Anti-adalimumab
antibodies
in
idiopathic
uveitis
and
juvenile
idiopathic arthritis
Marie Vanassche
Student number: 01510067Supervisors: Prof. Dr. Carolien Bonroy, Prof. Dr. Joke Dehoorne
A dissertation submitted to Ghent University in partial fulfilment of the requirements for the degree of Master of Medicine in Medicine
Deze pagina is niet beschikbaar omdat ze persoonsgegevens bevat.
Universiteitsbibliotheek Gent, 2021.
This page is not available because it contains personal information.
Ghent University, Library, 2021.
4
P
REFACE
Writhing this thesis has been a true learning experience in getting to know the subject in depth as well as becoming acquainted with scientific research. Along the way, there were some people who supported me and helped me get through it all.
Firstly I would like to thank all of my friends who were there for me these past two years. My friends at university, who always give great advise and also help me remembering my deadlines. Also my friends who have accompanied me to the library when I was writing this thesis and made the necessary breaks more relaxing.
Secondly my parents, who have been a big support for me during my whole education. They help relativizing a lot of things and ease my nerves when necessary. Also thank you to Martine De Cock for proofreading my thesis and helping with the final touches.
Lastly I would like to thank my supervisors prof. Dehoorne and prof. Bonroy. They have been an incredible help these last years. Thank you for always answering my many questions, giving great feedback and extensively consulting during our meetings. You have truly taken your time and patience with me.
6
T
ABLE OF CONTENTS
Preface ... 4 Table of contents ... 6 Abstract ... 8 Abbreviations ... 10 1 Introduction ... 11 1.1 Uveitis ... 11 1.1.1 Classification ... 11 1.1.2 Etiology ... 15 1.1.3 Clinical features ... 16 1.1.4 Complications ... 171.1.5 Role of ophtalmologist and pediatrician ... 17
1.2 Juvenile idiopathic arthritis ... 18
1.2.1 Subtypes ... 18
1.2.2 Prognosis and outcome ... 19
1.2.3 Relation to uveitis ... 20
1.3 Treatment ... 23
1.3.1 Treatment of uveitis ... 23
1.3.2 Treatment of JIA ... 25
1.3.3 Anti-drug antibodies ... 26
1.4 Therapeutic drug monitoring ... 27
1.5 Objectives ... 28
2 Materials and Methods ... 29
2.1 Study design and patient recruitment ... 29
2.2 Database design and variables ... 29
2.2.1 Demographical, clinical and therapeutic data ... 29
2.2.2 ADA and AAA ... 29
2.3 Statistical analysis ... 29
7
3.1 Baseline characteristics ... 31
3.2 ADA drug levels in relation to disease activity, concomitant medication and dose .. 32
3.3 AAA titers in relation to age at disease onset, disease activity, dose, concomitant medication and treatment duration ... 35
3.4 Relation between AAA titers and ADA drug levels ... 39
4 Discussion ... 41
4.1 AAA and disease activity ... 41
4.2 AAA titers, ADA drug levels, dose and duration of therapy ... 41
4.3 Concomitant MTX, AAA and ADA ... 42
4.4 ADA drug levels, ADA dose and disease activity ... 42
4.5 Age of onset of disease and presence of ANA in relation to AAA ... 43
5 Conclusion ... 44
8
A
BSTRACT
Background: Adalimumab (ADA) has proven to be an effective treatment in pediatric
idiopathic uveitis, juvenile idiopathic arthritis (JIA) without uveitis, and JIA-associated uveitis (JIA-U). When treated with ADA, some patients form anti-adalimumab antibodies (AAA). This phenomenon has been associated with treatment failure. Therapeutic drug monitoring (TDM) based on ADA serum levels and AAA titer measurements can be a helpful tool in understanding the process of treatment failure.
Objectives: The aim of this study is to investigate the relation between AAA formation and the
concomitant use of methotrexate (MTX), ADA dose, ADA serum levels, age at onset of disease, ANA positivity before treatment, disease activity and treatment duration. We also aim to evaluate the association between the ADA drug levels and the disease activity, the concomitant use of MTX and high ADA doses.
Methods: Consecutive patients (n=34) treated with ADA were recruited from the Pediatric
Rheumatology Unit of the Prinses Elisabeth Childrens Hospital, Ghent University Hospital between February 2018 to August 2019. Data on routinely detected ADA drug levels and AAA titers were analyzed. The data were statistically analysed with IBM SPSS Statistics (version 26).
Results: No significant difference between the ADA drug levels was measured at the first
sample collection for patients in remission versus those with active disease (p= 0.925). The ADA drug levels were higher in the patients who received concomitant MTX. (p=0.180) and there was a positive correlation between ADA dose and ADA drug levels (p=0.002).
AAAs were found in 18% of the patients (n=6) in at least one of the time points. The median time of AAA detection in our population was 26.8 months [interquartile range (IQR) 7,8 – 49,25 months]. Patients with AAA formation were younger (p=0.557), had a higher dosing regimen of ADA (µg/week/kg) (p=0,416), and were more likely to have active disease (p=0,382). Less AAA formation was noticed in case of concomitant use of MTX during ADA treatment (p= 0,328). Patients who were longer on biological therapy had a higher chance of AAA development (p=0,832), but there was no relation to the AAA titer level (p=0,798). AAA formation was higher in those patients who had higher ANA titer intensity scores (>2+) (p=0,008).
Conclusion: Different risk factors for AAA formation and reduced ADA drug levels could be
9 determined as well. TDM might help the clinician in correctly dosing ADA and also help understand the process of treatment failure in individual patients.
10
A
BBREVIATIONS
AAA : anti-adalimumab antibodies ADA : adalimumab
AHA : anti-histone antibodies AIA : anti-infliximab antibodies ANA : anti-nuclear antibodies
ILAR : International League of Associations for Rheumatology IU : infectious uveitis
JIA : juvenile idiopathic arthritis
JIA-U : juvenile idiopathic arthritis associated uveitis LOR : loss of response
MTX : methotrexate
NIU : non-infectious uveitis
NSAIDs : non-steroidal anti-inflammatory drugs PGA : patient global assessment
RF : rheumatoid factor
11
1 I
NTRODUCTION
1.1 U
VEITISUveitis is defined as an ocular inflammation of the uveal tract, which comprises the iris, ciliary body and the choroid. The causes of inflammation may be infectious, non-infectious, or idiopathic (see etiology). with either an exogenous or an endogenous source (1), meaning that inflammation can be caused by both inflammatory as well as non-inflammatory processes in the eye (2).
Pediatric uveitis occurs only in aproximately 5-10% of the uveitis population (3). The total incidence of uveitis in children and adults is 52 cases/100,000 and the mean prevalence is 145 cases/100,000 (4).
1.1.1 Classification
For the classification of uveitis, the criteria of the standardization of uveitis nomenclature (SUN) are used that was based on the anatomical localization of the uveitis (see Figure 1 and Table 1), as composed by the SUN working group. This SUN working group also clarified the descriptors of uveitis in terms of onset, duration and course. Further, it made a grading scheme for anterior chamber cells and anterior chamber flare and the terminology for uveitis activity was standardized as well (5, 6).
1.1.1.1 Anatomical Classification
Anterior uveitis
Anterior uveitis is the most common type of uveitis (3), in which the main site of inflammation is the anterior chamber and is, thus, predominantly anterior to the lens. Subtypes of anterior uveitis are iritis, iridocyclitis (when the ciliary body is involved) and anterior cyclitis (5, 7). This type of uveitis is mostly mediated by T-lymphocytes and macrophages. When the blood-ocular barrier is affected, protein and cells will enter the aqueous humour (8).
Intermediate uveitis
In the intermediate type of uveitis the main inflammation location is the vitreous humour (7). The subtypes of intermediate uveitis include pars planitis, posterior cyclitis and hyalitis (5). Intermediate uveitis is most common in young adults and is usually bilateral, but asymmetric and it is most often associated with T-cell infiltration of the vitreous or pars plana (8).
12 In case of posterior uveitis the back of the eye is the most inflamed. The subtypes of posterior uveitis are focal, multifocal or diffuse choroiditis, chorioretinitis, retinochoroiditis, retinitis and neuroretinitis (5). CD4+ T-cells and macrophages have been shown to be generally present in acute phases. Molecular mimicry is speculated to be on the basis of these auto-immune responses (8).
Panuveitis
Panuveitis designates ocular inflammation that occurs over the entirety of the entire eye including the anterior chamber, the vitreous humor and the retina or the choroid (7).
13
Table 1. The SUN* Working Group Anatomic Classification of Uveitis (adapted from the International Uveitis Study Group anatomic classification in reference 1)
Type Primary site of
inflammation°
includes
Anterior Uveitis
Figure 1: uveitis anatomy (9)
Anterior chamber Iritis Iridocyclitis Anterior cyclitis Intermediate uveitis
Figure 2: uveitis anatomy (9)
Vitreous Pars planitis Posterior cyclitis Hyalitis
Posterior uveitis
Figure 3: uveitis anatomy (9)
Retina or choroid Focal, multifocal, or diffuse choroiditis Chorioretinits Retinochoroiditis Retinitis Neuroretinitis
14 Panuveitis
Figure 4: uveitis anatomy (9)
Anterior chamber, vitreous, and retina or choroid
*SUN = Standardization of uveitis nomenclature °As determined clinically
1.1.1.2 Disease course
Besides the anatomical localization, the disease course, i.e. the disease onset and duration, is also used to describe the uveitis. The onset of uveitis can be considered as sudden or insidious and the duration can be categorized as limited (less than 3 months) or persistent (more than 3 months). Lastly the course of uveitis, can be defined as acute (an episode with sudden onset and limited duration), recurrent (different episodes separated by periods of inactivity without treatment for more than 3 months) or chronic (persistent with periods of inactivity that last no more than 3 months after discontinuing treatment) (5).
1.1.1.3 Disease activity
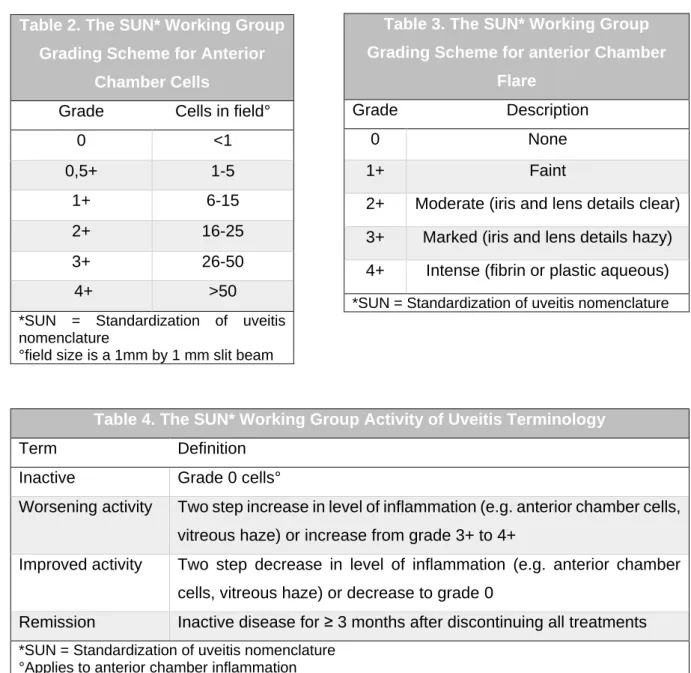
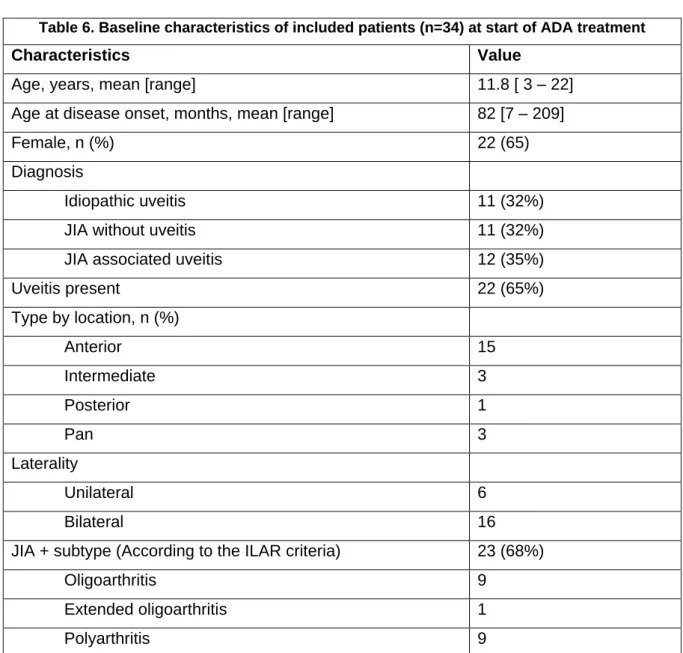
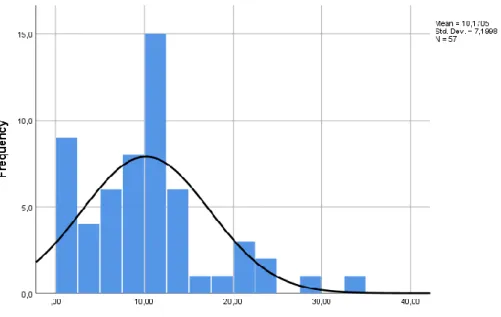
In the follow up of uveitis, standard methods are used for the evaluation of the disease activity. The SUN working group developed standardized grading schemes for the anterior chamber cells (table 2) and anterior chamber flare (table 3). The same group also developed a terminology about the evolution of uveitis activity (table 4) (5).
15
1.1.2 Etiology
The diagnosis of uveitis is syndromic meaning that many different diseases can cause similar clinical features and eye manifestations. A distinction is made between Infectious uveitis (IU) and Non-infectious uveitis (NIU).
IU is present in approximately 22% of the pediatric patients. The most common causes of IU are viral, such as the herpesvirus and the varicella zoster virus that usually cause acute and unilateral uveitis or cytomegalovirus, which is an important source of uveitis in immunocompromised children (10). Other common causes include tuberculosis (mostly in developing countries) and parasitic infections (e.g. toxoplasmosis, cysticerosis and toxocara) (2, 10).
NIU is either idiopathic, meaning that no cause can be identified, associated with a systemic disease or associated with an ocular syndrome. Uveitis associated with systemic diseases are most common, found in approximately 35% of the patients, of which juvenile idiopathic arthritis (JIA) occurs the most frequently, whereas other causes include sarcoidosis and Beçhet’s, but
Table 4. The SUN* Working Group Activity of Uveitis Terminology
Term Definition
Inactive Grade 0 cells°
Worsening activity Two step increase in level of inflammation (e.g. anterior chamber cells, vitreous haze) or increase from grade 3+ to 4+
Improved activity Two step decrease in level of inflammation (e.g. anterior chamber cells, vitreous haze) or decrease to grade 0
Remission Inactive disease for ≥ 3 months after discontinuing all treatments *SUN = Standardization of uveitis nomenclature
°Applies to anterior chamber inflammation
Table 3. The SUN* Working Group Grading Scheme for anterior Chamber
Flare
Grade Description
0 None
1+ Faint
2+ Moderate (iris and lens details clear) 3+ Marked (iris and lens details hazy) 4+ Intense (fibrin or plastic aqueous) *SUN = Standardization of uveitis nomenclature Table 2. The SUN* Working Group
Grading Scheme for Anterior Chamber Cells
Grade Cells in field°
0 <1 0,5+ 1-5 1+ 6-15 2+ 16-25 3+ 26-50 4+ >50
*SUN = Standardization of uveitis nomenclature
16 also several others. NIU associated with the ocular syndromes (e.g. sarcoidosis and TINU syndrome) occur in approximately 20% of the patients and idiopathic uveitis in approximately 23% of the cases (4).
The anatomical localization and the different etiologies of uveitis are strongly correlated. Anterior uveitis is clearly associated with the alloantigen HLA-B27 (11) and is linked to arthritis (3). Furthermore, the most common form of non-infectious anterior uveitis is idiopathic and usually affects young adults, but less frequenty sarcoidosis, Beçhet’s disease and the Fuchs’ uveitis syndrome, whereas the common infectious causes of anterior uveitis are the herpes viruses and tuberculosis and those of intermediate uveitis are often idiopathic and on a presumed immunological base. Non-infectious causes of posterior uveitis include multiple sclerosis, sarcoidosis, Bechet’s disease and primary retinal vasculitis whereas infectious causes can include bacterial, viral or fungal causes (8).
1.1.3 Clinical features
The clinical features of uveitis depend on the anatomical localization and the course of the inflammation (10).
The most typical symptoms of acute anterior uveitis are an aching pain and photophobia. Other symptoms are redness, decreased vision and epiphora. On the contrary, chronic anterior uveitis present more muted symptoms and can result in an apparently quiet eye with minimal signs that may not be proportional to the intraocular inflammation (1). Upon further examination, the eye looks red (often ciliary flush) and the cornea has a hazy stroma (1, 8). These symptoms are most obvious in acute anterior uveitis whereas in chronic anterior uveitis they are more subtle. Further, keratic precipates, aqueous cells, aqueous flare, iris nodules, iris atrophy, iris neovascularization, posterior synechiae and anterior vitreous cells are observed (1).
Intermediate uveitis is usually chronic, resulting in less outspoken symptoms. The patient does not experience pain, but has complaints of blurred vision and floaters. On examination, a typical lack of ciliary flush or severe signs of anterior inflammation are visible (1, 8). In addition, vitreous haze, snow banking, snow balls, retinal vascular cuffing, macular edema, optic disc swelling (in severe cases) and neovascularization may also be present (8).
Symptoms of posterior uveitis may either be acute or chronic. The patient usually has no pain. The primary symptoms include floaters and decreased vision that can occur simultaneously or separately based on the anatomical localization of the inflammation (1). In some cases, photopsia, nyctalopia and dyschromatopsia can occur. Examination of the eye can reveal signs
17 of vitritis, choroiditis (whether or not active), retinal vascular inflammatory changes, macular oedema, retinitis, optic nerve head swelling and exudative retinal detachment (8).
1.1.4 Complications
Uveitis can be an important cause of visual morbidity in children. Children have an increased risk of complications because they often have chronic disease. Complications are reported in up to 50% of the children, with vision loss in 25–40% and legal blindness in up to 25% of the patients (12). Late diagnosis is also a damaging factor that is often the consequence of the lack of symptoms and the inability to indicate changes in vision. Here the most common vision threatening complications of uveitis will be discussed (13).
As a result of anterior uveitis, posterior synechiae can occur that are adhesions between the iris and the anterior part of the lens and are visible as a deformation of the pupil, also one of the causes of secondary glaucoma in anterior uveitis. Moreover anterior uveitis can be the cause of cataract and band keratopathy (8), i.e., calcium deposits in the bowman’s layer of the cornea (1) and are often linked to JIA. Cataract and glaucoma can also be the result of intermediate uveitis (8), although the glaucoma is caused less frequently by synechiae. However, intermediate uveitis provokes an enhanced risk of developing cystoid macular edema, which is the swelling of the inner layers of the macular retina that can prompt visual loss (1). Cystoid macular edema together with media opacity are the main complications of posterior uveitis (8).
1.1.5 Role of ophtalmologist and pediatrician
When a child shows signs of uveitis or has a risk to develop uveitis, it is up to the ophthalmologist to evaluate and grade the visual acuity, severity of uveitis and complications in each eye. Split lamp examination (to investigate anterior chamber cells and flare), intraocular pressure and fundus examination are needed to identify complications (10).
Further investigations should be done by the pediatrician to identify the possible etiology. Usually a good history and clinical examination are enough to pinpoint a systemic cause. Thorough joint examination is crucial to rule out JIA (10).
Though juvenile uveitis comprises only a small portion of the uveitis population, it is important to assess this condition in a multidisciplinary pediatric setting as patient care, including examination and treatment, can be more challenging (3). As children are often preverbal or more asymptomatic, they more often present themselves with already significant ocular damage or vision loss compared to adults (14-16).
18
1.2 J
UVENILE IDIOPATHIC ARTHRITISJuvenile idiopathic arthritis (JIA) is the most common form of chronic rheumatic disease in children, JIA-associated uveitis (JIA-U) is its most common extra articular manifestation (6). JIA is not a singular condition but a collection of different forms of arthritis with onset before the age of 16 that persists for more than 6 weeks with an unknown cause. These different forms are classified in subtypes by the International League of Associations for Rheumatology (ILAR) (17). Prevalence and incidence of JIA have been unclear due to the lack of a clear classification systems and the location-bound differences in different regions. Data has suggested a disease prevalence of 64–400 in 100.000 and an incidence of 1-22 in 100.000 (18).
1.2.1 Subtypes
The different subtypes of JIA represent different types of the disease with each their own characteristics (17).
Systemic arthritis
Systemic arthritis accounts for 5-15% of all children with JIA (19). This subtype is has a clear distinction with the other subtypes in that it is preceded by a fever at least 2 weeks in duration accompanied by at least one of the following symptoms: typical erythematosus rash, hepatomegaly or splenomegaly, generalized lymphadenopathy, or serositis. The occurring arthritis is mostly asymmetrical and polyarcticular. At onset arthritis might not be present, but may form over time. Anemia is also frequent and is microcytic. In children, this anemia is more severe than in adults (17).
Oligoarthritis
The most frequent form of JIA is oligoarthritis which represents 50-80% of the chronic arthritis population in children (19). In oligoarthritis only four or fewer joints are involved in the 6 months following the onset of the disease. The condition mainly situates itself in the lower half of the body and predominantly the knee followed by the ankles. Most of the patients have a very distinct type of oligoarthritis. This type does not occur in the adult population. It is typed by asymmetric arthritis, early onset, female patients and often positive antinuclear antibodies (or ANA). Also these patients have a high risk of iridocyclitis (17).
Rheumatoid-factor-negative polyarthritis
Polyarthritis accounts for approximately 20% of JIA patients. Of these patients 85% is rheumatoid-factor (RF) negative. RF negative RA is five times more common in girls (19). Alike RF positive polyarthritis this subtype affects 5 joints or more but here IgM RF is absent. This
19 is the most heterogeneous subtype consisting of 3 main subsets. The first one resembles early onset oligoartritis except for the fact that more joints are involved. Most patients have asymmetric arthritis, are very young of age at onset and are female. Also frequently positive ANA’s are observed. Association has been made with HLA antigens and a higher risk of iridocyclitis is noted. The second subset is characterised by clear symmetric synovitis of both large and small joints. The main patient is at school age and is ANA negative. Lastly the third subset is a form that is called dry synovitis as it shows negligible joint swelling yet in this case stiffness and flexion contractures are present (17).
Rheumatoid-factor-positive polyarthritis
3% of JIA patients are affected by RF positive RA (19) and most of the patients are female. This type is alike the adult form of RF positive RA (17, 19). Here five or more joints are affected in the first 6 months of the disease. Also the presence of IgM RF is required. The typical presentation is a symmetrical polyarthritis that is mostly situated in the small joints of both the hands and feet. This subtype stands out because of the fact that rheumatoid nodules can form on the classic places (none of the other subtypes do) (17).
Enthesitis-related arthritis
Up to 10% of JIA patients have enthesitis-related arthritis (19). It mainly affects males over the age of six years (17, 19). Only four or fewer joints are involved. Typically this subset affects the lower extremities and in contrast to the other subsets hip involvement can be seen at onset. The main typing factor is the association with HLA-B27. This form of arthritis belongs to the group of spondylarthropathies (17).
Psoriatic arthritis
The proportion of patients with JIA who have psoriatic arthritis is approximately 7 %. Psoriatic arthritis is defined by the simultaneous presentation of arthritis and psoriatic rash or if skin involvement is absent the presentation of arthritis with at least two of the following: dactylitis, nail pitting or psoriasis in a first degree relative. Typically these patients have an early onset with asymmetric arthritis. Frequently they are ANA positive and have a higher risk of developing iridocyclitis. This condition is comparable to oligoarthritis the main difference being that in the patients with psoriatic arthritis dactylitis (both in small and large joints) is more common (19).
1.2.2 Prognosis and outcome
Firstly, certain terms for the state of the disease must be defined. Inactive disease or remission is defined as an active joint count of 0 combined with absence of systemic manifestations in
20 those with systemic onset JIA, absence of enthesitis in those with enthesitis-related JIA or psoriatic JIA, absence of uveitis and a PGA <10 mm (Patient global assessment) (20). Disease remission can be classified into 2 types defined by consensus formation using Delphi and Nominal Group Techniques. Firstly there is disease remission on medication (≥6 months of continuous inactive disease while receiving medication) and the other form is disease remission off medication (≥12 months of inactive disease without receiving medication) (21). A large study from Canada examined 1104 children to investigate the short-term outcomes of JIA. Probabilities of attaining an active joint count of 0 was more than 78% over 2 years in al JIA categories. 70% of the children attained inactive disease within 2 years in all categories except for RF-positive polyarticular JIA. A little more than half of these patients discontinued treatment after a median of 3.1 year of treatment. The probability of attaining remission within 5 years was 46%-57% with exception of polyarthritis (20).
Another study from Norway investigated the long term outcomes of JIA. 176 patients were followed for 30 years. After 30-year follow-up, 59% of the patients were in clinical remission off medication, 7% were in clinical remission on medication and 34% had an active disease state. 70% of the patients were in the same disease category as they were at 15 and 50 years (22).
1.2.3 Relation to uveitis
When looking among all cases of pediatric uveitis, studies from Europe, North America and Israel have found that JIA associated uveitis (JIA-U) ranged from 15%-67%. Within the population of children who have been diagnosed with JIA the prevalence estimates of JIA-U range from 12% to 30%. Though the prevalence seems to be going in a decreasing trend over the past decade (6). The median onset of uveitis was reported in a large German study to be 5.5 months after the onset of arthritis with nearly three quarters developing ocular inflammation within 1 year and up to 90% of the patients within 4 years (23).
JIA-associated uveitis most commonly presents itself in the form of chronic anterior uveitis (68%), which is insidious at onset and frequently asymptomatic. This form has a high association with oligoarticular JIA (6, 23). It is also frequently seen in RF-factor negative JIA and less frequently in RF-factor positive and psoriatic JIA (23). Acute anterior uveitis, which has a more symptomatic presentation, is more frequently seen in relation to enthesitis-related arthritis (6, 23). This type of uveitis is the second most prevalent form (16%), followed by recurrent anterior disease (12%) and panuveitis (3.5%) (6).
The onset of arthritis typically precedes the start of uveitis though the converse situation may occur in a limited number of cases. Systemic and ocular inflammation may not have a synchronous disease progression and thus can be treated independently. However a link has
21 been reported between clinical remission of JIA and the presence of uveitis. It was observed that those who had JIA-U had a smaller chance of clinical remission than those who did not. Also a correlation between the severity of uveitis and a short time interval between the onset of JIA and the onset of JIA-U was seen (23).
Several risk factors of developing JIA-U have been described. Higher risk for chronic anterior uveitis was observed in those with younger age of onset, in females, the presence of antinuclear antibody (ANA), HLA-B27 or HLA-DRB1 and the most recent found risk factor anti-histone antibodies (AHA) (6, 23). A study on the risk factors for uveitis reported that the risk of evolving uveitis was only age dependent in girls (24). Within the subtype oligoarticular JIA the chronic type of uveitis was associated with the HLA haplotypes DR5 and HLA-DRB1*1104 (more particularly the combination of HLA-HLA-DRB1*1104 and HLA-DRB1*0201). HLA-B27 is seen in relation to acute anterior uveitis with enthesitis-related arthritis (6).
1.2.3.1 ophtalmological screening
The latest recommendations for screening for uveitis in children with JIA were published in 2006 by the American Academy of Pediatrics. (25). According to these guidelines an ophthalmology screening every 3–4 months in children with oligoarticular or polyarticular rheumatoid factor (RF) negative arthritis who are antinuclear antibody (ANA) positive, <7 years of age, and diagnosed with arthritis for ≤4 years is recommended (26). Yet alterations where made by a German study group (the German Uveitis in Childhood Study Group). This group recommended intervals for uveitis screening, which are adapted to the uveitis risk of the individual JIA subtypes (Table 5) (25).
22
Table 5. Screening intervals suggested by Heiligenhaus
(Heiligenhaus et al. 2007) for uveitis in patients with juvenile
idiopathic arthritis (JIA) as classified by International League of
Associations for Rheumatology (ILAR) criteria
JIA-subgroup
ANA Age at JIA onset (years) JIA duration (years) Recommended screening intervals (years) OA, RF–PA, PsA, UA + ≤6 ≤4 3 OA, RF–PA, PsA, UA + ≤6 >4 6 OA, RF–PA, PsA, UA + ≤6 ≥7 12 OA, RF–PA, PsA, UA + >6 ≤2 6 OA, RF–PA, PsA, UA + >6 >2 12 OA, RF–PA, PsA, UA - ≤6 ≤4 6 OA, RF–PA, PsA, UA - ≤6 >4 12 OA, RF–PA, PsA, UA - >6 n.a. 12
ERA n.a. n.a. n.a. 12
RF+PA, Sys A
n.a. n.a. n.a. 12
Patients with uveitis
n.a. n.a. n.a. According to uveitis
course
ERA = enthesitis‐related arthritis, OA = oligoarthritis, PsA = psoriatic arthritis, RF–PA = rheumatoid factor‐negative polyarthritis, RF+ PA = rheumatoid factor‐positive polyarthritis, Sys A = systemic arthritis, UA = unclassified arthritis, n.a. = not applicable.
23
1.3 T
REATMENT1.3.1 Treatment of uveitis
Here the treatment of idiopathic uveitis and JIA associated uveitis will be discussed. The treatment of these two are considered the same. The primary goal of uveitis treatment is to suppress inflammation to SUN grade 0 and to decrease the risk of complications (10). Early detection and appropriate assessment is the key in uveitis treatment. Topical glucocorticoids are used for the treatment of acute anterior uveitis. All medications discussed below are used in chronic uveitis. Treatment follows different multistep protocols dependent on uveitis grade, duration of treatment, occurrence of flare and potential complications. Management of uveitis is discussed below and follow a modified algorithm based on consensus guidelines (6).
1.3.1.1 Medication
There is no international consensus regarding treatment of idiopathic and JIA-associated uveitis. Therefore management is based on the personal experience of the professional. There are considerable differences found regarding treatments in clinical practices (27).
1.3.1.1.1 Topical glucocorticoids
Active uveitis requires immediate treatment (27). In immune mediated uveitis the first line of treatment is topical corticosteroids (6, 10, 27). These are used for both acute and chronic uveitis (6, 27). Though very effective in anterior uveitis they do not have the same efficacy in intermediate and posterior uveitis (10). Frequency of application is variable with inflammation severity. Chronic use of topical steroids can lead to complications such as cataract and glaucoma (6, 10, 27). The adjacent use of non-steroidal anti-inflammatory drugs (NSAIDs) has been considered and could play a role in treatment with steroids. Monotherapy of NSAIDs however is not recommended (27).
1.3.1.1.2 Systemic glucocortocoids
When topical steroid treatment does not suffice or the dose needs to be increased to such an extent that the risks outweigh the benefits systemic steroids are used (27). Immediate use of systemic steroids are recommended if the child has poor prognostic factors at presentation including significant ocular complications such as cataract, glaucoma, posterior synechiae, band keratopathy, dense vitreous body opacification, hypotony and macular edema, uveitis antedating arthritis, posterior synechia, male gender and poor initial vision (6, 10, 27). systemic steroids are also necessary when there is absence of remission later on during the disease course (27). These are used in combination with topical steroids. Oral steroids are also used in the treatment of intermediate and posterior uveitis (10). It must be noted that evidence for the use of systemic steroids has only been seen in adult studies (6). Periocular or intraocular
24 steroids can be used for severe uveitis. Knowing the familiar side effects of systemic steroids early introduction of steroid-sparing immunosuppression in those with moderate to severe uveitis is necessary (10).
1.3.1.1.3 Synthetic DMARD’s
The preferred immunosuppressive treatment after glucocorticoids is methotrexate (MTX) (6, 10, 27). When disease inactivity is not reached within 3 months or inflammation is reactivating during steroid dose reduction systemic immunosuppression is recommended (27). Common side-effects of MTX are nausea and vomiting (6, 10) but they can be resolved through antiemetics and counselling (10). A reduced need for cataract extraction with MTX use was seen (6). Intraocular inflammation resolves in around 75% of children being treated with MTX. Treatment is continued until the disease remains inactive even after stopping the use of topical steroids and is then further continued for about 18-24 months before withdrawal (10). Other DMARD’s such as mycophenolate mofetil, tacrolimus, azathioprine and cyclosporine are less frequently used in the treatment of uveitis though combinations of two DMARD’s have been known to be used in severe cases (6).
1.3.1.1.4 Biologicals
Biologic response modifiers or so-called biologicals are defined as a medication made through recombinant DNA technology and is designed to be effective based on the molecular understanding of a disease pathogenesis. Biologicals are not considered first line treatment because of their high cost and limited data of long-term safety (28).
In the context of idiopathic uveitis and JIA-U, the use of biologicals should be considered in case the child does not show treatment response 3-6 months after starting MTX (6, 10). The most used biologicals are the TNF alpha blocking agents: adalimumab (ADA) and infliximab they are as add-on treatment of synthetic DMARD’s (6). The first choice of drug is ADA over infliximab (27). ADA is the only approved biological treatment option in Belgium for pediatric patients from two years of age with chronic non-infectious anterior uveitis who have had inadequate response to conventional therapy.
1.3.1.1.4.1 Infliximab
Currently infliximab is not approved for the treatment of uveitis and JIA-U in Belgium.
Infliximab is a chimeric monoclonal antibody that binds both circulating as well as membrane-bound tumour necrosis factor alpha (TNF-α). The medication needs to be administered intravenously. Infliximab dosage is 3-5mg/kg, with administration at weeks 0, 2, and 6 followed by maintenance of 3-10 mg/kg every 4-8 weeks with a maximal dose of 20 mg/kg in children. It’s use has been approved for a number of inflammatory diseases (such as ulcerating colitis,
25 rheumatoid arthritis, ankylosing spondylitis, psoriasis, psoriatic arthritis, rheumatoid arthritis and Behçet disease). Efficacy of infliximab with JIA-U has been proven in different retrospective studies. The majority of the patients experienced rapid control of uveitis after the second infusion. Pediatric patients might require higher dose by weight and more frequent administrations compared to adults (28). Two year outcomes from a prospective trial including both adults and children showed promising results for the use of infliximab in different forms of uveitis associated with multiple diseases (including idiopathic uveitis) where 3 in 4 patients showed initial improvement. Out of the responders 60% (15 of 23 patients) preserved effectiveness in the first year and also 60% (9 of 15 patients) in the next year (29). A study including 31 patients showed a very high rate of adverse effects from the medication including 3 out of 31 patients developing drug-induced lupus. It was speculated that this could be the result of very high drug levels after intravenous administration of the TNF-α blocker (28).
1.3.1.1.4.2 adalimumab
ADA is a fully human monoclonal antibody against TNF-α. ADA is administered by subcutaneous injection. Dosage depends on the child’s body weight, 40 mg every 2 weeks in children weighing ≥ 30 kg; 20 mg every 2 weeks in children < 30 kg (28). To assess the efficacy of adalmumab in children with JIA-U the SYCAMORE trial was done. 90 patients were followed where 60 received ADA treatment complementary to MTX and 30 received placebo (they did receive MTX treatment). Patients were followed over 18 months and received ADA every 2 weeks according to the dose noted above. ADA treatment had a clear benefit over MTX treatment alone. Treatment failure was significantly lower in the patients in the ADA group over the placebo group (16 patients [27%] vs. 18 patients [60%]). Also a delay in time of treatment failure was seen in the ADA group compared to the placebo group. Time of treatment response was also favorable in the ADA group. The use of corticoids was also investigated. In the ADA group there was a significant percentage who reduced steroid use in treatment over the placebo group. In children who used 2 or more steroid drops 51% had a dose reduction in the ADA group over 16% in the placebo group. In children who were taking one or more drops at the start of the trial 47% of the ADA group reduced to zero drops over 16% in the placebo group. It must be noted that adverse effects, including minor infections and respiratory disorders did occur more frequently in the ADA group (30).
1.3.2 Treatment of JIA
Treatment of JIA is similar compared to treatment of idiopathic uveitis and JIA-U. NSAIDs are classically the first line treatment of JIA. These are merely symptomatic medications that are not recommended to be used for more than 2 months with active disease. Only a few NSAIDs are approved for use in children such as naproxen, ibuprofen and indomethacin. Intra-articular
26 corticosteroid injections are used for rapid relief of inflammatory symptoms and for functional improvement as well as to reduce the need for regular systemic therapy. Systemic corticosteroids are mostly used to control the extra-articular manifestations of JIA (high fever unresponsive to NSAIDs, severe anemia, myocarditis or pericarditis and macrophage activation syndrome). MTX is the most widely used DMARD in the management of JIA. MTX can be used both systemic as orally. After 6 months of disease remission with MTX treatment the medication can be stopped. Biologic DMARDs are also used in the management of JIA. The first biologic agent registered for use in JIA is etanercept, a fully human TNF inhibitor. Inflixmab was observed to have a similar effect to etanercept after 1 year of treatment. The efficacy of ADA has been proven. It was found to be highly effective in children and adolescents with JIA who had previously been treated with other biologic agents. TNF inhibitors were observed to be more effective if administered early in the disease (31).
1.3.3 Anti-drug antibodies
While the use of biologicals in the treatment of uveitis has shown promising results, it has become clear that prolonged exposure to the drugs may result in a loss of response (LOR). Deteriorated response has been linked to the formation of anti-drug antibodies (32, 33).
1.3.3.1 Anti-infliximab antibodies
There are only limited data on anti-infliximab antibodies (AIA) in children (34). More data are available in adults.
A prospective study on the frequency of AIA and its association with adverse effects and treatment failure was done in patients with rheumatoid arthritis. In total, 218 patients (without prior anti-TNF treatment were given infliximab treatment. The serum through levels of infliximab and AIA were measured (prior to infusion) at baseline, week 14 and week 52 or when infliximab treatment was terminated. Clinical assessments were done at baseline, week 2, week 6 and then every 8 weeks. AIAs were detected in 54% of the patients during a 52 week follow-up. Patients with detectable AIAs had lower median infliximab through levels compared to those detectable AIAs. Within the group of AIA positive patients, 43% withdrew due to treatment failure and adverse drug reactions, whereas only 31% of the patients from the AIA negative group. The patients with detectable AIAs had a higher risk of developing adverse drug reactions compared to the patients without detectable AIAs. No difference of AIA status was seen between the primary responders and the primary non responders, though there was a difference with the secondary non-responders. Fewer primary non-responders had detectable AIAs compared to the secondary non-responders (35).
A study exploring the binding sites of AIA in infliximab treated pediatric patients with rheumatic diseases, suggested that higher levels of AIA advanced the clearance of serum infliximab, and
27 thus reduces its efficacy. Also the time interval between onset of treatment and the formation of AIA was investigated. The majority of the patients showed antibody formation in a time interval of around 26 and 69 weeks. The earliest detection of AIA was after 112 days (~4 months) and the latest detection of antibodies was around 1734 days (~4,7 years) (34).
1.3.3.2 Anti-adalimumab antibodies
Although ADA is a fully human immunoglobulin G antibody, it has been demonstrated to be immunogenic. Anti-adalimumab antibodies (AAA) have been shown in 5% to 54% of the patients on ADA. A study done in 25 patients with non-infectious refractory uveitis (including 2 with JIA and 13 idiopathic) investigated the occurrence of AAA with ADA treatment. Of these patients, 18 (72%) of 25 showed favorable clinical response. 11 of the 18 responders showed complete response and 7 showed partial response. A higher ADA serum trough level was seen in the responding fraction of the group. 8 out of 25 patients where AAA positive. Half of these where considered to have transitory AAA (positive titer of >10U/ml at 1 single measurement) and the other half was considered to have permanent AAA (positive titer of >10U/ml at more than 1 measurement). AAA form immunocomplexes with ADA which is described to stimulate clearance of the drug. All 4 of the patients with permanent AAA showed antibodies early in the treatment (less than 8 weeks after onset). Permanent AAA where linked to an inverse correlation between the AAA titers and the ADA trough levels though this inverse correlation was not seen with transitory AAA. Also it was stated that no correlation was seen between the occurrence of AAA and the concomitant use of other immunomodulatory drugs (such as MTX). (32). In contrast to another study with JIA-U patients that stated a link between the occurrence of AAA and the absence of concomitant MTX treatment .(36). A potential link between associated systemic diseases and AAA was also speculated. AAA where also associated with worse uveitis outcomes though this was only seen in those patients with permanent AAA (32). The other study on patients with JIA-U confirms this relation between high AAA levels (≥12U/ml) and higher grade of uveitis and also lower ADA serum trough levels. They also saw a link between younger age at disease presentation and AAA formation (36).
1.4 T
HERAPEUTIC DRUG MONITORINGTherapeutic drug monitoring (TDM) is a tool for guiding dosage adjustments to optimize drug exposure, maximizing the drug response rate. It is based on measurements of through drug levels and anti-drug antibody titers and provides the clinician an objective tool to guide the treatment. This is necessary because there is a large difference in treatment response (more than 10x interindividual difference) in relation to the serum drug trough level. Aside from the benefit in treatment, TDM can also avoid unnecessary therapeutic interventions and so reduce costs (37).
28 Although becoming standard practice in tertiary centers, the widespread accessibility and recognition of TDM is hindered (37). Nevertheless, the profit of TDM and its cost-effectiveness for optimizing treatment in non-infectious uveitis has been described in several studies (32, 33). The Monitoring of monoclonal Antibodies Group in Europe (MAGE) provides an insight on the concept of TDM. As described above, not all patients response well to treatment and the formation of anti-drug antibodies is thought to be at the base of this problem. Optimal use of TDM during these expensive treatments may lead to prolonged remission and better cost-effectiveness. TDM requires the accessibility of assays to measure the concentration of the serum drugs and the availability of studies correlating drug blood concentration with clinical effectiveness. However, as for today both these requirements are insufficient still. Immunogenicity-exposure-response is extensively demonstrated (especially in older patients) for the more established drugs such as ADA and infliximab in IBD, inflammatory rheumatic diseases and psoriasis (37).
TDM is useful in the early stages of therapy to alert the clinician for early risk of treatment failure. Sub therapeutic concentrations in the early stages of treatment are associated with higher disease activity and anti-drug antibody development. This can result in loss of response within the first year of treatment. Furthermore, TDM can be used to identify the real primary non responders (who have adequate drug administration). To gain insight in the pharmacokinetic evolution over time in the patient, it can be helpful to measure consecutive trough concentrations. Disease progression might influence the pharmacokinetic evolution of the drug and therefore the drug concentration can vary over time. It is recommended that, over treatment, clinical follow-up of drug concentration is done at every visit during the introduction phase of treatment and in a later phase about once every 3-6 months unless signs of loss of clinical response arise (37).
1.5 O
BJECTIVESIn this study on ADA treatment and AAA formation in children with idiopathic uveitis, JIA without uveitis and JIA-associated uveitis, we aimed to find out whether the use of concomitant MTX, ADA dose and ADA serum levels were associated to AAA formation. We also wanted to investigate whether age at onset of disease, ANA positivity before treatment, disease activity and treatment duration are risk factors for more AAA formation. Moreover we wanted to see if ADA drug levels have a positive impact on disease activity. Lastly we wanted to know whether the use of concomitant MTX and increased ADA dose (corrected for weight) leads to increased ADA serum levels.
29
2 M
ATERIALS AND
M
ETHODS
2.1 S
TUDY DESIGN AND PATIENT RECRUITMENTConsecutive patients (n=34) treated with ADA were recruited from the Pediatric Rheumatology Unit of the Prinses Elisabeth Childrens Hospital, Ghent University Hospital between February 2018 to August 2019. Inclusion criteria for the retrospective cohort study included either confirmed diagnosis of JIA (based on criteria defined by the ILAR), JIA associated uveitis or idiopathic uveitis. This study was approved by the ethics committee of the Ghent University hospital (approval number: 2019/1413). No patient consent was needed as data analysis was performed on anonymized data.
2.2 D
ATABASE DESIGN AND VARIABLES2.2.1 Demographical, clinical and therapeutic data
Demographical and clinical data included sex, age, weight, diagnosis and subtype of JIA (according to the ILAR classification criteria), the date at onset of uveitis and JIA, uveitis type and anatomical location(according to the SUN criteria) and disease activity at the moment of first drug level sampling. Data on the therapy included date of start of anti-rheumatic therapy, previous anti-rheumatic drugs, the use of concomitant immunosuppression, and the dose of ADA. In addition, data on ANA positivity before start of ADA was collected.
2.2.2 ADA and AAA
Data on routinely detected ADA drug levels and AAA titers were collected. In a routine context, ADA drug levels (through concentrations) and AAA titers (only in case of low or undetectable drug levels) were randomly collected after start of treatment, depending on frequency of visits in function of disease activity. A CE-labelled and validated enzyme-linked immuoadsorbent assay (ELISA; ApDia, Turnhout, Belgium) method was be used for ADA and AAA measurements.
2.3 S
TATISTICAL ANALYSISStatistical analysis of the data was performed on IBM SPSS Statistics (version 26). To investigate associations between categorical variables the Chi2-test was done. When the
conditions for a Chi2-test were not reached, a Fisher’s exact test was performed. To compare
continuous variables the Mann-Whitney U test was used. When analysing a correlation between pairs of continuous variables a Pearson correlation was performed. When the
30 variables were not normally distributed a Spearman correlation was done. A p value of less than 0,05 was considered statistically significant.
31
3 R
ESULTS
3.1 B
ASELINE CHARACTERISTICSADA drug levels and AAA titers were measured in a total of 34 pediatric patients: 11 with idiopathic uveitis, 11 with JIA without uveitis, and 12 with JIA-associated uveitis (JIA-U). Patients’ baseline characteristics are summarized in Table 6. The median dose of ADA at first sampling was [0,86 mg/kg/2wk]. At the time of first sampling, 24 patients were treated with concomitant MTX and at the time of second sampling, 12 patients were treated with concomitant MTX. Before start-up of biological treatment 31 patients were treated with other antirheumatic drugs including MTX (n=30), other cDMARD’s (n=8) and other TNF blocking agents (n=3). At the time of first sampling remission was achieved in 24 of ADA treated patients (71%) and active disease was present in 10 patients (29%). No information on the disease progression was collected during the second sample.
Table 6. Baseline characteristics of included patients (n=34) at start of ADA treatment
Characteristics Value
Age, years, mean [range] 11.8 [ 3 – 22]
Age at disease onset, months, mean [range] 82 [7 – 209]
Female, n (%) 22 (65)
Diagnosis
Idiopathic uveitis 11 (32%)
JIA without uveitis 11 (32%)
JIA associated uveitis 12 (35%)
Uveitis present 22 (65%) Type by location, n (%) Anterior 15 Intermediate 3 Posterior 1 Pan 3 Laterality Unilateral 6 Bilateral 16
JIA + subtype (According to the ILAR criteria) 23 (68%)
Oligoarthritis 9
Extended oligoarthritis 1
32 ERA 3 Psoriasis arthritis 1 ANA positivity, n (%) 20 (59) HLA-B27, n (%) Positive 2 (6) Negative 12 (35) Unknown 20 (59)
Previous antirheumatic drug 31 (91%)
MTX 30
Other conventional DMARD (Neoral, Cellcept, SSZ) 8 Other TNF blocker (Enbrel, Golimumab) 3
Concomitant steroids (at the time of first sample) 14 (41%)
Local 8
Oral 3
Local + oral 2
Disease activity (at time of first sampling), n (%)
Active 10 (29)
Remission 24 (71)
ANA = antinuclear antibody, cDMARD = conventional disease-modifying antirheumatic drug, JIA = juvenile idiopathic arthritis, MTX = methotrexate, SSZ = sulfasalazine
3.2 ADA
DRUG LEVELS IN RELATION TO DISEASE ACTIVITY,
CONCOMITANT MEDICATION AND DOSEIn total ADA drug levels were measured at 57 moments (34 levels from the first sample collection, 19 from the second and 4 from the third). The pooled median through ADA level (results of all samples irrespective of the time point after start of the therapy) was 10.17 µg/mL (IQR 5.25 – 12.65 µg/mL) (Figure 5). The median ADA drug level during the first sample collection was 10.1 µg/mL (IQR 3.05 – 12.43). The mean duration of biological therapy at the time of the first sample collection was 20,3 months (range: 4 – 91)
33 Figure 5: Histogram and distribution curve of all measured ADA drug levels (µg/mL)
There was no significant difference between the ADA drug levels measured at the first sample collection for patients in remission versus active disease (median drug levels: 9.65 versus 10.65; p= 0.925) (Figure 6).
Figure 6: Boxplot of ADA drug level (µg/mL) measured at first sample collection by disease activity
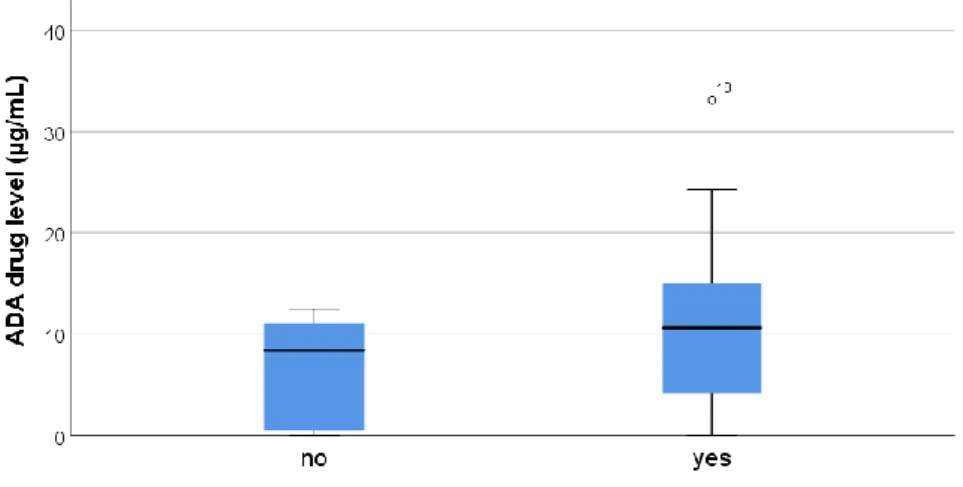
34 ADA drug levels were increased in those patients who received concomitant MTX. Yet there was no statistical significant difference in ADA drug levels, measured during the first sample collection, between those patients who had concomitant MTX treatment and those without (median drug levels: 10.65 versus 8.4; p=0.180) (Figure 7) (Figure 8).
Figure 7: Boxplot of ADA drug level of first sample collection by concomitant MTX
Figure 8: Population pyramid frequency first sample (µg/mL) by concomitant MTX during first sample collection
When looking at the effect of dose, corrected for weight, there was a positive correlation between ADA dose and ADA drug levels (p=0.002; Pearsons r = 0.523) (Figure 9).
35 Figure 9: Scatterplot of ADA drug levels (µg/mL) versus dose corrected for weight (mg/kg/week)
3.3 AAA
TITERS IN RELATION TO AGE AT DISEASE ONSET,
DISEASE ACTIVITY,
DOSE,
CONCOMITANT MEDICATION AND TREATMENT DURATIONAAA were found in 18% of the patients (n=6) in at least one of the time points. The median time of AAA detection in our population was 26.8 months [interquartile range (IQR) 7.8 – 49.25 months].
Patients with AAA formation were found to be younger. But no statistically significant difference in age between those who had AAA formation and those who did not could be shown (median age in months: 49 versus 75.5; p=0.557) (Figure 9).
36 Figure 10: boxplot of age at disease onset (months) by AAA titers at any time point
Patients with AAA were found to have a higher dose of ADA (µg/week/kg), corrected for weight. But there was no statistically significant relation found (median dose: 0.82 versus 0.79; p=0416) (Figure 11).
Figure 11: Boxplot of dose corrected for weight (mg/kg/weeks) by AAA at first sample collection
There was a 50%/50% divide between the patients with detectable AAA titers for active versus inactive disease and a 25%/75% divide between the patients without detectable AAA levels for respectively active versus inactive disease (Figure 12). Patients with detectable AAA levels
37 showed increased serum titers when they had active disease versus when they did not (median titer: 122.0 versus 63.3; p=0.658) (Figure 13).
Figure 12: Clustered Bar count of AAA titers (ng/mL) by disease activity
Figure 13: Boxplot of AAA titer at first sample collection (ng/mL) by Disease activity at first sample collection
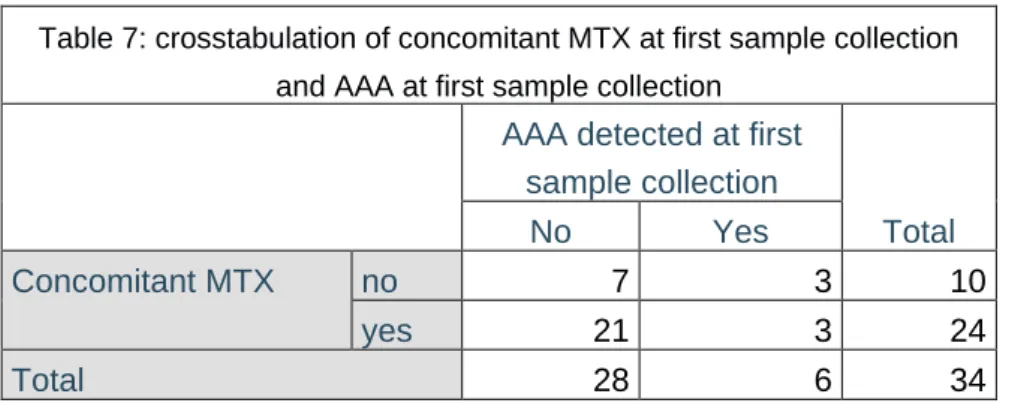
Detectable AAA levels were found in 3 out of 10 patients without (30%) and in 3 out if 24 patients with concomitant MTX (12.5%). But no statistical significance could be shown for less AAA formation in case of concomitant use of MTX during ADA treatment (p= 0.328) (table 2).
38 Table 7: crosstabulation of concomitant MTX at first sample collection
and AAA at first sample collection AAA detected at first
sample collection Total No Yes Concomitant MTX no 7 3 10 yes 21 3 24 Total 28 6 34
Duration of biological therapy (months) was longer for those patients who had AAA development, yet no statistically significant difference could be shown (median titers 22.5 versus 18; p=0.832) (Figure 14) and was not related to the AAA titer level (p=0.798; Spearmans rho = 0.035) (Figure 15).
39 Figure 15: Scatterplot with fit line of AAA titer at sample collection by duration of biological treatment
When looking at ANA levels in patients before biological treatment a difference in AAA formation was seen between those patients who had ANA titer intensity scores of more than 2+ and those with lower ANA titer intensity scores. Those with higher ANA levels had a greater tendency to have AAA formation (p=0.008) (table 3).
Table 8: crosstabulation of AAA formation and ANA > +2 before biological treatment Count ANA > +2 Total no yes AAA formation No 22 6 28 Yes 1 5 6 Total 23 11 34
3.4 R
ELATION BETWEENAAA
TITERS ANDADA
DRUG LEVELSIncreased AAA titer levels were correlated to lower through levels of ADA during the first collection (p<0.001) and during the combined serum collections (p<0.001) (Figure 16 and 17).
40 Figure 16:Boxplot of adalimumab drug levels and grouped AAA
Figure 17: Overlay scatter adalimumab drug levels and AAA titers
41
4 D
ISCUSSION
4.1 AAA
AND DISEASE ACTIVITYAlthough ADA is effective for the treatment of idiopathic uveitis, JIA without uveitis and JIA associated uveitis loss of response (LOR) on the drug is a clear issue in a substantial amount of patients. No clear data exists on the incidence of LOR. Data from the Stockholm TNF alpha blocker follow-up registry suggests a LOR of 4% of patients per year in adults with rheumatoid arthritis. (38) While data on adults with Crohn’s disease suggest a LOR incidence from 8% to 71%. (39) In neither of these studies a differentiation was made between the different anti-TNF-α drugs. In many studies concerning ADA the responsive antibody (AAA), detected in many patients after start of ADA treatment, is held responsible for this phenomenon (36, 38, 40, 41). It is hard to extrapolate the information found in literature to children with JIA, JIA-U and uveitis. There is limited data on the subject of biological treatment and TDM in general and especially in children. For this reason a lot of adult studies and studies concerning other auto-immune mediated diseases were used for the interpretation of the results.
In our study 6 out of 34 patients (18%) showed presence of AAA. 50% of patients with detectable AAA titers had active disease versus 25% of patients without detectable AAA titers (Figure 12). The AAA positive patients with active disease showed increased AAA titers although no significant relation could be shown. The non-significant finding of our study could be due to the small patient population and its heterogeneity. Moreover, as the AAA titers were measured at random time points, it can not be excluded that AAA might have been present some time before the actual sample collection. Also, as disease activity at the time of sample collection was used to evaluate the effect of AAA, no differentiation was made between primary non-responders and LOR.
4.2 AAA
TITERS,
ADA
DRUG LEVELS,
DOSE AND DURATION OF THERAPYAAA may reduce ADA drug levels by inducing faster drug inactivation and clearance through formation of immunocompexes with ADA, and is therefore linked to LOR. When using the ELISA analysis, no conclusion can be drawn on the AAA titers in patients with increased ADA drug levels. When measuring AAA, only the excess of AAA (unbound) can be measured. ADA bound AAA cannot be detected and it is also believed that these bound AAA are clinically irrelevant. So when patients show increased ADA drug levels, any present AAA will be bound to the serum ADA. In this regard it is difficult to attribute any clinical relevance to the inverse relation between AAA titers and ADA drug levels as was also shown in our study (but also by others), as it is linked to the assay design to measure them (32, 38, 40, 41).
42 There was no relation between the dose of ADA and AAA formation. This might suggest that increased drug explosion does not influence AAA formation. The patients with longer treatment duration were more inclined to form AAA. Although no statistically significant difference could be shown, it seems like longer exposure to the drug adds to the risk of AAA formation.
4.3 C
ONCOMITANTMTX,
AAA
ANDADA
Although no statistically significant relation could be shown, the ratio of use of concomitant MTX versus ADA monotherapy in the group without detectable AAA was 75%/25% and the ratio in the group with detectable AAA was 50%/50% suggesting that less AAA formation is seen in the patients with concomitant MTX use (Table 7). There was also an effect of concomitant MTX use on ADA drug levels. The patients with concomitant use of MTX leaned more towards increased ADA drug levels. There is dispute on the relation between the use of concomitant MTX and AAA development and ADA drug levels. Literature shows conflicting data on this relation. Although the majority of data seems to lean towards an inverse association (36, 38, 41), a notable amount of studies suggests no relation (32, 40). A study done on ADA treatment in children with non-infectious uveitis showed a lack of protective effect of concomitant immunosuppression and no differences in mean ADA drug levels between those who received concomitant immunosuppression and those who did not (24). Another study on adult patients with Crohn’s disease showed no influence of concomitant immunosuppression on AAA formation nor on the ADA drug level (40). In these studies no differentiation was made between the types of concomitant immunosuppression. This data suggests that it might be possible to discontinue concomitant MTX without increasing the risk of AAA formation. It might be the small patient population and patient heterogeneity that caused a non-significant result in our study on ADA drug levels and concomitant use of MTX. It may also be that the cross-sectional study design might influence the statistically non-significant finding and it may be possible that adding concomitant immunosuppression to ADA therapy may influence these variables in a patient specific manner as shown in the patients treated with infliximab (40).
4.4 ADA
DRUG LEVELS,
ADA
DOSE AND DISEASE ACTIVITYA positive correlation was seen between the ADA dose corrected for weight and the ADA drug levels. When looked at the ADA drug levels in correlation to disease activity it could not be shown that the drug levels had any effect on whether the patient was in remission or had active disease at the time of sample collection. Based on our data we were unable to suggest any threshold for therapeutic ADA drug level to control disease. Many studies on ADA and TDM do indicate a correlation between ADA drug levels and disease progression (32, 36, 38, 40,
43 41). Again the limitation of our population number and our patient heterogeneity should be taken into account. Also, as mentioned before, no differentiation was made between primary non responders and LOR in our study, which also has an effect on the results. It could be that, in our population, there is an excess of primary non responders that show increased ADA drug levels, which have no effect on their disease course.
4.5 A
GE OF ONSET OF DISEASE AND PRESENCE OFANA
IN RELATION TOAAA
Our results show a positive relation between AAA titers and high pre-treatment ANA values. We defined high grade of ANA as >2+, as higher ANA grades usually have a more meaningful clinical outing. It could be that children with high ANA are more immunogenic by nature and thus have a stronger immune reaction to the ADA drug. A study done on infliximab treated psoriasis patients also showed a link between increased AIA and pre-treatment ANA (42). ANA positivity has been brought into relation with younger age at onset of disease in oligo- and polyarthritis (43). Our study also suggests a relation between AAA formation and younger age at onset of disease. Although this relation was not statistically significant. The relation between AAA and ANA might indicate an increased risk of AAA formation in the patients with the JIA subtype psoriatic arthritis, oligoarthritis and asymmetric RF negative polyarthritis as they usually correlate with ANA positivity.
44
5 C
ONCLUSION
We can conclude that higher ADA dose, longer treatment duration and higher levels of ANA before treatment are associated with a higher risk of AAA formation. Whereas concomitant use of MTX is associated with less AAA formation. Also patients with younger age at onset of disease, which was less investigated in literature, seemed to have greater risk of AAA formation. Within the group of patients with detectable AAA, higher AAA titers were found in those children with active disease. We also found that concomitant use of MTX and higher ADA dose was correlated to higher ADA drug levels.
Measuring drug concentration might help the clinician in correctly dosing the medication. Although, as mentioned before, our results do not show a correlation between drug concentrations and disease activity, the majority of data in the literature do suggest a strong correlation. So if the drug concentration is below the therapeutic range in a patient, increasing the dose, shortening the interval or a combination of these may be considered. It is getting more and more clear that TDM can help understanding the process of LOR in individual patients. With this information the clinician can act promptly and adequately. It can also be used for patients with ongoing remission. When patients show favorable disease progression with high AAA the question rises whether it could be profitable to consider stopping biological treatment and thus counteracting overtreatment of the patients. Also monitoring drug levels could help initiating the patients who have a good response to biological treatment in converting to monotherapy. Yet, considering the findings on the influence of MTX on AAA formation, stopping concomitant immunosuppression might increase immunogenicity of the biological drug. So it remains a difficult question which drug to discontinue or to discontinue at all.