugSep Oct Nov Dec
N u m b e r o f ca se s (s e n tin e l la b s) 18-59 yrs 2020
5-year moving average threshold (+2sd) 0 10 20 30 40 50 60 70
Jan Feb Mar AprMay Jun Jul AugSep Oct Nov Dec
N u m b e r o f ca se s (s e n tin e l la b s) 60-79 yrs 0 5 10 15 20 25 30
Jan Feb Mar Apr May Jun Jul AugSep Oct Nov Dec
N u m b e r o f ca se s (s e n tin e l la b s) 80+ yrs
3.2 Pneumococcal pneumonia
Previously, we described that the estimated number of hospitalizations for pneumococcal pneumonia is 7500 per year, of which 5500 in elderly people (8). As IPD remained relatively stable over time in the last years, we assume that pneumococcal pneumonia also remained relatively stable over time. We do not have surveillance data available of 2020 to assess the impact of the COVID-19 pandemic on (pneumococcal) pneumonia, but it is likely that similar trends will have occurred as for IPD with large reductions in pneumococcal pneumonia cases because of the COVID-19 measures, as was also seen for several other (vaccine-preventable) infectious diseases (11).
3.3 COVID-19 and pneumococcal disease
The Health Council concluded in their advice in April 2020 based on Dutch data and international literature that coinfections of SARS-CoV-2 and Streptococcus pneumoniae may occur but that coinfections do not seem to be a large problem (6). Two recent studies from the United Kingdom (12, 13) and a rapid review (14) confirmed this. Adler et al. described that five of 137 (4%) hospitalized patients with COVID-19 who were tested for presence of other pathogens were found to have pneumococcal coinfection (12). Hughes et al. investigated blood cultures of 643 of 836 hospitalized COVID-19 patients and no pneumococcal infection was detected (13). Both studies concluded that there is a low rate of bacterial coinfection in hospitalized COVID-19 patients. By contrast, in influenza infection the prevalence of bacterial coinfection in hospitalized patients can exceed 30% (2). Also the Dutch Working Party on Antibiotic Policy (SWAB) describes that the risk of bacterial
pneumonia in patients with COVID-19 seems low and suggests restrictive use of antibacterial drugs in patients with COVID-19, especially in patients with mild to moderate disease severity (15). Dutch IPD surveillance data also show no indication of an increased risk of pneumococcal infection in COVID-19 patients as the IPD incidence decreased rather than increased during the COVID-19 pandemic (see section 3.1.3).
RIVM letter report 2020-0168
4
New pneumococcal vaccines
There are two new pneumococcal conjugate vaccines, a 15-valent and a 20-valent vaccine, which are expected to be registered in the next few years. We discuss these vaccines below. There are other higher-valent conjugate vaccines in an earlier stage of development and therefore we do not discuss those in this report. In addition to PCVs, several other vaccine concepts are currently being tested in clinical development programmes. These types of vaccines may induce broader, non-serotype-specific, protection against pneumococcal disease.
4.1 15-valent PCV
MSD is developing a 15-valent pneumococcal conjugate vaccine (V114) including serotypes 22F and 23F in addition to the serotypes included in PCV13. The vaccine is currently being tested in Phase 3 clinical trials in different populations who are at increased risk for pneumococcal disease including healthy older adults and healthy children, as well as people who are immunocompromised or have certain chronic conditions. The pivotal PNEU-AGE (V114-019) study in healthy adults 50 years of age or older demonstrated that V114 is non-inferior to the currently available 13-valent pneumococcal conjugate vaccine (PCV13) for the 13 serotypes targeted by both vaccines and superior for serotypes 22F and 33F, the two serotypes targeted by V114 but not PCV13 (16).
4.2 20-valent PCV
Pfizer is developing a 20-valent pneumococcal conjugate vaccine (20vPnC) that is under investigation for the prevention of invasive disease and pneumonia in adults aged 18 years and older. 20vPnC includes the 13 serotypes contained in PCV13 plus 7 additional serotypes (8, 10A, 11A, 12F, 15BC, 22F and 33F).
One study (NCT03760146) evaluated the safety and immunogenicity of 20vPnC compared with PCV13 and PPV23 in 3,880 adults aged 18 years and older who were not previously vaccinated against pneumococcal disease (17). This study showed non-inferiority at one month after vaccination for all serotypes in common with PCV13 and for 6 of the 7 additional serotypes when compared to the PPV23 in adults of 60 years and older; 1 of the new 7 serotypes missed non-inferiority criteria by a small margin.
RIVM letter report 2020-0168
5
References
1. Gezondheidsraad. Vaccinatie van ouderen tegen pneumokokken. Den Haag: Gezondheidsraad; 2018 28 februari 2018.
2. Chertow DS, Memoli MJ. Bacterial coinfection in influenza: a grand rounds review. Jama. 2013;309(3):275-82.
3. ECDC. Poster influenza during covid-19 pandemic why its important get vaccinated 2020 [Available from:
https://www.ecdc.europa.eu/en/publications-data/poster- influenza-during-covid-19-pandemic-why-its-important-get-vaccinated.
4. CDC. Additional considerations for influenza vaccination 2020 [Available from: https://www.cdc.gov/vaccines/pandemic-guidance/index.html.
5. Thindwa D, Garcia Quesada M, Liu Y, Bennett J, Cohen C, Knoll MD, et al. Use of seasonal influenza and pneumococcal
polysaccharide vaccines in older adults to reduce COVID-19 mortality. Vaccine. 2020;38(34):5398-401.
6. Gezondheidsraad. COVID-19 en vaccinatie tegen pneumokokken. Den Haag: Gezondheidsraad; 2020 20 april 2020.
7. The National Immunisation Programme in the Netherlands - Surveillance and developments in 2018-2019 (RIVM report 2019-0193). Bilthoven; 2019.
8. Knol MJ, Sanders EAM, De Melker HE. Pneumokokkenziekte in Nederland - Achtergronddocument voor de Gezondheidsraad (RIVM rapport 2017-0181). Bilthoven; 2017.
9. Patterson S, Webber C, Patton M, Drews W, Huijts SM, Bolkenbaas M, et al. A post hoc assessment of duration of protection in CAPiTA (Community Acquired Pneumonia immunization Trial in Adults). Trials in Vaccinology. 2016;5:92-6.
10. Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. The New England journal of medicine. 2015;372(12):1114-25.
11. Schurink-van 't Klooster TM, De Melker HE. The National
Immunisation Programme in the Netherlands - Surveillance and developments in 2019-2020 (RIVM report 2020-0077). Bilthoven: National Institute for Public Health and the Environment (RIVM); 2020.
12. Adler H, Ball R, Fisher M, Mortimer K, Vardhan MS. Low rate of bacterial co-infection in patients with COVID-19. Lancet Microbe. 2020;1(2):e62.
RIVM letter report 2020-0168
Page 22 of 22
antimicrobial prescribing. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2020. 15. Sieswerda E, De Boer MGJ, Bonten MMJ, Boersma WG, Jonkers RE,
Aleva RM, et al. The Dutch Working Party on Antibiotic Policy (SWAB) recommendations for antibacterial therapy in adults with COVID-19. 2020.
16. Merck Announces Positive Topline Results from Two Phase 3 Adult Studies Evaluating V114, Merck’s Investigational 15-valent
Pneumococcal Conjugate Vaccine, Including Pivotal Trial [press release]. 2020.
17. Pfizer Announces Top-Line Results from Phase 3 Study of 20-Valent Pneumococcal Conjugate Vaccine in Pneumococcal Vaccine-Naïve Adults Aged 18 Years or Older [press release]. 2020.