promote plant growth
rhizosphere bacterium Streptomyces sp.1-43 to
Defining the plant molecular networks used by the
Academic year 2019-2020 Master of Science in Biology
Master's dissertation submitted in order to obtain the academic degree of
Counsellor: Sarah Langendries
Supervisor: Prof. Sofie Goormachtig
Student number: 01203725© Faculty of Sciences – Research Group Rhizosphere
All rights reserved. This thesis contains confidential information and confidential research results that are property to the UGent. The contents of this master thesis may under no circumstances be made public, nor complete or partial, without the explicit and preceding permission of the UGent representative, i.e. the supervisor. The thesis may under no circumstances be copied or duplicated in any form, unless permission granted in written form. Any violation of the confidential nature of this thesis may impose irreparable damage to the UGent. In case of a dispute that may arise within the context of this declaration, the Judicial Court of Gent only is competent to be no
I
TABLE OF CONTENTS
Table of contents ... I
List with figures ... 1
List with abbrivations ... 1
1.Introduction ... 1
1.1 The need of fertilizers and environmental effects ... 1
1.1.1 The impact on soil ... 2
1.1.2Fertilizer induced water pollution ... 2
1.1.3 Future challenges of climate change ... 3
1.2 Potential of biofertilizers to replace chemical fertilizer ... 4
1.2.1 PGPR as a biofertilizer & bioagent ... 5
1.3 Streptomyces spp. as a plant growth rhizobacteria ... 6
1.3.1 Life History traits cycle ... 6
1.3.2 The endophytic lifestyle of Streptomyces: from rhizosphere to the internal plant tissues ... 7
1.3.3 The rhizoplane, a specific habitat or a transitional boundary ... 7
1.3.4 Plant colonization and changes by endophytic PGPR. ... 8
1.3.5 Modes of action: plant growth promotion ... 10
Biofertilization: Facilitation of nutrient uptake ... 10
Bio-stimulation: Production of Phytohormones and enzymes ... 11
Bioprotection: biotic and abiotic stress management ... 12
1.4 Plant growth mechanisms of Arabidopsis thaliana... 15
1.4.1 Leaves growth mechanisms in Arabidopsis thaliana. ... 15
1.4.2 Regulated cell division in the leaves ... 15
1. 5 From the lab to the farm. ... 15
2. Objectives ...16
3.Materials and methods. ...17
3.1 Growth effect analyses on Arabidopsis thaliana ... 17
3.1.2 Plant genotypes and growth conditions ... 17
3.1.3 Bacterial strains and culture conditions... 17
II
3.1.5 Phenotypical analysis of root and shoot fresh weight ... 17
3.1.6 Phenotypical analysis of the root architecture of A. thaliana ... 17
3.1.7 Phenotypical analysis of the rosette of A. thaliana ... 18
3.1.8 GUS staining ... 18
3.2 Colonization analyses ... 18
3.2.1 transformation of Streptomyces ... 18
3.2.2 Bacterial identification ... 18
3.2.3 Visualisation of colonization ... 19
3.3 Preliminary studies Triticum aestivum ... 19
3.3.1 Inoculation and growth conditions of wheat ... 19
3.3.2 Phenotypical analysis of root and shoot fresh weight ... 19
4. Results ...21
4.1 Phenotypic response of Arabidopsis thaliana upon inoculation with Streptomyces sp.1.43 or Streptomyces sp.1.58. ... 21
4.1.1 Effect on the shoot and root fresh weight ... 21
4.1.2 Effect on the root architecture upon inoculation with Streptomyces sp.1.43 or Streptomyces sp.1.58 ... 23
4.1.3 Effect on the rosette of A.thaliana upon inoculation: leaf series ... 25
4.1.4 Kinematic cell analysis ... 27
4.1.5 Plant growth promotion of Arabidopsis by the potential presence of bacterial VOCS... 29
4.1.6 Analysis of expression in inoculated CYCB1;2-DBOX:GUS Arabidopsis plants upon inoculation of Streptomyces sp.1.43 or Streptomyces sp.1.58. ... 30
4.2 Root colonization potential ... 31
4.2.1 Bacterial Identification ... 31
4.2.2 Growth promoting effect of GFP-tagged Streptomyces sp.1.43 or ... 32
Streptomyces sp.1.58 on Arabidopsis ... 32
4.2.3 Quantification inoculation of Streptomyces sp.1.43::GFP and Streptomyces sp.1.58::GFP ... 34
4.2.4 Colonization pattern of GFP-tagged Streptomyces sp.1.43 and Streptomyces sp.1.58 ... 34
4.3 Phenotypic effects of Triticum aestivum upon inoculation with Streptomyces sp.1.43 ... 37
5. Discussion ...39
5.1 Both Streptomyces sp.1.43 and Streptomyces sp.1.58 display similar growth promoting effects on Arabidopsis ... 39
III 5.1.1 Growth enhancement of Arabidopsis rosette upon colonization of Streptomyces sp.1.43 or
Streptomyces sp.1.58 ... 39
5. 1.2 The root phenotype of Arabidopsis is altered by colonization by Streptomyces sp.1.43 or Streptomyces sp.1.58 ... 40
5.1.3 Potential bacterial VOCs induced Arabidopsis growth promotion ... 43
5.1.4 Medium dependent growth promoting effects of Streptomyces sp.1.43 and Streptomyces sp.1.58 on Arabidopsis ... Fout! Bladwijzer niet gedefinieerd. 5.1.5 Colonization pattern of Streptomyces sp.1.43 and Streptomyces sp.1.58 ... 45
5.2 No growth promoting effects on Triticum aestivum by inoculation of Streptomyces sp.1.43 ... 46
5.3 Conclusion ... 47
Summary (English) ...48
Summary (Nederlands) ...50
Communication to a broad audience ...52
Acknowledgments ...54
6. References...55
7. Attachments ...68
7.1 Supplementary data ... 68
1
LIST WITH FIGURES
Figure 1 Total fertilizer production by nutrient.
Figure 2 The mean relative yield change (%) (1980–2010). Figure 3 Schematic close up of the root interface interactions. Figure 4 Illustration of the life cycle of Streptomyces spp.
Figure 5 An illustration of the immune defence response of the plant host when encountering microorganism and the different responses.
Figure 6 Schematic representation of the bacterial distribution and colonization patterns. Figure 7 Illustration of the different phytohormones involved throughout the plant’s life cycle. Figure 8 An illustration of the mode of action of plant growth promoting bacteria.
Figure 9 Three in vitro setups to study the phenotypic effect of Streptomyces inoculation on Arabidopsis.
Figure 10 Effect Streptomyces sp.1.43 or Streptomyces sp.1.58 on Arabidopsis thaliana root and shoot fresh weights. Figure 11 Effect on the root architecture of Arabidopsis after inoculation with Streptomyces sp.1.43 and Streptomyces
sp.1.58.
Figure 12 Effect of Streptomyces sp.1.43 or Streptomyces sp.1.58 on the primary root length of Arabidopsis thaliana. Figure 13 Effect on the rosette of A. thaliana after inoculation with Streptomyces sp.1.43 or Streptomyces sp.1.58. Figure 14 Growth promoting effect of Streptomyces sp.1.43 or Streptomyces sp.1.58 on the average area of the
individual leaves of A. thaliana.
Figure 15 Effect of inoculation with Streptomyces sp.1.43 or Streptomyces sp.1.58 on A.thaliana on leaf cell parameters.
Figure 16 Effect of Streptomyces strains on Arabidopsis thaliana root and shoot fresh weights for two different setups. Figure 17 Effect of the expression pattern of CYCB1;2-DBOX:GUS in the apical shoot meristem of A.thaliana upon
inoculation with Streptomyces sp.1.43 or Streptomyces sp.1.58.
Figure 18 Effect of the expression pattern of CYCB1;2-DBOX:GUS in the primary root meristem of A.thaliana upon inoculation with Streptomyces sp.1.43 or Streptomyces sp.1.58.
Figure 19 Effect of GFP-tagged Streptomyces sp.1.43 or Streptomyces sp.1.58 on Arabidopsis thaliana shoot and root fresh weight.
Figure 20 Visualization of bacterial colonization patters by Streptomyces sp.1.43::GFP and Streptomyces sp.1.58::GFP on A. thaliana.
Figure 21 Colonization on the root tips of A. thaliana by Streptomyces sp.1.43::GFP or Streptomyces sp.1.58::GFP. Figure 22 Colonization of A. thaliana leaves by Streptomyces sp.1.43::GFP or Streptomyces sp.1.58::GFP.
2 Figure 23 Effect of Streptomyces sp.1.43 on growth parameters of Triticum aestivum.
Figure 24 Overview of the results of plant growth phenotypical and cellular effects of Arabidopsis upon inoculation with Streptomyces sp.1.43 and Streptomyces sp.1.58.
Table 1 Identification of the 18 transformed Streptomyces isolates analysed with BLAST Table 2 Quantification Streptomyces sp.1.43 and Streptomyces sp.1.58
Figure S1 Image of GFP tagged Streptomyces colonies. Figure S2 Leaf series work flow.
Figure S3 Procedure for the kinematic cell analysis.
Figure S4 Effect of the expression pattern of CYCB1;2-DBOX:GUS in the apical shoot meristem of A.thaliana upon inoculation with Streptomyces sp.1.43 or Streptomyces sp.1.58 .
Figure S5 Effect of the expression pattern of CYCB1;2-DBOX:GUS in the apical shoot meristem of A.thaliana upon inoculation with Streptomyces sp.1.43 or Streptomyces sp.1.58 .
Figure S6 Effect of the expression pattern of CYCB1;2-DBOX:GUS in the primary root meristem of A.thaliana upon inoculation with Streptomyces sp.1.43 or Streptomyces sp.1.58.
Figure S7 Effect of the expression pattern of CYCB1;2-DBOX:GUS on the root architecture of A.thaliana upon inoculation with Streptomyces sp.1.43 or Streptomyces sp.1.58.
Figure S8 Signals of stress present in Arabidopsis grown in the sand assay.
Table S1 Parameters of the leaf series analysis for non-inoculated and Streptomyces sp.143 or Streptomyces sp.158 inoculated plants.
Table S2 Independent replicates the performed kinematic cell analysis of Streptomyces sp.143 or Streptomyces sp.158 inoculated Arabidopsis
1
LIST WITH ABBRIVATIONS
A. thaliana: Arabidopsis thaliana
ABA: Abscisic Acid
ACC: 1-AminoCyclopropane-1-Carboxylate Aux: IAA Auxin/Indole-3-acetic acid
CFU: Colony forming units
CO2: Carbon dioxide
Col-0: Columbia-0
DNA: Deoxyribonucleic acid
Dpi: Days post inoculation
GA: Gibberellic Acid
GFP: Green fluorescent protein
HCN: Hydrogen cyanide
IAA: Indole-3-Acetic Acid
ISR: Induced systemic resistance
K: Potassium
MAMPS: Microbe associated molecular patterns
N: Nitrogen
N2: Dinitrogen
NO2: Nitrous oxide
PAMPS: Pathogen associated molecular patterns PGPR: Plant growth promoting rhizobacteria PRR: pattern recognition receptors
qRT-PCR: Quantitative reverse transcriptase – polymerase chain reaction SAR: Systemic Acquired Resistance
Introduction
1
1.INTRODUCTION
1.1 The need of fertilizers and environmental effects
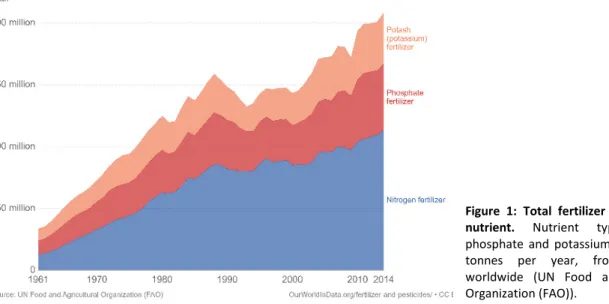
Worldwide crop production is affected by various biotic and abiotic stress factors. In the last decades, chemical fertilizers and pesticides were introduced in agriculture to sustain the growing demand for food and to compensate for the nutrient deficiency of the soil. Chemical fertilizers are compounds containing a high concentration of nutrients required for plant growth (e.g. urea, calcium nitrate, ammonium sulphate, diammonium phosphate) (Pellegrini et al., 2018). Their use worldwide has been increasing since the so-called ‘green revolution’ around the 1950s-1960s (Pellegrini et al., 2018). This method proved to be very successful, the global crop production tripled during the last 50 years (production/area) and is still increasing (Figure 1). However, this excessive use of chemical fertilizers slowly started to display its deleterious effects on the environment across different ecosystems (Springmann et al., 2016; Rosenzweig et al., 2014).
Figure 1: Total fertilizer production by nutrient. Nutrient type (nitrogen, phosphate and potassium), measured in tonnes per year, from 1961-2014 worldwide (UN Food and Agriculture Organization (FAO)).
Consequently, crop yields are impacted by accelerated erosion and depletion of soil organic matter (SOM) (Lal et al.,2009). Agriculture will have to resolve growing crops under suboptimal conditions, as climate change will further increase this challenge (Lobell et al., 2012; Najafi et al., 2018; Ray et al., 2015; Van Ittersum et al., 2013). The following section, will discuss certain effects that illustrate the unsustainability of creating the suboptimal conditions for crops by adding fertilizers and pesticides.
Introduction
2
1.1.1 THE IMPACT ON SOIL
Soils provide multiple ecosystem services: allowing sustained food production, flood regulation, improved air and water quality and provide an essential reservoir for biodiversity (Smith et al., 2016). All soils are subject to various degrees of human disturbance, either directly through land use or indirectly through responses of human-induced global change. The apparent deleterious effects of chemical fertilizers use are soil acidification, leaching, salting, waterlogging, compaction, changes in alkalinity and salinity, ultimately decreasing the soil fertility and making it unsuitable for growing crop plants (Schroder et al., 2011; Cai et al., 2015; Tian et al., 2015; Groffman et al., 2016). As a consequence also the microbial soil community is affected and thereby influences important plant-microbe interactions (Zhong et al., 2010; Hu et al., 2011).
1.1.2 FERTILIZER INDUCED WATER POLLUTION
Chemical fertilizers are applied in large amounts in modern agriculture for improving yield. These chemical fertilizers mainly contain ammonia, nitrates, phosphates, organics, alcohols and heavy metals (Manuel et al., 2014). Despite the applied amounts, only between 50% to 60% of the nutrient inputs are utilized by crops (Smith et al., 2016). A first problem introduces itself when runoff from agricultural soils enters our waterways, the high nutrient concentrations affect the health conditions of freshwater bodies directly and indirectly, pulling the ecosystem towards a polluted, eutrophic state (Mancosu et al., 2015). Consequently leading to possible harmful algal blooms (Mekonnen et al., 2015). A second problem introduces itself when the polluted water travels further downstream and contaminates the coastal areas or ends up in the groundwater, which can cause oxygen depletion, especially in lakes and in coastal zones (Mekonnen et al., 2015). The resulting lack of dissolved oxygen reduces the survival rate of lake and coastal fauna and flora (Biello et al., 2008; Beman et al., 2005; Doney et al., 2010). Lastly, the polluted water can also affect human health, eutrophication causes harmful effects such as blue baby syndrome (acquired methemoglobinemia) when the nitrate is above 10mg/L (Knobeloch et al., 2009). Considering that the annual global flows of nitrogen and phosphorus have now reached more than double than the natural levels, the reduction of the inputs of contaminants and excessive nutrients into downstream aquatic ecosystems is of significant importance (Sutton et al., 2013; Tilman et al., 2002).
Introduction
3
1.1.3 FUTURE CHALLENGES OF CLIMATE CHANGE
Climate change and agriculture are inseparable. It is in the best interest of agriculture to mitigate its effect on the environment and to maintain its yield on the long term. Extreme heat waves and droughts have reduced global harvests of cereals such as maize, wheat and rice in a span of 50 years (Figure 2) (Rosenzwieg et al., 2014). Drought stress is affected by climatic and agronomic factors, and in view of climate change and limiting global freshwater supply it has been predicted that the impacts of drought will further magnify in the future. Approximately 20% to 50% of crop yields are lost due to drought and high soil salinity (Qadir et al., 2014; Springmann et al., 2016). Drought is likely to continue to affect community composition and reduce the abundancy and diversity of bacteria (Hueso et al., 2012; Yuste et al., 2012). It is shown that soil communities that are acclimated to a dry environment are not necessarily able to maintain the same functions during more extreme or more frequent future droughts (Preece et al., 2019). Moreover, the manufacture of chemical fertilizers requires non-renewable resources like coal and petroleum products (Preece et al., 2016). For instance, N is made by splitting atmospheric N2 and requires high inputs of fossil fuels. All these concerns emphasize the search for an alternative strategy that can provide nutrition to the plants in an efficient and sustainable way. When there is no mitigation of this increasing trend this can lead to further reduction in yield (Figure 2) (Rosenzwieg et al., 2014). Nutrient deficiency, drought, salinity, flooding, and temperature are major drivers of the current and future yield losses. In addition, it has been predicted that this decline will accelerate (Rosenzwieg et al., 2014).
B
Figure 2: Mean relative yield change (%). The
yield change from reference period (1980–2010) compared to local mean temperature change (°C) in 20 top food-producing regions for each crop and latitudinal band (A) (Rosenzwieg et al., 2013). Multimethod estimates of global crop yield changes in response to temperature increase of relative change in mean production calculated with different models in wheat, rice, maize and soy for the next 60 years (B) (Zhao et al., 2017).
A
Introduction
4
1.2 Potential of biofertilizers to replace chemical fertilizer
Soil management strategies today are mainly dependent on inorganic chemical-based fertilizers, which cause a threat to human health and the environment. The search of alternatives for chemical fertilizers leads to the potential use of biofertilizers for increasing soil fertility and crop growth promotion in sustainable farming. Biofertilizers are commonly referred to as the fertilizer that contains soil micro-organisms which can include: nitrogen fixers, potassium and phosphorus solubilizers, growth promoting rhizobacteria (PGPRs), endo and ecto-mycorrhizal fungi, cyanobacteria and other useful micro-organisms (Garcia-Gonzalez, 2016; Rashid et al., 2016). Bio-fertilizers can be an important component of integrated agricultural management and have multiple advantages compared to chemical fertilizers. A first advantage is that biofertilizers directly supply their host, thus have no overspill and do not cause harmful effects to the environment in contrast with their chemical opponents which often run-off into the water ways. Secondly, bio-fertilizers play an important role in improving fertility and structure of the soil (Chen et al., 2006). Bio-fertilizers have long lasting effects due to their slow nutrient release, which contain a wide range of nutrients that are often absent in inorganic fertilizers. Consequently, the long term use of bio-fertilizer increases the overall soil fertility, unlike chemical inorganic fertilizers which acidify the soil making it hard for microorganisms to survive (Bhattacharjee et al., 2014). For instance, it has been described that the application of blue green algae (BGA) in combination with Azospirillum displayed significant growth effects. Also biofertilizers with Azotobacter, Rhizobium and Vesicular Arbuscular Mycorrhiza displayed an increase in the yield of wheat plants (Bhattacharjee et al., 2014). Thirdly, weather variables due to the human induced-climate change, such as temperature and precipitation changes, are encouraging for pests and fungus to develop. For instance, plant disease risk can readily shift under climate change (Garett et al., 2016; Gregory et al., 2009). In addition, studies have shown that application of nitrogen fertilizer in some weather conditions cause emission of nitrous oxide which has a global warming effect, potential 296 higher times than that of an equal mass of carbon dioxide (Gruber et al., 2008). Lastly, they are safe in use, have higher production rates and have a low installation cost compared to chemical fertilizers (Gurdeep et al., 2015). In conclusion, the use of biofertilizers leads to improved nutrients and water uptake, plant growth and plant tolerance to abiotic and biotic factors (Yang et al., 2009; Kroll et al., 2017). These potential biological fertilizers would play a key role in productivity and sustainability of soil and also in protecting the environment as eco-friendly and cost effective inputs for the farmers. However, the collective view of functions and interactions between a host and the associated microorganisms is still lacking (Vandenkoornhuyse et al., 2015; Kroll et al., 2017; Bulgarelli et al., 2013). More detailed insights of these mechanisms can help to integrate the use of PGPR for a sustainable agricultural ecosystem.
Introduction
5
1.2.1 PGPR AS A BIOFERTILIZER & BIOAGENT
The soil constitutes a pool of microscopic life forms including bacteria, fungi, actinomycetes, protozoa and algae (Ho et al., 2017). But of these microorganisms, bacteria are by far the most common. A true hotspot of this bacterial life is the rhizosphere, where the density is 10-fold higher than in the bulk soil (Ho et al., 2017). The rhizosphere is the interface between roots and soil, where most of the interactions take place. This narrow zone is different from bulk soil in terms of nutrient availability, supplied by root exudates (Yuan et al., 2018). The rhizosphere is populated by a diverse range of microorganisms. The bacteria colonizing this specific habitat are collectively called rhizobacteria. Numerous species of soil bacteria which flourish in the rhizosphere provide benefits to the plant and facilitate plant growth by diverse mechanisms. These rhizobacteria are collectively known as PGPR (plant growth promoting rhizobacteria) (Figure 3) (Kroll et al., 2017). PGPR have great potential for plant growth and performance, including the potential to increase yields, nutrient uptake and pathogen resistance and increased tolerance towards abiotic stress such as drought and salt stress (Yandigeri et al., 2012; Vurukonda et al., 2018).
Figure 3: Schematic close up of the root interface interactions. The host-microorganisms interactions
and colonization patterns, microorganisms colonizing the rhizosphere (immediately surrounding the root) and phyllosphere (Kroll et al., 2017).
Introduction
6
1.3 Streptomyces spp. as a plant growth rhizobacteria
The genus Streptomyces is a filamentous gram-positive bacteria belonging to the family Streptomycetaceae, order Actinomycetales belonging to the class Actinobacteria (Van der Meij et al., 2018). Streptomyces bacteria are ubiquitous in soil and emit the characteristic earthy smell (Gerber et al., 1965). Almost 1000 Streptomyces species have been identified from the five continents, in diverse terrestrial and aquatic environments and play an important ecological role as composters in soil (Rey et al., 2016; Van der Meij et al., 2018). Besides their well-recognized life form of being soil-dwelling bacteria, some Streptomyces spp. live as plant pathogens such as S. scabies or S. turgidiscabies (Kroll et al., 2017; Bonaldi et al., 2015) others as beneficial symbionts with a wide variety of higher organisms, including insects, plants, and sponges (Seipke et al., 2012).
1.3.1 LIFE HISTORY TRAITS CYCLE
The Streptomyces life cycle begins with spore germination and outgrowth of a feeding substrate mycelium, which is uncommon for bacteria (Seipke et al., 2012). They are well adapted to living in soil where they grow in the form of mycelia (Figure 4) (Seipke et al., 2012). This growth method, coupled with the activities of extracellular hydrolytic enzymes, helps them to gain access to the nutrients locked up in the soil (Chater et al., 2006). When the substrate mycelium becomes nutrient-limited, Streptomyces produce reproductive aerial hyphae, which undergo cell division to form spores (Chater et al., 2006). They evolved filamentous growth ca. 440 Mya when plants were first colonizing land. It has been suggested that this enabled them to successfully colonize plant roots (Chater et al., 2006). The worldwide distribution of Streptomyces might be correlated to their production of spores, which are easily spread and thus could explain their presence in different environments (Souza et al., 2016). One striking aspect of these spores is their resistance to desiccation and heat (McCormick et al., 2011). Among the many features, Streptomyces especially caught the attention as antibiotic producers. Of the total bioactive compounds known to be produced by Actinomycetes, more than half are contributed by the single genus Streptomyces (Berdy, 2005).
Figure 4: An illustration of the life cycle of
Streptomyces spp. starting with spore dispersal,
spore germination, the outgrowth of substrate feeding mycelium, production of reproductive hyphae, segregation and again spore formation (Seipke et al., 2012).
Introduction
7
1.3.2 THE ENDOPHYTIC LIFESTYLE OF STREPTOMYCES: FROM RHIZOSPHERE TO THE
INTERNAL PLANT TISSUES
A variety of endophytes have been isolated from different tissue types in numerous plant species and often multiple endophytes species are found within a single plant (Ali et al., 2017). Endophytes can typically interact with their hosts more effectively than their plant growth-promoting rhizospheric counterparts, as lower concentrations of metabolites secreted by the bacterial endophytes may exert a greater effect on the plant, as they are not affected by soil conditions (Gupta et al., 2000). Endophytic Streptomyces spp. have been isolated from a variety of plants from various vegetative and reproductive plant parts, such as roots, tubers, stems, leaves and seeds (Patel et al., 2018; Ali et al., 2017). Among plant growth-promoting endophytic actinobacteria, most isolates belong to the genus Streptomyces (Qin et al. 2014; Van der Meij et al., 2018). For instance, the relative abundance of Actinobacteria in the endophytic compartment of A. thaliana represented 22% of the total endophytic microbial community and was predominately driven by the family Streptomycetacea (Van der Meij et al., 2018). The following section will discuss the endophytic lifestyle of Streptomyces spp. divided into 2 sections, firstly in terms of the plant immune system as a response to the microorganisms and secondly a brief overview of the colonization traits enabling a successful establishment in the host and the possible transmission patterns of Streptomyces.
1.3.3 THE RHIZOPLANE, A SPECIFIC HABITAT OR A TRANSITIONAL BOUNDARY
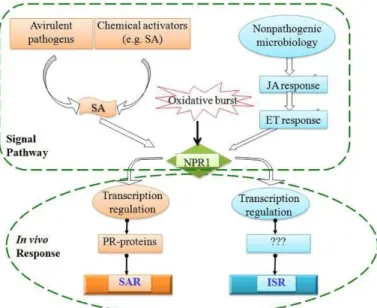
Not unexpectedly, plants and soil bacteria strongly influence each other. Plants emit root-exudates such as flavonoids, strigolactones, terpenoids, organic compounds such as sugars, amino acids, ions growth factors and enzymes, which can act as nutritional resources, chemo-attractants or chemo-repulsants and hereby support, restrict, or terminate microbial growth and activity (Huang et al., 2014; Yuan et al., 2018). Consequently, the root-exudates of the host shape the microbial community structure and have a significant influence on plant root–microorganism interactions. From a plants perspective, the interaction may be beneficial, harmful or neutral. Therefore it is in the plant best interest to be continuously prepared for a possible attack. The first line of defense includes the physical barriers such as rigid cell walls, trichomes, thick cuticles and chemical weapons (Osbourn, 1996). The second line of defense can be activated when pattern recognition receptors (PRR) of plants recognize the microbial pathogens due to general features such as flagellin, lipopolysaccharides, peptidoglycan, β‐glucans and chitin. These recognizable structures are referred to as pathogen or microbe associated molecular patterns (PAMPS and MAMPs) (Zamioudis et al., 2012). MAMPs trigger a local defense response in the roots, which can be translated into systemic defense responses (Pieterse et al., 2014). Plant growth-promoting bacteria are known to induce non-specific plant defense mechanisms known as induced systemic resistance (ISR) and are controlled by hormones such as salicylic acid, jasmonic acid and ethylene (Pieterse et al.,204; Millet et al., 2010; Vos et al., 2013) (Figure 5). Interestingly this is in contrast with the systemic acquired resistance (SAR) which is most commonly induced by pathogens and involves salicylic acid signalling (Vos et al., 2013).
Introduction
8 This indicates that beneficial rhizosphere microorganisms have the capacity to counter the immune response of plants (Pieterse et al., 2014; Zamioudis et al., 2012). It is suggested that signalling molecules emitted by microorganisms can modulate plant gene expression, defense responses and developmental processes (Davies et al., 2005; Conn et al., 2008). For example, Nod and Myc, these factors are found to be released by rhizobia and are examples of microbial signalling molecules. These suppress salicylic acid-dependent defense responses and initiate a common symbiosis signalling pathway (Oldroyd et al., 2013; Zamioudis et al., 2012). Additionally, it has been described that colonization of rice with the endophytic bacteria Azoarcus sp. is independent of the common symbiotic signalling pathway shared by root nodule symbioses (RNS) and arbuscular mycorrhizal (AM) indicating that certain endophytic bacteria could have different tools of colonization (Chen et al., 2015). For instance, inoculation with Streptomyces sp. resulted in induced systemic resistance against pathogenic fungi such as Botrytis cinerea and Heterobasidion abietinum (Lehr et al., 2008) and F. oxysporum (Shobha et al., 2012). Over the last decade, the roles of both plant-associated microbes and the host in ecosystem function have been recognized and research has progressed significantly in understanding the complex mechanisms that play a role in the root interface and how plants shape an unique rhizosphere microbial community.
Figure 5: An illustration of the immune defence response of the plant host when encountering microorganism and the different responses (Chan
et al. , 2013).
1.3.4 PLANT COLONIZATION AND CHANGES BY ENDOPHYTIC PGPR.
During millions of years of coevolution with plants, bacteria have been equipped with necessary traits to colonize their host rhizosphere or internal tissues (endosphere). Traits that enable bacteria to plant internal tissues include, motility, chemotaxis, production of cell-wall degrading enzymes and lipopolysaccharide formation (Piromyou et al., 2015). Initially, Streptomyces spp. are attracted to the rhizosphere because of its nutrient rich nature, and chemotaxis by the root exudates, as described above.At this point, the increased population rates coupled with bioactive compound production are determinants for a successful rhizosphere establishment. These capacities play an important role in overcoming competition and can be partially explained by the ability to produce antibiotics and other bioactive compounds that give an ecological advantage, allowing Streptomyces to colonize niches (Souza et al., 2016).
Introduction
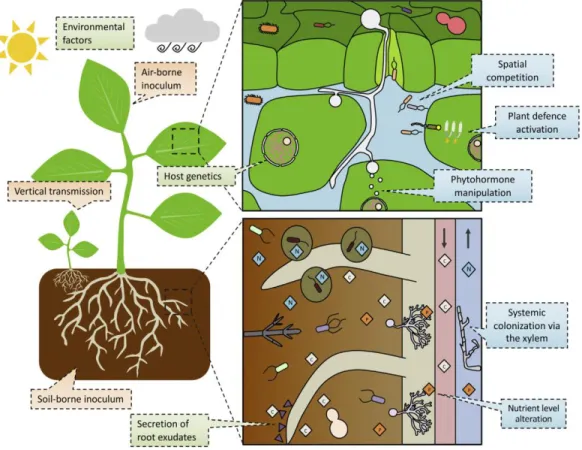
9 Streptomyces colonizing the rhizosphere can further translocate to the internal tissues (Compant et al., 2005). This ability is associated with the secretion of lytic enzymes such as chitinases, glucanases and cellulases, most Streptomyces spp. are producers of such extracellular enzymes (Chater et al., 2006). Typical hot spots for bacterial colonization are lateral root emergence sites through primary and lateral root cracks (Frank et al., 2017). Alternative entry points can be through natural openings such as stomata, cracks occurring as a result of plants growth or wounds generated by wind and/or pathogen attacks (Schrey et al., 2008; Santoyo et al., 2016). For example, Streptomyces sp. showed the ability to enter inside a wheat embryo tissue and then into the emerging radicle (Coombs et al., 2003). The widespread ability of Streptomyces spp. to degrade large organic molecules through the activity of extracellular enzymes provides them with the possibility of colonizing diverse habitats. In addition, these enzymatic activities make them good antagonists of plant pathogens. If the colonization was successful and Streptomyces sp. has entered through the root system, it is suggested that they can migrate upwards into the stems where they seem to proliferate and also reach the leaves as seen by detectable numbers in the leaf tissue (Figure 6) (Patel et al., 2018). Hereby also reducing the severity of aerial infection by fungal hyphal fragments in wheat and sorghum (Patel et al., 2018; Coombs et al., 2003). Furthermore, it has been described that bacteria living inside plant tissues as endophytes can be vertically transmitted from generation to generation via the seeds (Frank et al., 2017; Santoyo et al., 2016).
Figure 6: Schematic representation of the bacterial distribution and colonization patterns. On the left: representation of the bacterial distribution and colonization patterns in the rhizosphere (around) and endosphere (inside) of a plant root. The emerging sites of lateral roots are among the hotspots of bacterial colonization. Arrows represent the translocation of bacteria inside the xylem and phloem. On the right: Colonization routes for bacterial endophytes across the life cycle of the host. (A) Vertical transmission via seed; (B) Colonization of the spermosphere; (C)Colonization of developing reproductive organs via the shoot apical meristem as part of vertical transmission; (D) Colonization of root from soil; (E) Colonization of leaves though stomata after transmission via air; (F) Transmission via sap-feeders; (G) Transmission to flowers via pollinators (Liu et al., 2017; Frank et al., 2003).
Introduction
10
1.3.5 MODES OF ACTION: PLANT GROWTH PROMOTION
PGPR enhance plant growth through various mechanisms. For simplicity these will here be divided into three main groups. The first category is biofertilization, including increasing the nutrient availability for plants by nitrogen fixation, mineralization of organic compounds and solubilization of mineral nutrients. The second category is bio-stimulation, those are mechanisms that can alter and stimulate plant physiology by regulating and modulating phytohormones levels or growth regulators, hereby promoting plant growth. Certain bacteria have the capacity to protect the host against possible pathogens, these are discussed in the third and last category. Various mechanisms of action have been established for Streptomyces. However, some traits are known to be species-specific (Alekhya et al., 2016). In the following paragraphs some important mechanisms of PGPR are discussed with focus on the current knowledge of Streptomyces, here divided into three main categories biofertilization (1) bio stimulation (2) and bio protection (3) (Figure 8).
Biofertilization: Facilitation of nutrient uptake
Plant growth requires nitrogen as an essential element. However, the plant system is not capable of directly utilize atmospheric nitrogen. They rely on more bioavailable forms of nitrogen, such as ammonium and nitrate. Next to nitrogen, phosphorus is the second most important nutrient for plants. Phosphorus is a crucial component of major organic molecules such as nucleic acids and membrane phospholipids. Phosphate can be found in soils in the form of inorganic phosphate (Pi), which has low availability for plants. A third essential macronutrient for plant growth and development is potassium (Hafsi et al.,2014; Cakmak et al., 2005). Consequently, these essential nutrients correlate with the utilization of chemical fertilizers, with nitrogen fertilizers as the leader followed by phosphate and potassium fertilizers (FAO)( Figure 1). To cope with nutrient limitations, microorganisms can provide an alternative, by the use of nitrogen-fixing, phosphate solubilisation and potassium solubilizing microorganisms. Studies reported that Streptomyces spp. isolates had a strong nitrogen-fixing ability and the majority showed a nitrogen-fixing gene, which enables the capacity to convert atmospheric nitrogen into ammonia (Dahal et al., 2017). Additionally, Streptomyces spp. secretes chelating siderophore-like molecules, which leads to the solubilization of insoluble phosphates (Jog et al., 2014). Numerous Streptomyces spp. isolated from soils have been found to be specialized phosphate solubilizers and hereby promoting plant growth (Sadeghi et al., 2012; Jog et al., 2014). Lastly, studies on potassium solubilizing capacities of Streptomyces sp. found the capacity to release potassium from silicate minerals, thus a potential aid when nutrients limitations harm the yield of crops (Liu et al., 2016).
Introduction
11
Bio-stimulation: Production of Phytohormones and enzymes
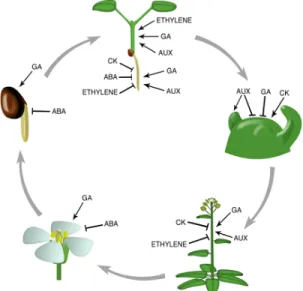
Phytohormones or plant growth regulators play a crucial role in the regulation of plant cells and physiological processes throughout the plant’s life cycle (Figure 7). Phytohormones are organic substances, which at low concentrations (< 1 mM), promote or modify the growth and development of plants throughout its life cycle (Damam et al., 2016). Common groups of phytohormones include gibberellins, cytokinins, abscisic acid, ethylene, brassinosteroids, and auxins. Production of these phytohormones can also be induced or produced by certain microbes, including fungi and bacteria such as PGPR (Daman et al., 2016). The involvement of auxin and cytokine produced by microorganisms or plants during their interaction with beneficial microbes will be covered in the following section.
Figure 7: Illustration of the different phytohormones involved throughout the plant’s life cycle (Weiss et al., 2007).
Phytohormone production
Auxins play vital roles in cell division and elongation, initiation of root systems, and leaves (Zhao et al., 2010). Phytohormones regulate the gene expression of plant defense and eventually trigger the production of defense molecules, such as pathogenicity-like proteins (Beneventi et al., 2013). Generally, roots are sensitive to fluctuations in IAA and its response to increasing amounts of exogenous IAA extends from elongation of the primary root, formation of lateral and adventitious roots (Sureshbabu et al., 2016). Certain bacteria and fungi have the capacity to produce auxins, the most common naturally occurring auxin is indole-3- acetic acid (IAA), which can be produced through at least three different tryptophan-dependent pathways (Duca et al., 2014). Streptomyces spp. are widely described to produce IAA and hereby improving plant growth in tomato (Goudjal et al., 2013), wheat (Sadeghi et al., 2012), rice (Harikrishnan et al., 2014; Gopalakrishnan et al., 2013), sorghum (Gopalakrishnan et al., 2013), chili (Suryanto et al., 2017) and banana (Kavino et al., 2011). Vice versa, IAA triggers cell differentiation, hyphal elongation, and sporulation in Streptomyces atroolivaceus (Matsukawa et al., 2007). It is important to note that not all the IAA-producing bacteria are beneficial to plants.
Introduction
12 Interestingly, many plant-beneficial bacteria produce IAA via the indole-3-pyruvate (IPyA) pathway, whereas many pathogenic bacteria mainly synthesize IAA via the indole-3-acetamide (IAM) pathway (Duca et al., 2014). Another phytohormone is cytokine, which promote cell division, cell enlargement, and increase root surface area through intense proliferation of adventitious and lateral roots (Hodge et al., 2009). For instance, in soybean, the microbial production of cytokinins is found to enhance plant growth (Garcia de Salamone et al., 2005). Cytokinins are also known to mediate signal exchange from roots to shoots under environmental stresses (Bielach et al., 2017).
ACC Deaminase production
A number of PGPR contain the enzyme 1‐aminocyclopropane‐1‐carboxylate (ACC) deaminase (Glick et al., 1998). This enzyme can cleave the plant ethylene precursor ACC, and thereby lower the level of ethylene in a developing or stressed plant. ACC is the immediate precursor of ethylene in plants (Reid et al., 1985). For many plants an high peak in the ethylene level is required to break seed dormancy (Esashi, 1969). However, after the germination is established, a sustained high level of ethylene would inhibit root elongation (Hodge et al., 2009). PGPR that contain the enzyme ACC deaminase act as a mechanism for ensuring that the ethylene level does not exceed to a point where initial root growth is inhibited. Consequently, plants inoculated with ACC deaminase‐containing PGPR are more resistant to stressful conditions such as heavy metals (Dimpka et al., 2008), phytopathogens (Ali et al., 2014), and drought and high salt levels (Negrão et al., 2017; Jaemsaeng et al., 2018; Penrose et al., 2003). In return the bacterial strains can utilize ACC as a nitrogen source (Glick et al., 1998). It has been observed that Streptomyces sp. strain PGPA39 producing ACC deaminase can mitigate salt stress and promote growth in tomato plants (Palaniyandi et al., 2014).
Bioprotection: biotic and abiotic stress management
Biotic stress management
As mentioned above agricultural productivity is affected worldwide due to anthropogenic and climate change-induced abiotic stresses. Abiotic stress factors include water deficit, salinity, extreme temperatures, and heavy metals. Associations with PGPR provide other means for enhanced resistance from diverse abiotic stress conditions of the plant such as drought (Yandigeri et al., 2012), salinity (Sadeghi et al., 2012), and metal toxicity (Ashraf et al., 2017). Here some of the foremost abiotic stress factors will be discussed in terms of the plant response and the current known mechanisms of PGPR to counter the specific stress.
Drought stress
More crop productivity is lost due to water scarcity than any other abiotic factor (Mancosu et al., 2015). Several mechanisms of drought resistance in plants have been proposed to be induced by PGPR, by producing exopolysaccharides (EPS) (Kaushal et al., 2016) 1-aminocyclopropane-1-carboxylate (ACC) (Zahir et al., 2008), volatile organic compounds (VOCs) (Park et al., 2015) and phytohormones like abscisic acid (ABA) (Porcel et al., 2014). The involvement of abscisic acid (ABA) in responses to abiotic stresses and in particular to drought is well characterized (Vurukonda et al., 2018). When the roots sense a decrease in soil water in the rhizosphere region, ABA biosynthesis in the root tips is increased, where after it is transported to the leaves leading to induction of stomatal closure (Davies et al., 2005).
Introduction
13 The second mode of action is inducing accumulation of osmolytes and antioxidants (Vurukonda et al., 2018). In addition, it has been shown that the production of phytohormones such as auxins, enhanced plant growth promotion of wheat in the stressed soil by Streptomyces coelicolor DE07, S. olivaceus DE10 and Streptomyces geysiriensis DE27 (Yandigeri et al., 2012).
Salinity stress
Crop plants are very sensitive to soil salinity and it severely limits plant productivity. Excess salt triggers ion toxicity and ion imbalances in plants, thus inducing metabolic imbalances and hyperosmotic stress that cause detrimental effects on plant growth and development (Negrão et al., 2017). Inoculation of wheat with Streptomyces sp. significantly increased the growth and germination rate (Sadeghi et al., 2012). Confirming these findings Streptomyces sp. enhanced salt tolerance in rice by lowering stress-induced ethylene via the action of ACC deaminase (Jaemsaeng et al., 2018).
Heavy metals
Heavy metals such as zinc (Zn), arsenic (As), chromium (Cr), cadmium (Cd), mercury (Hg), copper (Cu), nickel (Ni), and lead (Pb) represent a tricky challenge to plant and microbial growth if elevated above tolerance levels. The most widespread symptoms of heavy metal toxicity is a reduction in plant growth (John et al., 2012) including leaf chlorosis, necrosis, turgor loss, a decrease in the rate of seed germination, and a crippled photosynthetic apparatus, often correlated with progressing senescence processes or with plant death (Ebbs et al., 1997; Morkunas et al., 2018). Plants and their phytoremediation potentials are usually influenced by the toxic effects of metals in soil (Chaves et al., 2011). The application of certain PGPR can facilitate the phytoremediation of the host (Ashraf et al., 2017; Juwarkar et al., 2012). Studies on the microbial communities of soils highly contaminated from mining activities have shown that high proportions of Streptomyces and Bacillus are present, revealing that these organisms are better adapted to contaminated soils than other common inhabitants of soils (Kothe et al., 2010). A wide diversity of genera of PGPR have evolved unique ways to use, transform and mobilize heavy metals to make them tolerable for the host, or even usable for the microorganisms themselves (biosorption) (Alvarez et al., 2017; Soburia et al., 2017). One of the described mechanisms to do so is by siderophores, these help to reduce the stress imposed on plants by high levels of heavy metals and have been found in Streptomyces spp. (Ahemad and Kibret, 2014; Khamna et al., 2009; Dimpka et al., 2008; Sessitsch et al., 2013).
Biotic stress management
Another asset of certain PGPR strains is the capacity to act as biocontrol agents, which can act in two major important ways. Firstly they have the ability to perform antibiosis, parasitism, or competition with the pathogen for nutrients and space (Patel et al., 2018). Secondly, they may also induce disease resistance in the host plant that they colonize, acting along different steps of the infection process (Katiyar et al., 2018). The potential of Streptomyces spp. as biocontrol agents against numerous pathogens are confirmed (Cao et al., 2016). For example, Streptomyces protects rice against Pyricularia oryzae in (Law et al., 2017), likewise against Pectobacterium carotovorum in tomato (Dias et al., 2017), Sclerotinia sclerotiorum in lettuce (Chen et al., 2016), and against Fusarium graminearum in wheat (Palazzini et al., 2017). In addition, it has been confirmed that the genus Streptomyces spp. produces a wide variety of fungal cell wall degrading enzymes such as glucanases, chitinases, and peptidases (Chater et al., 2006).
Introduction
14
Volatile compounds
Another important mechanism to improve the resistance against plant pathogens, is the ability of certain PGPR to release an array of volatile organic compounds (VOCs) (Ryu et al., 2003; Park et al., 2015). The chemical structures of several VOCs are typically small molecules such as alkenes, alcohols, benzenoids, aldehydes, ketones and terpenes. In 26 Streptomyces species, 120 different VOCs were identified comprising alkanes, alkenes, alcohols, esters, ketones, sulphur-containing compounds and terpenoids (Schöller et al., 2002, Wan et al., 2008). These microbial VOCs are believed to play an important role in regulating plant growth and stress resistance. A few compounds have been studied which affect root architecture, plant immunity, and expression of plant genes involved in defense and hormonal signalling pathways (Bitas et al., 2013). Some of these VOCs significantly enhanced plant shoot and root biomass of Arabidopsis thaliana (Vespermann et al., 2007; Ryu et al., 2003;). It has also been shown that VOCs of several Streptomyces spp. isolates inhibiting growth of potential competitors. For instance, resistance against hyphal growth of R. solani (Cordovez et al., 2015), against Aspergillus flavus, Aspergillus ochraceus, Aspergillus niger, and Penicillum citrinum (Wang et al., 2013), against Rhizoctonia solani PTRRC-9, Pyricularia grisea PTRRC-18, Bipolaris oryzae PTRRC-36 and Fusarium fujikuroi PTRRC-16 in rice (Boukaew et al., 2013). In addition, it has been identified that certain endophytic Streptomyces species produce HCN, a volatile antifungal compound, which showed contribution to the suppression of Fusarium graminearum (Palazzini et al., 2017). In conclusion, volatile producing Streptomyces spp. have the potential to be used as biocontrol agents and enhance plant health.
Figure 8 : An illustration of the mode of action of plant growth promoting bacteria. Conceptual presentation of PGPR plant growth promoting and abiotic stress alleviating traits of Streptomyces (Grover et al., 2016).
Introduction
15
1.4 Plant growth mechanisms of Arabidopsis thaliana.
After discussing the mechanisms of microorganisms that stimulate growth promotion of the host, the following section will discuss the growth mechanisms and the development of the host itself. A. thaliana is widely used as a model system in plant biology due to its compact size, known genome and easy to transform qualities (Meinke et al., 1998). A. thaliana serves as a convenient model for addressing fundamental questions of biological structure and is therefore often tested in growth promotion upon contact with PGPR.
1.4.1 Leaves growth mechanisms in Arabidopsis thaliana.
Growth and development of Arabidopsis and the involved genes are widely described. Here we mainly focus on the development of the leaf which can be divided into two major phases. The first phase is dominated by proliferative activity and the second phase by cell expansion (Kalve et al., 2014). During the phase cell proliferation occurs throughout the entire primordium and generates new cells, the size of which remains relatively constant and small. In the second phase, cell division in the developing leaves has decreased and further growth is mainly achieved by cell expansion, resulting in a large increase in cell size. In Arabidopsis the period of cell expansion is associated with endoreduplication (Kalve et al., 2014). Arabidopsis leaves, cells enter into the endoreduplication process as a consequence of decreasing auxin concentrations (Ishida et al., 2010). In contrast ethylene and GAs are hypothesized to positively affect endoreduplication (Gendreau et al., 1999). In addition, JAs were shown to inhibit cell proliferation in Arabidopsis leaves (Noir et al., 2013).
1.4.2 Regulated cell division in the leaves
Although Arabidopsis produces different organs throughout their life cycle, little is known about the factors that regulate the timing of developmental processes and the rate of development. It is reported that the restricted expression of FUS3 to the epidermis is sufficient to control foliar organ identity in A. thaliana by regulating the synthesis of two hormones, abscisic acid and gibberellin. FUS3 expression is influenced by the patterning hormone, auxin, and therefore acts as a nexus of hormone action during embryogenesis (Gazzarrini et al., 2004).
1. 5 From the lab to the farm.
Taken together, Streptomyces spp. as biofertilizer present an attractive alternative to chemical fertilizers, pesticides, and supplements, which may result in a significant increase in agricultural plant growth and disease control in the field. Nevertheless, the proportion of registration of Streptomyces spp. containing products that are currently commercialized as biofertilizer is very low. This is because even though bio-fertilizer have many positive aspects, the use can sometimes not lead to the expected positive results in the field. Some of the biofertilizers bottlenecks that still needs to be resolved in order to obtain an effective inoculation, include the unavailability of suitable strains, unavailability of suitable carrier, short shelf life or hostile conditions before usage (Chen et al., 2006; Bhattacharjee et al., 2014). Nevertheless, the described agricultural usefulness of Streptomyces will no doubt stimulate future research to better understand the complex relationship between Streptomyces symbionts and their plant hosts and lead to decreasing loss of diseases and abiotic stresses. In conclusion, these potential biological fertilizers could play a key role in productivity and sustainability of soil and protect the environment in an eco-friendly and cost effective way.
Objectives
16
2. OBJECTIVES
The excessive use of fertilizers and pesticides has a significant impact on the environment (Springmann et al., 2016). An alternative is the use of plant growth-promoting rhizobacteria (PGPR). These represent a wide variety of soil bacteria which may grow in, on, or around plant tissues and are beneficial for the growth and survival of the plant (Ho et al., 2017; Vurukonda et al., 2018). However, the collective view of functions and interactions between a host and the associated microorganisms is still lacking (Vandenkoornhuyse et al., 2015). Two Streptomyces strains from the bacterial collection of the rhizosphere lab (VIB-UGent Centre of plant biotechnology and bioinformatics) have been confirmed for their growth promoting potential for Arabidopsis, wheat and maize. Furthermore, endophytic Streptomyces spp. have been isolated from a variety of crops of plants from various vegetative and reproductive plant parts, such as roots, tubers, stems, leaves and seeds. Streptomyces spp. is therefore good model to study endophytic colonization (Qin et al. 2011; Van der Meij et al.,2018 Ali et al., 2017).
Due to confidentiality, the strains will be referred to as Streptomyces sp.1.43 and Streptomyces sp.1.58 in this study. Streptomyces are known to form close associations with plants as endosymbionts and for their growth promoting capacities (Bérdy, 2005). We hope to achieve a better understanding of the mechanism that controls the complex interaction with the rhizosphere bacterium Streptomyces sp. 1.43 and Streptomyces sp.1.58 and its effect on the growth of Arabidopsis thaliana and Triticum aestivum.
In a first work package, the phenotypic effects of inoculation with Streptomyces sp.1.43 or Streptomyces sp.1.58 on the root and shoot growth of A. thaliana and T. aestivum grown in sterile sand will be evaluated. When Arabidopsis plants inoculated with Streptomyces sp.1.43 or Streptomyces sp.1.58 show a significant growth enhancement, an additional leaf series and a kinematic cell analyse will be carried out to further investigate the mechanism on the cellular level. In addition, to detect differences on a molecular level, the expression levels in CYCB1;2-DBOX:GUS transgenic Arabidopsis seeds, will be analysed upon inoculation of Streptomyces sp.1.43 or Streptomyces sp.1.58. Statistical analyses will be used to detect significant differences of the measured parameters between the inoculated and non-inoculated plants.
In a second work package, Streptomyces will be GFP-tagged in order to visualize the colonization pattern on Arabidopsis. The GFP-tagged strains will be screened for their sustained phenotypical growth effect on Arabidopsis before evaluating colonization efficacy using confocal microscopy. The overall aim of this study is to evaluate the growth promoting effect of Streptomyces sp.1.43 or Streptomyces sp.1.58 on A. thaliana and to reveal their colonization pattern. These findings could guide efforts to improve sustainable breeding strategies and a more efficient use of biofertilizers.
17
3.MATERIALS AND METHODS.
3.1 Growth effect analyses on Arabidopsis thaliana
3.1.2 PLANT GENOTYPES AND GROWTH CONDITIONS
Wild-type Colombia (Col-0) and transgenic Arabidopsis thaliana CYCB1;2-DBOX:GUS were used in this study. Seeds were surface sterilized (protocol 7.2.9) and sown in 6-well plates containing sterile sand (8-10g) irrigated with (6-7mL) Hoagland solution (protocol). The seeds were vernalized at 4 °C in the dark during 2 days before being inoculated.
3.1.3 BACTERIAL STRAINS AND CULTURE CONDITIONS
Streptomyces sp.1.43 and Streptomyces sp.1.58 are a part of the bacterial collection of the rhizosphere lab (VIB-UGent). The strains were grown in liquid R2A medium (protocol) at 28°C in aerobic conditions for 4 days before inoculation of A. thaliana. The (CFU/mL) was quantified by plating a serial dilutions of the bacterial suspension (grown in liquid for 4 days) and counting the number of colonies grown after 2 days of incubation at30°C. The quantification experiment was repeated twice in three replicates before inoculation and after 21 dpi.
3.1.4 ROOT INOCULATION OF A. THALIANA
The seedlings were inoculated by pipetted 60 µL of the bacterial suspension directly on the A. thaliana seeds. Control seeds were treated with the same volume of R2A. After inoculation the 6-well plates were placed in the tissue culture room (21°C, long day 6u-22u) for 21 days. Two designs were used for the 6-well plates during the experiments. In the first design every 6-well plate contained 3 non-inoculated plants in the first row and 3 non-inoculated plants with Streptomyces sp. 1.43 or Streptomyces sp. 1.58 in the second row. While the second design worked with full 6-well plates of inoculated plants and full 6-well plates of non-inoculated plants.
3.1.5 PHENOTYPICAL ANALYSIS OF ROOT AND SHOOT FRESH WEIGHT
A. thaliana plant growth was assessed at 21 days after inoculation (dpi). A photo of each well plate was made before the plants shoot and root fresh weight was measured using an analytical balance. Afterwards the plants were transferred to square petri dishes containing agar (1%). Excel was used to perform two-sided student’s t-tests to detect significant differences between non-inoculated and inoculated plants.
3.1.6 PHENOTYPICAL ANALYSIS OF THE ROOT ARCHITECTURE OF A. THALIANA
A. thaliana plant growth was assessed at 21 days after inoculation (dpi). The plants were cleaned with multiple cleaning steps and were transferred to square petri dishes containing agar (1%). Scans were taken and ImageJ was used to measure the apical root length of A.thaliana for all treatments. Excel was used to perform two-sided student’s t-tests to detect significant differences between non-inoculated and non-inoculated plants.
Material and Methods
18
3.1.7 PHENOTYPICAL ANALYSIS OF THE ROSETTE OF A. THALIANA
In a following procedure each leaf was dissected from the rosette and placed on square agar (1%) containing petri dishes. Leaves were arranged according to age, with the oldest leaves (the cotyledons) on the left and the youngest on the right and photographed. For further analysis 4 different macro tools were used in ImageJ (the macros were provided by Dr. Nathalie Gonzalez of the research group of prof. Dr. Dirk Inze, UGent VIB). These macro’s enabled to measure each surface area the separate leaf. Excel was used to perform two-sided student’s t-tests to detect significant differences between non-inoculated and inoculated plants.
3.1.8 GUS STAINING
Arabidopsis thaliana seeds from the used GUS reporter line CYCB1;2-DBOX:GUS were surface sterilized. After the 21dpi the seedlings were harvested and stained following the staining protocol (protocol 7.2.5) Incubation occurred at 37 ° C for 2 hour. The staining was stopped by transferring the plants to NT buffer. Seedlings were placed on slides with 50% glycerol, the visualization was done with a light microscope.
3.2 Colonization analyses
3.2.1 TRANSFORMATION OF STREPTOMYCES
E.coli ET12567 (pUZ8002) (provided by professor Paolo Cortesi, Department of Food, Environmental and Nutritional Sciences (DeFENS), University of Milan) and was used as donor strain for the conjugation. Plasmid (Et12567/pUB307 and ET12567/oriT) that carries the GFP gene under a constitutive promoter and an apramycin resistance marker, was conjugated into Streptomyces sp.1.43 and Streptomyces sp.1.58. The plasmid was transformed into the donor strain E.coli ET12567 (pUZ8002) and conjugated into recipient Streptomyces strains (protocol 7.2.6). The following bacterial strains were used; Streptomyces sp.1.43 and Streptomyces sp.1.58 as recipient: sensitive to apramycin, resistant to nalidixic acid and E.coli with as donor strain: resistant to apramycin, resistant to nalidixic acid. The transformants were screened for GFP expression and tested for their growth promoting capacities on A. thaliana. For microscopic observations, the transformed Streptomyces strains were grown on R2A medium and incubated at 28 °C for 4 days. Subsequently, they were visualized to confirm the expression of GFP in transformants. Only transformants that show no difference compared with the wild type were used in further experiments.
3.2.2 BACTERIAL IDENTIFICATION
To confirm the identity of the Streptomyces strains, a 16S sequencing protocol (protocol 7.2.3 ) was performed. Genomic DNA of wild-type and the transformed Streptomyces strains were extracted. The PCR products were purified using the magnetic bead purification kit and sequenced by Eurofins.
Material and Methods
19 Afterwards the sequences were trimmed and aligned. The 16S rRNA sequences were identified by comparing the partial 16S rRNA sequences of the isolated strains with those available in the GenBank database using BLAST program.
3.2.3 VISUALISATION OF COLONIZATION
Surface sterilized Col-0 seeds were vernalized and placed in the growth chamber 21°C, day/night conditions long day (6u-22u) on gelzane (protocol 7.2.7 ) plates before inoculation. After 4 days, five seedlings of the same size were chosen to be inoculated with the GFP tagged strains by a adding a bacterial solution (20µl) directly to the roots. To observe Streptomyces sp.1.43 and Streptomyces sp.1.58 colonization pattern in A.thaliana, the roots were treated with (PI) to visualize plant cell walls. The shoots were washed and mounted in water before visualisation. The samples were analysed with a confocal laser scanning microscope at different time points post inoculation.
3.3 Preliminary studies Triticum aestivum
3.3.1 INOCULATION AND GROWTH CONDITIONS OF WHEAT
Streptomyces sp. 1.43 and Streptomyces sp. 1.58 were grown in liquid R2A medium. The pellet (centrifugation for 10 min at 15000rcf) of 4 old days bacterial culture was washed, resuspended and diluted in PSB (Phosphate Buffered Saline). The bacterial suspension was diluted in PBS (protocol 7.2.4) before inoculation. Sterilized wheat seeds (protocol 7.2.10) were shaken in the bacterial solution for 3 hours. The inoculated wheat seeds were transferred into pots containing sand (Fountain blue sand) and were irrigated from the bottom with 500mL Hoagland solution every 4 days for 14 days.
3.3.2 PHENOTYPICAL ANALYSIS OF ROOT AND SHOOT FRESH WEIGHT
At 14 dpi the length of the roots and shoot was measured with a ruler, measuring from the hypocotyl to the leaf tip, or root tip. The shoots and root fresh weights were measured using an analytical balance. Excel was used to perform two-sided student’s t-tests to detect significant differences between non-inoculated and non-inoculated plants.
Results
21
4. RESULTS
4.1 Phenotypic response of Arabidopsis thaliana upon
inoculation with Streptomyces sp.1.43 or Streptomyces sp.1.58.
4.1.1 Effect on the shoot and root fresh weight
Streptomyces sp.1.43 and Streptomyces sp.1.58 are a part of the bacterial collection of the rhizosphere lab (VIB-UGent) and were selected for their capacities as potential PGPR from previously conducted studies on wheat and Arabidopsis (data not shown). Due to confidentially they are referred to as Streptomyces sp.1.43 and Streptomyces sp.1.58 in this study. As a next step towards a more natural situation, Arabidopsis seeds were inoculated with Streptomyces sp.1.43 or Streptomyces sp.1.58 and grown in sterile sand for 21 days. Different setups were used to evaluate to phenotypical effects of the Streptomyces strains on Arabidopsis (Figure 9).
Figure 9: Three in vitro setups to study the phenotypic effect of Streptomyces sp.1.43 or Streptomyces sp.1.58 inoculation on Arabidopsis. The experimental setup of previously conducted studies: A.thaliana grown in square petri-dishes containing agar based medium inoculated with Streptomyces sp.(b1.43) or Streptomyces sp.(b1.58) compared to control (mock) plants (Adopted from master thesis Amber Lampens) (A). This study used two different setups. The first experimental setup is shown in (B). A.thaliana seeds were inoculated and grown in 6-well plates in sterile sand, with the first row containing non-inoculated plants (mock) and the second row inoculated plants with Streptomyces sp.1.43 or Streptomyces sp.1.58. The second experimental setup is shown in (C), 6-well plates containing 6 inoculated plants. Scale bar represents 1 cm.
A
C
B
1.43
1.43
1.43
mock
Results
22 For the evaluation of the phenotypical effect of Streptomyces sp.1.43 or Streptomyces sp.1.58 on Arabidopsis the first setup was used (Figure 9B). Inoculation with Streptomyces sp.1.43 or Streptomyces sp.1.58 results in a significant increase of both shoot and root fresh weight of Arabidopsis compared to the control plants (mock) after 21 days in the sand assay (Figure 10).
Figure 10: Effect Streptomyces sp.1.43 or Streptomyces sp.1.58 on Arabidopsis thaliana root and shoot fresh weights. The root fresh weight (A) and shoot fresh weight (B) of A. thaliana inoculated with Streptomyces sp.1.43 or Streptomyces sp.1.58 compared to non-inoculated plants (mock). Exemplary pictures of Arabidopsis grown in 6-well plates, inoculated with Streptomyces sp.1.43 (C) or Streptomyces sp.1.58 (D). Error bars represent the standard error. Statistical analyses were performed with a student t-test (*p < 0.05, ** p < 0,01, ***p < 0,001). These experiments were repeated three times independently with 18 plants for each treatment. Scale bar represents 1 cm.
Sho
ot
fr
esh
wei
gh
t
(g
)
mock mock 1.43 1.58A
B
C
***
***
0,000
0,002
0,004
0,006
0,008
0,010
0,012
0,014
mock
inoculated
1.43
1.58
***
***
0,000
0,002
0,004
0,006
0,008
0,010
0,012
0,014
mock
inoculated
R
o
o
t
fr
es
h
w
ei
gh
t
(g)
1.43
1.58
D
Results
23 The average of the shoot fresh weight of inoculated A. thaliana plants with Streptomyces sp.1.43 is 0.0107
±
0.0026g and the average root fresh weight is 0.0119±
0.0039g, while the average of the shoot fresh weight of non-inoculated plants (mock) is 0.0077 ±0.0023g and the average root fresh weight is 0.0067 ±0.0031g. Inoculated A. thaliana plants with Streptomyces sp.1.58 show on average a shoot fresh weight of 0.0094±
0.0017g and a root fresh weight average of 0.0105±
0.0041g. Control plants have a shoot fresh weight average of 0.0069 ±0.0021g and root fresh weight average of 0.0056 ±0.0027g. The average shoot fresh weight increased significantly by 38% for Streptomyces sp.1.43 inoculated plants and 29% for Streptomyces sp.1.58 inoculated plants. The average root fresh weight increased significantly by 78% for Streptomyces sp.1.43 inoculated plants and 62% for Streptomyces sp.1.58 inoculated plants.4.1.2 Effect on the root architecture upon inoculation with Streptomyces sp.1.43
or Streptomyces sp.1.58
In order to investigate the root architecture, the A. thaliana plants inoculated with Streptomyces sp.1.43 or Streptomyces sp.1.58 are harvested after 21 dpi, undergo multiple cleaning steps and are displayed on square agar plates (Figure 11). Measuring the length of the primary roots of the inoculated plants using ImageJ shows a significant decrease compared to non-inoculated plants (mock) (Figure 12).
Figure 11: Effect on the root architecture of Arabidopsis after inoculation with Streptomyces sp.1.43 or
Streptomyces sp.1.58. Exemplary scans of Streptomyces sp.1.43 or Streptomyces sp.1.58 inoculated and non-inoculated plants (mock) after 21dpi grown in the sand assay and transferred from sand to agar plates, after multiple cleaning steps.
Streptomyces sp.1.43
Streptomyces sp.1.58 mock